Abstract
Saccharomyces species, which are mostly used in the food and beverage industries, are known to differ in their fermentation efficiency and tolerance of adverse fermentation conditions. However, the basis of their difference has not been fully elucidated, although their genomes have been sequenced and analyzed. Five strains of four Saccharomyces species (S. cerevisiae, S. kudriavzevii, S. bayanus, and S. paradoxus), when grown in parallel in laboratory conditions, exhibit very similar basic physiological parameters such as membrane potential, intracellular pH, and the degree to which they are able to quickly activate their Pma1 H+-ATPase upon glucose addition. On the other hand, they differ in their ability to proliferate in media with a very low concentration of potassium, in their osmotolerance and tolerance to toxic cations and cationic drugs in a growth-medium specific manner, and in their capacity to survive anhydrobiosis. Overall, S. cerevisiae (T73 more than FL100) and S. paradoxus are the most robust, and S. kudriavzevii the most sensitive species. Our results suggest that the difference in stress survival is based on their ability to quickly accommodate their cell size and metabolism to changing environmental conditions and to adjust their portfolio of available detoxifying transporters.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Various yeast species have been used in food fermentation throughout the world for centuries. The genus Saccharomyces stands out among them, with its members being used in brewing, baking, and winemaking. Saccharomyces cerevisiae is by far the most studied, as it serves as an excellent laboratory model of an eukaryotic cell, and it is also widely exploited in modern biotechnologies due to the existence of a broad palette of tools for its genome engineering. Other members of the Saccharomyces genus are less exploited, though the Saccharomyces sensu stricto species complex contains important species for traditional fermentation processes, such as S. kudriavzevii, S. bayanus (uvarum) or S. paradoxus. In recent years, a lot of effort has been spent on elucidating the origin and level of kinship among these species, including sequencing their genomes, on characterizing their specific properties related to fermentation processes such as the production of ethanol and aroma compounds (Stribny et al. 2015; Stribny et al. 2016) or the use of various sugars as carbon sources (Kurtzman et al. 2010).
Though the Saccharomyces species share many properties, they differ significantly in their ability to grow at the low temperatures necessary for some fermentation processes, with S. kudriavzevii and S. uvarum being rather cryotolerant (Gamero et al. 2013), or in their tolerance of osmotic stress and the connected production of osmoprotective glycerol (Oliveira et al. 2014; Perez-Torrado et al. 2016). In general, Saccharomyces species have different strategies to survive under osmotic or cold-osmotic stressful conditions. Under wine-making conditions, the balance of intracellular glycerol, which mainly depends on its production, efflux and influx, is shifted to an increased level in all species. It has been shown that some species, e.g., S. cerevisiae, depend more on changing their glycerol production, whereas others tend to rely more on the varying its influx (e.g., S. bayanus and S. kudriavzevii; Perez-Torrado et al. 2016).
Most of the studies comparing the properties of Saccharomyces species were performed in various conditions resembling industrial fermentations. In this study, we estimated and compared the basic physiological parameters of four Saccharomyces species under standard laboratory conditions, which confirmed some of the results obtained in conditions simulating industrial fermentations, and moreover, identified some unexpected differences in their ability to cope with the extremely low availability of potassium, with anhydrobiosis or in their tolerance to various toxic cationic compounds.
Materials and methods
Strains
The yeast strains were the laboratory wild-type strain Saccharomyces cerevisiae FL100 (ATCC 28383), industrial strain Saccharomyces cerevisiae T73 (Lalvin, Lallemand), Saccharomyces kudriavzevii IFO1802 and its ura3 derivative, Saccharomyces bayanus (uvarum) CECT12600, and Saccharomyces paradoxus 120M. All strains, except FL100, were kindly provided by Prof. Amparo Querol (IATA-CSIC). FL100 was provided by Prof. François Lacroute (CGM-CNRS).
Media and growth conditions
Yeast cell cultures were grown in standard liquid or solid (supplemented with 2% agar) media: YPD (1% yeast extract, 2% bacto peptone, 2% glucose; pH 6.2); YNB-NH4 (0.67% yeast nitrogen base without amino acids, 2% glucose; pH 4.8) and K+-free YNB-F (0.175% YNB-F without amino acids, diammonium sulfate and potassium (Formedium™, UK), supplemented with 0.4% diammonium sulfate, 2% glucose, appropriate amount of KCl and buffered to pH 5.8 with diammonium hydroxide). For testing the utilization of various carbon sources, glucose was replaced in YNB media with 2% mannitol, 2% maltose, 2% glucitol (sorbitol), 2% galactose, 0.1% lysine, 2% lactic acid (pH adjusted to 5.5 by ammonium hydroxide) or a combination of 1.5% glycerol, and 1.5% ethanol. For pHin measurements, strains were grown in the low-fluorescence media YNBpH without diammonium sulfate, folic acid and riboflavin (MP Biomedicals) supplemented with 0.4% diammonium sulfate and 2% glucose; pH 4.5.
In general, all growth experiments were performed at two temperatures (25 and 30 °C). If there were no significant differences among the results obtained at both temperatures, only those obtained at 30 °C are shown. For growth-curve and lag-phase measurements, cells were inoculated to A600 = 0.02 in 100 μL aliquots of media in a 96-well microplate and cell growth was monitored in an ELx808 Absorbance Microplate Reader (BioTek Instruments, USA) as described previously (Maresova and Sychrova 2007).
For drop tests on solid media, cells were suspended in sterile water to A600 = 1. Serial tenfold dilutions of cell suspensions were prepared and spotted on YPD or YNB plates supplemented as indicated. All experiments were repeated at least two times and representative results are shown.
Cell size
To determine the cell size, a CasyCell Counter (Roche) with a 60-μm capillary was used. Ten microliters of cells in the mid-exponential phase of growth in YNB and in YPD was diluted in 10 mL of CasyTon® dilution buffer and subsequently analyzed according to the manufacturer’s instructions. To study cell volume changes upon the addition of KCl, an aliquot of the cell culture was transferred into the appropriate volume of growth medium with KCl to obtain 1 M KCl in the final suspension, and the cell size was measured after 5 min. The experiments were repeated three times, and each time, at least 2 × 104 cells were analyzed. Intervals containing the most typical 60% of the population are shown in the box plot diagram with the mean diameter.
Survival of abiotic stress
Cells grown to the stationary phase at 25 °C in 200 mL of YPD were dehydrated and subsequently rehydrated to estimate their anhydrobiosis tolerance (Borovikova et al. 2014). Harvested cells were washed and dehydrated by convective drying at 30 °C for 15–16 h. The dehydrated biomass was rehydrated in distilled water at room temperature for 10 min. Cell survival was estimated after appropriate dilution of the non-treated and rehydrated biomasses, respectively, plating on solid YPD and counting the colonies (cfu) after 3 days of growth at 25 °C. The experiments were repeated three times; all strains were processed in three parallels in each experiment.
Glycerol production
To analyze the total production of glycerol, cells were grown in three parallels in 20 mL of YPD media to A600 = 1 at 25 °C. Two aliquots of 1 mL were then withdrawn from each parallel culture, boiled for 10 min, mixed intensively with a vortex and centrifuged (14,000g; 10 min). The glycerol concentration was measured in the supernatant using a Glycerol Assay Kit (Sigma). The presented results are the means ± SD of three biological replicates assayed in triplicate.
Glucose-induced medium acidification
Glucose-induced medium acidification was measured using the method described in (Maresova and Sychrova 2007). Briefly, fresh cells were suspended in YNB medium without glucose containing the pH indicator (0.01% bromocresol green sodium salt) to A600 0.15. After 60 min, medium acidification was started by the addition of glucose to a final concentration of 2%. Changes in absorbance (595 nm) were recorded in an ELx808 Absorbance Microplate Reader (BioTek Instruments, USA) at 25 or 30 °C, respectively. Three aliquots of cells were measured for each strain and each set of conditions in individual experiments (technical replicates) and the presented data are means of three independent experiments with very similar results.
pHin
The pHin of strains was estimated using pHluorin, a pH-sensitive ratiometric GFP variant (Miesenbock et al. 1998). Cells transformed with either the pHl-G (S. cerevisiae, S. paradoxus, S. bayanus) or pHl-U (S. kudriavzevii ura −) plasmid (Maresova et al. 2010) were grown in YNBpH medium to A600 ≈ 0.6 at 25 or 30 °C. To maintain the pHl-G plasmid in cells, YNBpH medium was supplemented with 900 μg/mL of G418; when measuring the pHin of S. bayanus CECT12600, the amount of G418 was increased to 1200 μg/mL to enhance the fluorescence signal of cells. The fluorescence intensity ratios of growing cells were measured using a SynergyHT microplate reader (BioTek Instruments, USA) with a 516/20 nm emission filter and 400/30 and 485/20 nm excitation filters. To eliminate the background fluorescence, non-transformed cells were grown in parallel and the corresponding background fluorescence values were subtracted from the fluorescence at each excitation wavelength. The ratio of emission intensity I400 nm/I485 nm was used to calculate the pHin according to the calibration curve generated as described in (Duskova et al. 2015; Orij et al. 2009). Twenty aliquots of cells were measured for each strain in individual experiments (technical replicates) and the presented data are the means of at least three independent experiments.
Relative membrane potential (diS-C3(3) assay)
The relative membrane potential was estimated by a fluorescence assay based on the potential-dependent redistribution of the fluorescence probe diS-C3(3) (3,3′-dipropylthiacarbocyanine iodide; (Denksteinova et al. 1997; Gaskova et al. 1998)) as described in (Kodedova and Sychrova 2015). Cells were harvested, washed twice with distilled water, and resuspended in 10 mM MES buffer (pH 6.0 adjusted with triethanolamine) to A600 = 0.2. The probe was added to a final concentration of 4 × 10−8 mol/L. Fluorescence emission spectra (λex = 531 nm, λem = 560–590 nm) of the cell suspensions were measured in an ISS PC1 spectrofluorimeter equipped with a xenon lamp. The staining curves recorded the dependence of the fluorescence emission maximum wavelength λmax at the time of staining. The staining curves were fitted and the values of relative membrane potential after 25 min of staining were determined (λmax 25 ± SD). When appropriate, CCCP (carbonyl cyanide 3-chlorophenylhydrazone) was added to a final concentration in the range 5–20 μ mol/L.
Statistical analysis
Statistically significant differences were analyzed by the Student’s t test (when comparing two groups of data) or by ANOVA followed by Holm-Sidak test (for more than two groups of data) using SigmaPlot 13 and MS Office Excel software.
Results and discussion
Though various Saccharomyces species have been shown to differ in their osmotolerance and production of glycerol upon osmotic shock (Perez-Torrado et al. 2016), or in their low-temperature and ethanol tolerances (Belloch et al. 2008), little is known about their ability to cope with other stresses; and to our knowledge, their basic physiological parameters have never been studied and compared in detail to elucidate whether they are reflected in the ability to survive various stresses. For this study, we selected five strains of four species, S. cerevisiae FL100 (ATCC 28383) and T73 as representatives of a well-studied laboratory prototrophic wild type and a robust wine strain, respectively. We compared their properties with strains of S. kudriavzevii (IFO1802), S. bayanus (also known as S. uvarum; CECT 12600), and S. paradoxus (120 M).
Growth phenotypes in standard media, cell size, and potassium requirements
First we compared the growth of strains on plates with rich and minimal growth media (YPD and YNB, respectively), and on YNB-F media with very low potassium concentrations (Fig. 1), as potassium is the crucial cation that must be intracellularly accumulated to high levels (approx. 200–300 mmol/L) to ensure cell fitness (Arino et al. 2010; Navarette et al. 2010). The results obtained showed that in general, both YPD and YNB media support the growth of the yeast species very well. Only the growth of S. kudriavzevii is slower than the growth of the other four strains and this phenotype can be partly rescued by reducing the temperature (cf. Fig. 1; 30 vs. 25 °C).
Growth under standard conditions and the effect of a limited concentration of potassium. S. cerevisiae (Sc FL100 and Sc T73), S. kudriavzevii (Sk), S. bayanus (Sb) and S. paradoxus (Sp) were grown on solid YPD, YNB and YNB-F media (supplemented with the indicated amount of KCl), respectively, either at 30 or at 25 °C
With a limited amount of potassium, it was again S. kudriavzevii that exhibited problems growing. This sensitivity to low potassium was enhanced at the supraoptimal temperature (30 °C). On the other hand, S. cerevisiae T73 and S. paradoxus could grow well even in the presence of the lowest concentration of potassium (15 μmol/L) and at both temperatures. The laboratory S. cerevisiae strain (FL100) grew worse than the industrial one (T74) in the presence of low potassium and at both conditions tested. This result suggested that either the kinetic parameters of potassium uptake systems (Trk uniporters in Saccharomyces) and/or the number of corresponding genes differ among the species or that the species had a different membrane potential which is the driving force for potassium uptake via Trk systems (Arino et al. 2010; Ramos et al. 2011).
Further, we compared the size of cells growing in liquid YNB and YPD media, as well as the size change upon the transfer of the cells to a medium with a high osmotic pressure (YPD + 1 mol/L KCl). As shown in Table 1 and Fig. S1, the cells of both S. cerevisiae strains and those of S. bayanus were significantly smaller (approx. of about 15–18%) when grown in minimal YNB medium than in rich YPD. For S. paradoxus, this difference was much smaller (approx. 10%), and surprisingly, the size of S. kudriavzevii cells was almost the same in both media. An increase of growth-medium osmotic pressure (addition of KCl to a 1 mol/L final concentration) resulted in a decrease in cell size of about 10% with one exception. S. kudriavzevii did not adjust its size to the increase in osmolarity of the growth medium (Table 1 and Fig. S1). The small shrinkage of S. kudriavzevii cells upon high osmotic pressure could be related to its earlier observed salt sensitivity (Perez-Torrado et al. 2016) as the lower elasticity of the yeast cell wall and plasma membrane is reflected in the inability of a yeast cell to efficiently adjust its volume upon salt stress (Pribylova et al. 2007).
Adaptation to osmotic stress
In connection with the above-described results, we further estimated in detail the osmotolerance of the species. Osmotic stress was induced with two salts, NaCl and KCl, to distinguish between the osmotic stress and toxicity of sodium, and with glucitol to discriminate between specific salt tolerance and general osmotolerance. The obtained results are summarized in Fig. 2. As for general osmotolerance, S. cerevisiae T73 and S. paradoxus were able to grow at the highest concentrations of glucitol and KCl, whereas the other three strains were more osmosensitive (Fig. 2a). The situation was different with NaCl, where the toxicity of sodium cations contributed to the stress. S. kudriavzevii was highly NaCl sensitive, as it was not able to support NaCl concentrations higher than 1 mol/L under all tested conditions (YNB vs. YPD, 25 vs. 30 °C). Surprisingly, S. paradoxus, though highly tolerant to glucitol and KCl, was rather sensitive to high concentrations of NaCl, mainly at 25 °C. S. cerevisiae FL100 and S. bayanus tolerated almost as high concentrations of NaCl as the most osmotolerant S. cerevisiae T73. However, the level of tolerance to NaCl was growth-medium specific. Whereas for 1.5 mol/L KCl, the difference in growth inhibition among the five strains was the same in YPD and YNB media, though the growth on YNB was slower (Fig. 2a), the presence of NaCl in YNB media revealed a dramatic change, with S. cerevisiae FL100 being the most sodium tolerant (growing in up to 1.8 mol/L NaCl, not shown). S. cerevisiae FL100 was even more salt tolerant than T73 on YNB supplemented with NaCl (Fig. 2), and the other three species were highly salt sensitive and almost did not grow if YNB was supplemented with 1 mol/L NaCl (Fig. 2a).
Growth in the presence of high osmotic pressure. a Growth on solid YPD or YNB media supplemented with salts and glucitol as indicated, either at 30 or at 25 °C. b The length of the growth lag-phase in liquid YPD media (black columns) supplemented with 1.5 mol/L KCl (light gray columns) or 1.5 M glucitol (dark gray columns). Asterisk indicates growth lag phase significantly different from the lag phase measured in YPD (* P < 0.05; ** P < 0.001). Sc, S. cerevisiae; Sk, S. kudriavzevii; Sb, S. bayanus; Sp, S. paradoxus
The observed difference in salt tolerance among the species and growth media suggested that either the influx of toxic sodium cations into the cells differs in YNB and YPD or the expression and/or activity of sodium exporters is not the same in the cells growing in the two media. The uptake of sodium into yeast cells is not specific and is driven by the membrane potential (Arino et al. 2010; Ramos et al. 2011). To verify the first possibility of different sodium influx resulting from different levels of plasma-membrane potential, we measured the relative membrane potential of the five strains (cf. below section 3.5.).
The efflux of sodium cation from S. cerevisiae cells is mainly mediated by highly inducible Ena1 ATPase during salt stress (Arino et al. 2010; Ruiz and Arino 2007). But as it was shown previously (Garciadeblas et al. 1993; Wieland et al. 1995), the salt tolerance of S. cerevisiae strains also depends on the number of ENA copies in their genome. The difference observed for S. cerevisiae FL100 and T73 on YPD and YNB media supplemented with NaCl suggests that the ability of these two strains to eliminate toxic sodium differs in YPD and YNB media, and it would be interesting to compare the number of ENA genes, the level of their expression upon salt stress in YNB and YPD, and the activity of Ena ATPases of the two strains in the future experiments. Similarly, it would be interesting to search the genomes of the other three species, find a possible correlation between the number of their ENA genes and their salt tolerance, and finally compare the expression of these genes in YNB and YPD supplemented with NaCl.
The slight differences in the growth of strains on YPD plates with KCl and glucitol, i.e., the lowest sensitivity of S. cerevisiae T73 and S. paradoxus to osmotic stress, were confirmed when the lag phase of the five strains was compared (Fig. 2b). Though the transfer of cells from YPD to YPD supplemented with 1.5 mol/L KCl or 1.5 M glucitol significantly extended the lag phases of all the cultures, the growth of S. cerevisiae T73 and S. paradoxus cultures restarted first (Fig. 2b). Similar results were obtained when the start of the cultures’ growth was monitored at 25 °C (results not shown). These results, together with the estimation of the whole growth curves (not shown), confirmed the results obtained on the plates (Fig. 2a) that S. cerevisiae T73 and S. paradoxus are osmotolerant in general and have the quickest adaptation to an increased osmolarity produced by non-toxic solutes. Similarly, our results identifying S. kudriavzevii as a highly osmosensitive species confirmed those observed earlier (Perez-Torrado et al. 2016).
Production of glycerol in standard growth conditions
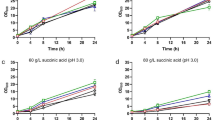
Glycerol is the main solute produced by yeast cells to compensate for increased environmental osmolarity (Hohmann 2002). The shorter lag phases of some of the species described above could be connected to a higher amount of glycerol produced by these species before the application of the osmotic stress. To verify this possibility, the total production of glycerol during the growth of cells in YPD was measured. Figure 3 shows that contrary to this presumption, S. cerevisiae T73 and S. paradoxus produced less glycerol during the exponential phase of growth in YPD than the other species, and it was the most osmosensitive yeast, S. kudriavzevii, that produced the highest amount of glycerol under the tested conditions. It is worth noting that Fig. 3 shows the production of glycerol in cells grown at 25 °C, i.e., under conditions optimal for S. kudriavzevii so the higher amount of glycerol produced by this species is not a result of supraoptimal temperature stress. The observed differences in the glycerol production of non-stressed cells in YPD supplement our knowledge on the glycerol production of the same strains during microvinification experiments (Perez-Torrado et al. 2016). Our results suggest that, at least in some Saccharomyces species, a good and fast adaptation to a sudden osmotic stress is not proportional to the already available amount of glycerol.
Glycerol production under standard growth conditions. The glycerol content was estimated in YPD cultures of A600 = 1. The results represent the amount of glycerol in 1 mL of cell culture. The average results of three independent experiments are presented ± SD. Values that do not differ significantly (P < 0.05) share a common letter. Sc, S. cerevisiae; Sk, S. kudriavzevii; Sb, S. bayanus; Sp, S. paradoxus
Survival of anhydrobiosis
In a natural environment, yeasts are periodically subjected to quite strong dehydration, resulting in the state of anhydrobiosis. This unique state of live organisms is linked with a temporary reversible suspension of metabolism for the periods of unfavorable environmental conditions. Upon rehydration, the cell functions can be restored and the cells start to grow and divide again (Dupont et al. 2014; Rapoport 2017). This ability is widely utilized, mainly in food-related biotechnology processes producing or employing so-called “dry yeast” (Beker and Rapoport 1987). The ability to survive subsequent desiccation and rehydration is connected, besides several others such as trehalose, glutathione or proline (Eleutherio et al. 2015; Espindola et al. 2003; Takagi 2008), to two osmolytes, glycerol and potassium ions (Borovikova et al. 2014; Bubnova et al. 2014). Thus, the ability to produce and take up glycerol, as well as the existence of effective uptake systems for potassium belong among key players in the ability of yeast cells to survive the loss of water during drying and the hypoosmotic shock during the rehydration process. Our tests of anhydrobiosis survival revealed that, again, S. kudriavzevii was the most sensitive to the stress (none of its cells survived; Fig. 4), and S. cerevisiae T73 and S. paradoxus survived the drying and subsequent rehydration much better than the other species (Fig. 4).
Survival of anhydrobiosis. Cells from the stationary phase of growth in liquid YPD at 25 °C were dehydrated at 30 °C for 15–16 h, and rehydrated in water at room temperature for 10 min. The viability of cells (survival in %, determined as cfu) is shown ± SD. Values that do not differ significantly (P < 0.05) share a common letter. Sc, S. cerevisiae; Sk, S. kudriavzevii; Sb, S. bayanus; Sp, S. paradoxus
In summary, the observed differences in (1) osmotolerance, (2) length of adaptation to a sudden osmotic stress, (3) the capacity to survive anhydrobiosis among the tested species, and (4) levels of produced glycerol in non-stressed conditions indicated that the studied species, though close relatives, significantly vary in their ability to accommodate to changing environmental conditions. Our results are in accord with a large study of the gene expression regulation in the four yeast species during osmotic stress (Perez-Torrado et al. 2016).
The above described differences among the strains and species also suggested that they could differ in some basic physiological parameters such as membrane potential, the activity of Pma1, and intracellular pH.
Relative membrane potential and sensitivity to cationic drugs
The usual way to estimate the differences in relative membrane potential among yeast strains and species is (1) testing their sensitivity to various cationic drugs which share a nonspecific mechanism of uptake driven by plasma-membrane potential (negative inside) but differ in their mechanism of action inside the cells (Barreto et al. 2011) and (2) estimating the relative membrane potential by a fluorescence technique employing the potential-dependent distribution of a fluorescent dye (Gaskova et al. 1998).
First, we tested the sensitivity of strains to three cationic drugs—Hygromycin B, tetramethylammonium (TMA), and spermine—added to YPD media. It has been repeatedly shown (Barreto et al. 2011; Llopis-Torregrosa et al. 2016; Navarette et al. 2010; Stribny et al. 2012) that a relative hyperpolarization of yeast cells results in an increased sensitivity to all these drugs, as well as to toxic lithium cations, which we also tested.
Surprisingly, we could not find a general correlation among the sensitivities to the four tested compounds. As shown in Fig. 5a, S. kudriavzevii was, in general, the most sensitive to all compounds except TMA, and S. cerevisiae T73 was highly tolerant to all three cationic drugs and LiCl. These results suggested that S. kudriavzevii could have a higher, whereas S. cerevisiae T73 a lower membrane potential. On the other hand, S. cerevisiae FL100 was only highly tolerant to LiCl, relatively tolerant to Hygromycin B, but sensitive to spermine and partly to TMA. Very interesting and unexpected results were obtained for S. bayanus and S. paradoxus. S. bayanus tolerated TMA and spermine well, but it was sensitive to Hygromycin B and mainly LiCl. On the other hand, S. paradoxus was highly sensitive to TMA and spermine, but relatively tolerant to Hygromycin B and highly resistant to the presence of LiCl (Fig. 5a).
Effect of cationic drugs, lithium cations and estimation of relative membrane potential. a Growth on solid YPD plates with indicated concentrations of Hygromycin B, spermine, TMA and lithium chloride at 30 °C. b Relative membrane potential of cells grown in liquid YPD or YNB. The average results of three independent experiments are presented ± SD. Asterisk indicates a significant difference in relative membrane potential between exponential and postdiauxic (YPD) or early and late exponential (YNB) growth phase cells (* P < 0.05; ** P < 0.01; *** P < 0.001). Sc, S. cerevisiae; Sk, S. kudriavzevii; Sb, S. bayanus; Sp, S. paradoxus; n.d., not determined. c The effect of CCCP addition on relative membrane potential. The CCCP (15 μmol/L final concentration) was added 8 min after the diS-C3(3) probe. □, S. cerevisiae FL100; ○, S. cerevisiae T73; ◊, S. kudriavzevii; ∇, S. bayanus; Δ, S. paradoxus. Open symbols, control; closed symbols, after the addition of CCCP (indicated with an arrow)
The S. paradoxus tolerance of LiCl was particularly surprising, given regarding the above-average sensitivity of this species to NaCl (Fig. 2a). Lithium and sodium cations are thought to enter yeast cells using a similar mechanism (potential-driven uptake mediated by various non-specific transporters), and they are detoxified similarly as well, either by sequestration in vacuoles (transported there by Nhx1 and Vnx1 cation/proton antiporters) or by an active efflux in which the plasma-membrane cation/proton antiporter (Nha1) and mainly Ena ATPases play a role (Arino et al. 2010; Ramos et al. 2011). Thus, the observed difference in the tolerance of Li+ and Na+ suggests that either lithium is, for an unknown reason, less toxic for S. paradoxus, or the relevant transport systems mediating the efflux and sequestration of the two cations discriminate among these substrates and have a higher affinity/capacity for lithium than for sodium. To verify this hypothesis, it will be necessary to express the relevant S. paradoxus transporters in a transporter-less S. cerevisiae mutant (e.g., BYT4589 lacking both plasma-membrane and vacuolar transporters of alkali metal cations; (Petrezselyova et al. 2013), estimate their capacities to transport both cations and compare them with those of S. cerevisiae Nha1, Ena1, Nhx1, and Vnx1.
As none of the studied strains, except S. cerevisiae T73, had a consistently higher (or lower) tolerance to all the tested compounds, the hypothesis of a possible difference in the plasma-membrane potential among the strains, drawn from the results of testing NaCl tolerance, was not confirmed. To further characterize the basic physiological parameters of the studied strains, we first estimated the relative membrane potential of cells from the exponential phase of growth in YPD. Both S. cerevisiae strains and S. paradoxus were stained with a potential-responding fluorescent dye to a similar level (Fig. 5b), whereas S. kudriavzevii possibly exhibited a very slight and S. bayanus a higher hyperpolarization relative to the other strains. Although S. bayanus cells exhibited a higher staining with a potentiometric probe in YPD, this did not correspond with a simultaneous relatively good tolerance of this species to the various tested drugs (cf. above and Fig. 5).
When the changes in relative membrane potential were estimated later during the postdiauxic phase of growth, an increase in staining was observed (λmax25 ~ 580), that as similar in four strains (Fig. 5b). The relative membrane potential of S. kudriavzevii was not estimated because of a very high cell flocculation in the postdiauxic phase of culture growth. This increase in staining is a well-known phenomenon in S. cerevisiae, where it was shown to correspond to a decreased activity of MDR pumps, for which the fluorescence dye is a substrate (Cadek et al. 2004; Malac et al. 2005). When the relative membrane potential was estimated during growth in YNB, it revealed that the cells of all the strains, except S. bayanus, had a higher membrane potential in the exponential phase of growth in YNB than in YPD. Postdiauxic and late-exponential cells of all the strains had similar membrane potential, regardless of the growth medium (Fig. 5b). When growing in YNB, both strains of S. cerevisiae seemed to have a significantly lower membrane potential than the other three species, but this difference was not reflected in a higher tolerance to cationic drugs in YNB medium (results not shown).
Only S. bayanus cells were stained to a similar level in both growth media, and they also differed from the other strains when the effect of the CCCP protonophore (5–20 μmol/L) on the relative membrane potential was tested. As expected, the addition of CCCP (shown for 15 μmol/L in Fig. 5c) produced a deep and rapid depolarization of both S. cerevisiae species, followed by a slower repolarization and even a hyperpolarization. The observed hyperpolarization is mainly a result of the CCCP-mediated activation of Pma1 H+-ATPase of the plasma membrane, though some increase in the staining is also related to the fact that CCCP is a substrate of MDR pumps, as is the fluorescent dye used. This means that the efflux of the dye is competitively inhibited by CCCP (Hendrych et al. 2009). CCCP also produced similar effects to those in S. cerevisiae in S. kudriavzevii and S. paradoxus cells, but it had no effect on S. bayanus (Fig. 5c), even when a higher concentration (20 μmol/L) was used (results not shown).
Intracellular pH and activity of Pma1
The higher level of membrane potential of S. bayanus growing in YPD, and its insensitivity to CCCP, suggested that Pma1 activity and/or its regulation in this species may be different from the other tested Saccharomyces species. To verify this hypothesis, we estimated, using pHluorin expression, the intracellular pH of the growing cells of all species and tested the activation of their Pma1 ATPases by the addition of glucose. Glucose is well known to quickly activate Pma1 ATPase in S. cerevisiae (Serrano et al. 1986; Sychrová and Kotyk 1985).
Figure 6a shows that the exponentially growing cells of all strains have very similar intracellular pH of around 7, what is a typical value of cytosolic pH of growing S. cerevisiae cell (Felcmanova et al. 2017; Orij et al. 2011). No statistically significant differences were found among the strains, which suggests that the observed differences in relative membrane potential (Fig. 5b) are not reflected in the level of intracellular pH.
Intracellular pH and glucose-induced activation of Pma1. a Intracellular pH of cells from exponential phase of growth in YNB. The mean results of at least three independent experiments are presented ± SD. No statistically significant differences among strains were found. Sc, S. cerevisiae; Sk, S. kudriavzevii; Sb, S. bayanus; Sp, S. paradoxus. b Changes in extracellular pH upon addition of glucose to cell suspension. □, S. cerevisiae FL100; ○, S. cerevisiae T73; ◊, S. kudriavzevii; ∇, S. bayanus; Δ, S. paradoxus. Open symbols, without glucose addition; closed symbols after addition of 2% glucose (indicated with an arrow)
The activation of Pma1 was measured, using the standard technique, as the acidification of external media upon the addition of glucose to the cell suspension. All strains responded to the addition of 2% glucose similarly (Fig. 6b), with S. cerevisiae FL100 and S. kudriavzevii exhibiting the slowest acidification rate. It is worth noting that similar results were obtained when the experiment was performed at 25 °C; thus, the supraoptimal temperature (30 °C) did not affect the activity of S. kudriavzevii Pma1, and the observed slow growth of this species in various experiments at 30 °C was not connected to an insufficient activity of Pma1 or the inability to maintain pHin homeostasis.
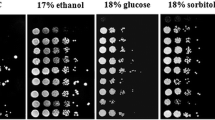
Glucose, which was able to activate Pma1 in all species, seemed to be also the best source of carbon for all of them (Fig. S2). All strains grew relatively well on 2% maltose as well, but their ability to use galactose and mainly non-sugar sources of carbon differed. Galactose only enabled S. bayanus to grow. A very interesting finding was that S. kudriavzevii grows better on YNB plates supplemented with mannitol than on YNB plates supplemented with glucitol (Fig. S2). This result suggested the existence of polyol transporters with a higher affinity to mannitol than to glucitol or different levels of mannitol and glucitol dehydrogenases, respectively, in this species. This is different from the situation in S. cerevisiae, where four transporters of the Hxt family transport glucitol and mannitol similarly well (Jordan et al. 2016).
Altogether, our results showed that though the four Saccharomyces species differ in their previously described parameters and phenotypes, such as their cryotolerance, production of glycerol or osmotolerance, their basic physiological parameters that are essential for their proliferation, fitness, and stress tolerance (e.g., plasma-membrane potential, intracellular pH and ability to activate Pma1 ATPase) are almost identical. Thus, in this study, the observed differences in their abilities to cope with the stresses of (1) limited potassium availability, (2) desiccation and rehydration, or (3) toxic sodium and lithium cations and various cationic drugs probably instead reflect differences in the activity of various specific transporters which provide the cells with potassium and glycerol, or eliminate toxic cations and drugs, than the difference in the available sources of energy for transport (membrane potential, protonmotive force or ATP). Our findings will lead to future experiments in which we will characterize the differences of relevant specific transporters in the four Saccharomyces species and, subsequently, try to improve the tolerances of sensitive species, e.g., the salt-tolerance of S. kudriavzevii by the introduction of sodium and lithium exporters from S. cerevisiae or highly osmotolerant yeasts. This osmotolerance improvement may be useful in various food and biotechnology applications of Saccharomyces yeast species.
References
Arino J, Ramos J, Sychrova H (2010) Alkali metal cation transport and homeostasis in yeasts. Microbiol Mol Biol Rev 74:95–120
Barreto L et al (2011) A genomewide screen for tolerance to cationic drugs reveals genes important for potassium homeostasis in Saccharomyces cerevisiae. Eukaryot Cell 10:1241–1250
Beker MJ, Rapoport AI (1987) Conservation of yeasts by dehydration. In:Scheper Th, Belkin S, Bley Th, Bohlmann J, Gu M.B, Hu W.-S, Mattiasson B, Nielsen J, Seitz H, Ulber R, Zeng A.-P, Zhong J.-J, Zhou W (eds) Advances in Biochemical Engineering / Biotechnology, vol 35. Springer Verlag Berlin Heidelberg NewYork London Paris Tokyo, pp 127–171
Belloch C, Orlic S, Barrio E, Querol A (2008) Fermentative stress adaptation of hybrids within the Saccharomyces sensu stricto complex. Int J Food Microbiol 122:188–195
Borovikova D, Herynkova P, Rapoport A, Sychrova H (2014) Potassium uptake system Trk2 is crucial for yeast cell viability during anhydrobiosis. FEMS Microbiol Lett 350:28–33
Bubnova M, Zemancikova J, Sychrova H (2014) Osmotolerant yeast species differ in basic physiological parameters and in tolerance of non-osmotic stresses. Yeast 31:309–321
Cadek R, Chladkova K, Sigler K, Gaskova D (2004) Impact of the growth phase on the activity of multidrug resistance pumps and membrane potential of S. cerevisiae: effect of pump overproduction and carbon source. Biochim Biophys Acta 1665:111–117
Denksteinova B, Gaskova D, Herman P, Vecer J, Malinsky J, Plasek J, Sigler K (1997) Monitoring of membrane potential changes in Saccharomyces cerevisiae by diS-C3(3) fluorescence. Folia Microbiol (Praha) 42:221–224
Dupont S, Rapoport A, Gervais P, Beney L (2014) Survival kit of Saccharomyces Cerevisiae for anhydrobiosis. Appl Microbiol Biot 98:8821–8834
Duskova M, Ferreira C, Lucas C, Sychrova H (2015) Two glycerol uptake systems contribute to the high osmotolerance of Zygosaccharomyces rouxii. Mol Microbiol 97:541–559. https://doi.org/10.1111/mmi.13048
Eleutherio E, Panek A, De Mesquita JF, Trevisol E, Magalhaes R (2015) Revisiting yeast trehalose metabolism. Curr Genet 61:263–274
Espindola AD, Gomes DS, Panek AD, Eleutherio ECA (2003) The role of glutathione in yeast dehydration tolerance. Cryobiology 47:236–241
Felcmanova K, Neveceralova P, Sychrova H, Zimmermannova OP (2017) Yeast Kch1 and Kch2 membrane proteins play apleiotropic role in membrane potential establishmentand monovalent cation homeostasis regulation FEMS. Yeast Res 17:fox053
Gamero A, Tronchoni J, Querol A, Belloch C (2013) Production of aroma compounds by cryotolerant Saccharomyces species and hybrids at low and moderate fermentation temperatures. J Appl Microbiol 114:1405–1414. https://doi.org/10.1111/jam.12126
Garciadeblas B, Rubio F, Quintero FJ, Banuelos MA, Haro R, Rodriguez-Navarro A (1993) Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces Cerevisiae. Mol Gen Genet 236:363–368
Gaskova D et al (1998) Fluorescent probing of membrane potential in walled cells: diS-C-3(3) assay in Saccharomyces Cerevisiae. Yeast 14:1189–1197
Hendrych T, Kodedova M, Sigler K, Gakova D (2009) Characterization of the kinetics and mechanisms of inhibition of drugs interacting with the S. cerevisiae multidrug resistance pumps Pdr5p and Snq2p. Biochim Biophys Acta 1788:717–723
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372
Jordan P, Choe JY, Boles E, Oreb M (2016) Hxt13, Hxt15, Hxt16 and Hxt17 from Saccharomyces cerevisiae represent a novel type of polyol transporters. Sci Rep 6:23502. https://doi.org/10.1038/srep23502srep23502
Kodedova M, Sychrova H (2015) Changes in the sterol composition of the plasma membrane affect membrane potential, salt tolerance and the activity of multidrug resistance pumps in Saccharomyces cerevisiae. PLoS One 10:e0139306. https://doi.org/10.1371/journal.pone.0139306PONE-D-15-28879
Kurtzman CP, Fell JW, Boekhout T (2010) The yeasts, a taxonomic study, 5th Edition edn. Elsevier, Amsterdam
Llopis-Torregrosa V, Husekova B, Sychrova H (2016) Potassium uptake mediated by Trk1 is crucial for Candida glabrata growth and fitness. Plos One 11:DOI:https://doi.org/10.1371/journal.pone.0153374
Malac J, Urbankova E, Sigler K, Gaskova D (2005) Activity of yeast multidrug resistance pumps during growth is controlled by carbon source and the composition of growth-depleted medium: DiS-C-3(3) fluorescence assay. Int J Biochem Cell Biol 37:2536–2543
Maresova L, Sychrova H (2007) Applications of a microplate reader in yeast physiology research. BioTechniques 43:667–672
Maresova L, Hoskova B, Urbankova E, Chaloupka R, Sychrova H (2010) New applications of pHluorin-measuring intracellular pH of prototrophic yeasts and determining changes in the buffering capacity of strains with affected potassium homeostasis. Yeast 27:317–325
Miesenbock G, De Angelis DA, Rothman JE (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394:192–195
Navarette C et al (2010) Lack of main K+ uptake systems in Saccharomyces cerevisiae cells affects yeast performance both in potassium sufficient and limiting conditions. FEMS Yeast Res 10:508–517
Oliveira BM, Barrio E, Querol A, Perez-Torrado R (2014) Enhanced enzymatic activity of glycerol-3-phosphate dehydrogenase from the cryophilic Saccharomyces kudriavzevii. PLoS One 9:doi.https://doi.org/10.1371/journal.pone.0087290
Orij R, Postmus J, Ter Beek A, Brul S, Smits GJ (2009) In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces cerevisiae reveals a relation between intracellular pH and growth. Microbiology-(UK) 155:268–278
Orij R, Brul S, Smits GJ (2011) Intracellular pH is a tightly controlled signal in yeast. Biochim Biophys Acta 1810:933–944
Perez-Torrado R, Oliveira BM, Zemancikova J, Sychrova H, Querol A (2016) Alternative glycerol balance strategies among Saccharomyces species in response to winemaking stress. Front Microbiol 7:DOI: https://doi.org/10.3389/fmicb.2016.00435
Petrezselyova S, Kinclova-Zimmermannova O, Sychrova H (2013) Vhc1, a novel transporter belonging to the family of electroneutral cation-Cl(-) cotransporters, participates in the regulation of cation content and morphology of Saccharomyces cerevisiae vacuoles. Biochim Biophys Acta 1828:623–631
Pribylova L, Farkas V, Slaninova I, de Montigny J, Sychrova H (2007) Differences in osmotolerant and cell-wall properties of two Zygosaccharomyces rouxii strains. Folia Microbiol (Praha) 52:241–245
Ramos J, Arino J, Sychrova H (2011) Alkali-metal-cation influx and efflux systems in nonconventional yeast species. FEMS Microbiol Lett 317:1–8
Rapoport A (2017) Anhydrobiosis and dehydration of yeasts. In: Sibyrny A (ed) Biotechnology of Yeasts and Filamentous Fungi. Springer international Publishing, Basel, pp 87–116
Ruiz A, Arino J (2007) Function and regulation of the Saccharomyces cerevisiae ENA sodium ATPase system Eukaryot. Cell 6:2175–2183
Serrano R, Kielland-Brandt MC, Fink GR (1986) Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature 319:689–693
Stribny J, Kinclova-Zimmermannova O, Sychrova H (2012) Potassium supply and homeostasis in the osmotolerant non-conventional yeasts Zygosaccharomyces rouxii differ from Saccharomyces cerevisiae. Curr Genet 58:255–264. https://doi.org/10.1007/s00294-012-0381-7
Stribny J, Gamero A, Perez-Torrado R, Querol A (2015) Saccharomyces kudriavzevii and Saccharomyces uvarum differ from Saccharomyces cerevisiae during the production of aroma-active higher alcohols and acetate esters using their amino acidic precursors. Int J Food Microbiol 205:41–46. https://doi.org/10.1016/j.ijfoodmicro.2015.04.003S0168-1605(15)00184-1
Stribny J, Querol A, Perez-Torrado R (2016) Differences in enzymatic properties of theSaccharomyces kudriavzevii and Saccharomyces uvarum alcohol acetyltransferases and their impact on aroma-active compounds production. Front Microbiol 7:897. https://doi.org/10.3389/fmicb.2016.00897
Sychrová H, Kotyk A (1985) Conditions of activation of yeast plasma membrane ATPase. FEBS Lett 183:21–24
Takagi H (2008) Proline as a stress protectant in yeast: physiological functions, metabolic regulations, and biotechnological applications. Appl Microbiol Biot 81:211–223
Wieland J, Nitsche AM, Strayle J, Steiner H, Rudolph HK (1995) The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J 14:3870–3882
Acknowledgements
The authors wish to thank Dr. A. Querol and Prof. F. Lacroute for the strains and Dr. O. Zimmermannova for critical reading of the manuscript.
Funding
This work was supported by the Czech National Science Foundation (grant number GA CR 15-03708S) and the European Union (grant number FP7-ITN-264717 Cornucopia).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Fig. S1
Cell diameter. The size of exponentially growing cells in YPD, YPD supplemented with 1 mol/L KCl and YNB was estimated and the intervals containing the most typical 60% of the cell populations are shown as a grey box. The mean diameter is represented by a line inside the box. Average results are shown ± SD. Asterisk indicates a significant difference in cell size between YPD and YNB media and upon transfer into media supplemented with 1 mol/L KCl (* P < 0.05, ** P < 0.01, *** P < 0.001). Sc, S. cerevisiae; Sk, S. kudriavzevii; Sb, S. bayanus; Sp, S. paradoxus. (TIFF 823 kb)
Fig. S2
Use of various carbon sources. Growth on solid YNB media supplemented with indicated carbon sources. Sc, S. cerevisiae; Sk, S. kudriavzevii; Sb, S. bayanus; Sp, S. paradoxus. (TIFF 3172 kb)
Rights and permissions
About this article
Cite this article
Zemančíková, J., Kodedová, M., Papoušková, K. et al. Four Saccharomyces species differ in their tolerance to various stresses though they have similar basic physiological parameters. Folia Microbiol 63, 217–227 (2018). https://doi.org/10.1007/s12223-017-0559-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-017-0559-y