Abstract
The key cell population permits cancer cells to avoid immune-surveillance is regulatory T cells (Tregs). This study evaluates the level of Tregs in chronic myeloid leukemia (CML) patients and the effect of Tyrosine kinase inhibitor (TKI) on Treg levels, as a pathway to understand the immune response and behavior among advance stage and optimal response CML patients using imatinib therapy. Blood samples were collected from 30 CML patients (optimal response to TKI), 30 CML patients (failure response to TKI), and 30 age- and gender-matched controls. Analysis involved measuring percentages of Tregs (CD4 + CD25 + FOXP3 +) by flow cytometer and demethylation levels of FOXP3 Treg-specific demethylated region (TSDR) by PCR. The data revealed that Tregs and the FOXP3-TSDR demethylation percentages significantly increased in failure response group in comparison to the optimal response and control groups, while no significant difference between optimal response and control groups. Tregs and FOXP3 TSDR demethylation percentages showed high sensitivity and specificity, suggesting powerful discriminatory biomarkers between failure and optimal groups. An assessment of the Tregs and demethylation percentage among different BCR-ABL levels of CML patients on TKI revealed no significant differences in parameter percentage in the optimal response to TKI patients with different molecular responses (log 3 reduction or other deeper log 4.5 and 5 reduction levels). Our findings demonstrate an effective role of functional Tregs among different CML stages. Also, the study suggests that the major molecular response to therapy at level 0.1% of BCR-ABL transcript could be enough to induce immune system restoration in patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic myeloid leukemia (CML) is a clonal malignant myeloproliferative disease distinguished by the chromosome 9 and 22 long arms rearrangement that creates aberrant chromosome termed “Philadelphia (Ph) chromosome” and results in the formation of fusion oncogene known as BCR-ABL1 (Jabbour and Kantarjian 2016). The introduction of tyrosine kinase inhibitor (TKI) therapies has revolutionized treatment options of CML (Kennedy and Hobbs 2018). Tyrosine kinase drugs inhibit the tyrosine kinase produced by the BCR-ABL oncogene that promotes molecular response (MR) and cytogenetic recovery with substantial survival improvement for most of the patients (Saubele et al. 2016). However, despite the impressive efficacy against proliferating CML cells by the TKI, it remains for the most part incurable with exception to allogeneic hematopoietic stem cell transplantation (HSCT), as TKIs are unable to eradicate the tiny fraction of quiescent stem cells that are responsible for disease persistence and recurrence upon TKI discontinuation (Hochhaus et al. 2017, 2020).
Immune response plays a significant role in preventing CML progression or recurrence that has derived from the residual leukemic cell (Ilander and Mustjoki 2017).
The FoxP3-expressing regulatory T cells (Tregs), which are the immune suppressive cells inhibit immune-mediated response against leukemic cells, thereby contributing to disease progression in CML patients (Hughes and Yong 2017). Evidence showed a clear variation in the Treg levels across different stages of CML disease (Zahran et al. 2014). TKI therapy is found to have a direct impact on the level of Treg cells (Hsieh et al. 2021). At diagnosis, an increased percentage of Treg cells is noticed (Hughes et al. 2017) while lower numbers of these cells are found in patients achieving deep molecular response (DMR) to TKI therapy (Tanaka et al. 2020). Overall, the high levels of Treg are associated with poor outcome and low survival rate (Cheng et al. 2017), indicating their prominent role in the course of the disease.
As the FoxP3 is a major regulator for the development and function of Tregs (Fontenot et al. 2003; Hori et al. 2003), this study has analyzed the percentage of Tregs using flowcytometry and has measured the demethylation levels of FoxP3 in order to evaluate this cell in CML patients.
This study aimed to investigate the contribution of Treg and TKI efficacy by examining the level of these cells in CML patients with different molecular responses to TKI therapy. We hypothesize that high levels of Tregs cells might contribute to the failure of the TKI therapy and disease progression. This information could be harnessed to discover new therapies that boost anti leukemic immunity against TKI-insensitive leukemia stem cells.
Materials and methods
Study design
This is a multi-center analytical observational case–control study conducted between Feb. 2019 till Mar. 2020. Sixty cases of CML on imatinib myesylate therapy for at least 12 months were recruited from Baghdad Teaching Hospital/Medical City and the National Center of Hematology.
The molecular response assessment of imatinib myselate therapy for the studied groups followed the European Leukemia Net (ELN) 2013 criteria (Baccarani et al. 2013) and was confirmed by real-time quantitative polymerase chain reaction (RQ-PCR) results for BCR-ABL1 transcripts that have taken within 3 months duration from the sampling time.
Molecular response to TKI assessed according to the International Scale (IS) as the ratio of BCR-ABL1 transcripts to ABL1 transcripts. Ethics approval is sought from the scientific ethical committee of the College of Medicine, Baghdad University and informed consent is obtained from each participant prior to the enrollment in this study.
Study subjects
Sixty CML patients were enrolled in this study; thirty of them were with a complete molecular response (p210 BCR-ABL transcript levels ≤ 0.1% IS) and are classified as an optimal response group to imatinib therapy. Another 30 cases lost their molecular response with or without the hematological response (p210 BCR-ABL transcript levels > 0.1% IS) and were classified as failure response group. The recent molecular data were taken from the patients’ records within the maximum duration results of 3 months from sampling time. Thirty apparently healthy volunteers, age-and gender-matched subjects, were considered as a control group in the current study. At the time of sampling, blood count indices were obtained and calculated for each subject by automated blood count analyzer.
Immune flow cytometry staining of regulatory T cells
To recognize the immune phenotype of regulatory T cells, a staining protocol was performed to all collected peripheral blood (PB) samples in EDTA according to Biolegend manufactures instructions and the method described previously (Zahran and Elsayh 2012; Zahran et al. 2014).
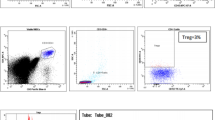
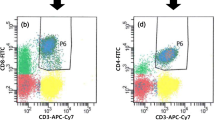
Flurochrome conjugated anti-human antibodies include CD45 conjugated to fluorescein isothiocyanate (FITC), CD4 conjugate to allophycocyanin /CY7 (APC/CY7), CD25 conjugate to allophycocyanin (APC), and FoxP3 conjugated to phycoerythrin (PE) purchased from (Biolegend, USA) are used. The extracellular staining is done by incubating 100 μl of anti-coagulated blood with a 3 μl of each fluorescence conjugated antibody. Another tube had only 100 μl of whole blood used as an unstained control with each run. After 15 min of dark incubation at room temperature, the erythrocyte lysis solution is added for 10 min at room temperature; then after centrifugation, the supernatant is discarded and the cells pellet are washed with cell wash solution (PBS). Thereafter; the cells are fixed and permeabilized by using True-Nuclear Transcription Factor buffer set (Biolegend, USA). To allow subsequent intracellular FoxP3 staining, a 3 μl of FoxP3 antibody is added and the tube is incubated in the dark at room temperature for 1 hr. After centrifugation, the supernatant is discarded and the cells pellet are washed with cell wash solution (PBS) and resuspend with 0.5 ml of 1 × cell fix buffer. Thereafter, tubes are acquired on flow cytometry FACS Canto II (BD BIOSCENCES) and analyzed with DIVA software version 2016 using an immunological gate (SSC/CD45) to select lymphocyte, along with CD4 marker used to differentiate CD4 + Tlymphocyte in all studied groups as shown in (Fig. 1a, b). Treg cells are determined by the proportion of CD4+CD25+FOXP3+ in all CD4 + T cells for each group, as shown in (Fig. 1c–e).
Flow cytometry analysis of regulatory T cells. a side scatter (SSC) vs. CD45 dot plot to identify lymphocyte, monocyte, and granulocyte populations b followed by gating on side scatter (SSC) vs. CD4 dot plot to identify CD4 + cells c CD25 vs. FOXP3 dot plot to identify Tregs (CD4 + CD25 + FOXP3 +) populations among the CD4 + T lymphocyte for control group sample. d CD25 vs. FOXP3 dot plot to identify Tregs (CD4 + CD25 + FOXP3 +) populations among the CD4 + T lymphocyte for an optimal response to TKI sample. e CD25 vs. FOXP3 dot plot to identify Tregs (CD4 + CD25 + FOXP3 +) populations among the CD4 + T lymphocyte for a failure response to the TKI sample
At least 100,000 events are analyzed for each sample. Data from the flow cytometer hierarch tables are used to calculate the cell populations of interest in all studied groups, and the value is obtained as a percentage.
Quantitative methylation-specific polymerase chain reaction (qMSP)
In this study, qMSP is used to analyze the methylation status of CpG dinucleotides in the TSDR of the FOXP3 gene of a Treg cell. Genomic DNA is isolated from whole blood sample by using protocol ReliaPrep™ Blood gDNA Miniprep System protocol (Promega, USA). The concentrations of extracted DNA are detected by using QuantiFluor® dsDNA system (Promega, USA), the range of the extracted DNA quantity was (22–40 ng/μl). DNA conversion by the bisulfite method is carried out by the MethylEdge™ Bisulfite Conversion System (Promega, USA) from the extracted DNA and achieved by using thermocycler which is programed as follows: 98 °C for 8 min, 54 °C for 60 min, and 4 °C hold. The primer selection and the cycling conditions of qMSP are done according to (Zafari et al. 2018). The primers selected for FOXP3-TSDR (methylated and unmethylated) were supplied by Macrogen Company (Korea) shown in (Supplementary file: Table 1). A 177 base-pair segment was the targeted DNA for PCR amplification, which was included in CpG island in the first intron of the FOXP3 gene. In the target DNA, the number of CpG sites was ten. The PCR reaction mixture was done in a total 10 μl volume and applied to MIC qPCR cycler machine. The cycling conditions of qMSP for analysis TSDR shown in (Supplementry file: Table 2).
This study used a positive control the CpG methylated human blood genomic DNA (Thermo Scientific, Lithuania) for methylated primers, and the used negative control was an unconverted DNA for both the methylated and the unmethylated primers. Also, water without a DNA template was included in each PCR reaction as a control for any contamination. The FOXP3-TSDR unmethylation level was calculated using an equation which previously defined by (Cottrell et al. 2007; Yadav et al. 2012; Zhuo et al. 2014; Zafari et al. 2018; Klumper et al. 2020): “100/[1 + 2^(Ct TG—Ct CG)] × 100%,” in which the cycle threshold achieved with TG (unmethylated) primers was represented by the CtTG and the cycle threshold achieved with CG (Methylated) primers was represented by the CTCG. For females because one of the two TSDR alleles is methylated as a result of X inactivation, the level of unmethylation was corrected by a factor of 2.
Statistical analysis
Anderson–Darling test is done to asses if continuous variables follow normal distribution (Stephens 1974). One-way ANOVA and Kruskal–Wallis tests are used for the comparison between different groups (control, optimal, and failure). Regression analysis was performed to assess the relationship between different variables. Receiver operating characteristic (ROC) curve was used to see the validity of different parameters in separating active cases from control (negative cases) and area under the curve, i.e., AUC and its P value prescribe this validity, the P value is considered significant at P > 0.05. SPSS 22.0.0 (Chicago, IL), MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium; 2014), GraphPad Prism version 8.0.0 for Windows, GraphPad Software, San Diego, California USA, software package used to make the statistical analysis.
Results
The study group consisted of 60 CML patients on at least twelve-month imatinib mesylate therapy. Thirty of the patients have an optimal response (major molecular response MMoR) with a mean disease duration of 73.7 ± 37.6 months, the remaining 30 CML patients have failure molecular and/or hematological response with mean disease duration of 69.4 ± 46.4 months, data revealed no significant statistical differences regarding the mean duration of imatinib therapy between groups (P = 0.693). Thirty matched age and gender healthy volunteers were considered as a control group. The male: female ratio among the optimal response was (1.72: 1), the failure response was (0.76: 1) and the control group was (1.14: 1) with (P = 0.3). Age for patients group ranged 18–75 years with mean 46.8 ± 13.7 years, while the control group ranged (27–68) years with mean 46.33 ± 8.84 years with P = 0.1.
Assessment the degree of TKI response in all CML patients is done according to the results of quantitative PCR for p210 BCR-ABL transcript levels and peripheral blood indices for each patient. All optimal response patients had demonstrated a major molecular response (BCR-ABL ≤ 0.1%), 46.7% of them had BCR-ABL levels ranged between 0.01 and 0.1%. Out of 93.3% of failure response patients group, 50% had both failure hematological response (FHR) and failure molecular response (FMR), another 43.3% had only FMR, and 6.7% had FHR and BCR-ABL levels were > 0.1–1% as shown in Fig. 2.
Data revealed no significant difference in CD4 + T cells percentage with P = 0.511 as shown in Table 1. There is a highly significant increase in Treg cells (P < 0.001) with (median) 22.5% for failure group ranged (7.1–39.6) in comparison to the control group 1.4% ranged 0.4–3.1 and optimal group 1.0% ranged 0.6–1.9, while there is no significant difference between optimal and control groups (P = 0.999) as shown in (Table 1, Fig. 3).
Regarding FOXP3-TSDR demethylation level, data showed a highly significant increase in mean ± SD demethylated percentage for the failure group (2.65 ± 1.42%) compared to the control group (1.00 ± 0.73%) and optimal group (0.93 ± 0.64%) with P < 0.001 for both, while there is no significant difference between optimal and control groups (P = 0.954) as shown in Table 1 and Fig. 4.
The ROC analysis for Tregs and FOXP3-TSDR demethylation levels revealed an excellent prediction of AUC values at 0.920 and 0.904, respectively (P < 0.001), among CML patients with failure response when compared to optimal responders which indicate as perfect markers to discriminate failure from optimal response as shown in (Table 2). Accordingly, Tregs showed 90% sensitivity and specificity at cutoff value more than 3.3%, while the FOXP3 TSDR demethylation levels showed 83.3% sensitivity and specificity at cutoff value > 1.4% to differentiating failure response CML patients from optimal responding patients as shown in (Table 2), while data analysis showed that all the studied parameters failed to discriminate between optimal response CML patients and a healthy control group (Supplementry file: Table 3).
The regression analysis showed a direct significant relationship between Treg cells and FOXP3-TSDR demethylation in control group (r = 0.638, P < 0.001) and failure response CML patients (r = 0.634, P < 0.001), while in optimal response, it showed a non-significant relationship (r = 0.036, P = 0.852), as shown in Table 3 and Figs. 5 and 6.
Tregs percentages are studied for CML patients according to different BCR-ABL transcript levels. Depending on RQ-PCR results that are expressed as BCR-ABL % in a log scale, the optimal and failure responder CML patients are divided into three groups: the first group with > 0.1% IS BCR-ABL levels, whereas for the second group BCR-ABL levels ranged from 0.1 to 0.0032% IS (log 3, 4, and 4.5 reduction level), and the third group was considered if BCR-ABL levels were less than 0.0032% IS (log 5 reduction level), as shown in Table 4.
In different molecular response groups, data revealed a significant difference between BCR-ABL level > 0.1% IS and BCR-ABL level ≤ 0.1% IS, whereas data showed no significant differences in studied parameters among the optimal responders patients with different molecular responses (log 3 reduction or other deeper log 4.5 and 5 reduction levels), as shown in Table 5.
Discussion
Despite the valuable efficacy of imatinib in the long survival duration of CML patients (Hochhaus et al. 2017). TKI-resistant leukemia stem cells remain in the bone marrow of CML patients which may lead to disease progression (Hinterbrandner et al. 2021). Trying to elucidate the immune response status in CML patients among different stages and molecular responses to therapy, this study measured and assessed the frequency of Treg cells for these patients.
The mean age of the CML patients was 46.8 ± 13.7 years. This is comparable to ELN 2020 review as a younger age distribution among Asian population was < 50 years old (Hochhaus et al. 2020), and it is almost similar to other Iraqi studies that showed 44 and 45 years old for Baghdad City and the Kurdistan region of Iraq (Matti et al. 2013; Ali et al. 2014). According to the male: female ratio, this study revealed that woman had a higher failure rate to therapy than men (56.7% vs. 43.3%); this may be relate to the different gender compliance and the socioeconomic status for the patients during unavailability of the imatinib, while other recent studies have reported that the female gender is more favorable to better molecular response than the male (Branford et al. 2013; Nachi et al. 2019).
Assessment the Treg cells by the flow cytometric analysis, this study showed a significant higher median level differences (P < 0.001) in the levels of CD4 + CD25 + FOXP3 + Treg cells among CML patients with failure response when compared to the optimal response CML patients. This result was similar to the findings of Cheng et al. (2017) who revealed a significant increase in Tregs in advance stage of CML patients when compared to treated CML patients and control groups and a lower survival rate in groups with higher Tregs expression. Zahran et al. (2014) study also revealed the more frequent deep molecular response rate to TKI among CML patients that were with lower Tregs percentage levels. Regarding the relation of Treg cells with BCR-ABL levels in CML, Rojas et al. (2010) found that patients with complete cytogenetic response (CCyR) had lower level of Treg cells when compared to those patients who failed to achieve CCyR. Also, (Hughes et al. 2017) confirmed a higher Treg cells percentage among newly diagnosis CML and pre MMR compared to those with MMR, MR4.5, and treatment-free remission. There is a discrepancy in Tregs prognostic relevance in cancer patients; Shang et al. (2015) showed survival benefits of tumor Tregs infiltration among colorectal, head and neck, and esophageal cancer patients. This study demonstrated that there was an increase of Tregs among CML patients with TKI failure response group.
It has been observed the FOXP3-TSDR demethylation levels elevated in different tumors, and up to our knowledge, there is no previous study to measure the FOXP3 methylation in different treatment responses of CML patients in which could be an early indicator in unresponsiveness to treatment and a way of discriminating the role of Tregs in disease progression.
The FOXP3-TSDR demethylation mean level was significantly higher among failure response group when compared to optimal response and control groups. Failure CML patients showed higher Tregs levels which might affect anti-tumor immune response; this is supported by a higher level of FOXP3-TSDR demethylation, while the optimal responder CML patients showed much lower mean level than the patients with failure response to therapy, with non-significant difference with the control group.
A study tested the methylation status of FOXP3-TSDR in peripheral blood of healthy controls and different solid tumors like (colorectal, lung, prostate, and breast cancers) patients; they found that patients with lung cancer had significantly higher demethylation level (2.3%) versus healthy control (1.4%); in colorectal cancer, there was a slight increase demethylation level (1.6%) compared to healthy controls, while prostate and breast cancer had elevated demethylation level (2.4%) compared to same sex healthy controls, 1.4% in male and 1.7% in female (Wieczorek et al. 2009). These results are almost comparable to our study which indicates a similar demethylated TSDR level with FOXP3 in peripheral blood among advance CML patients. Failure responder CML patients in current study observation showed a characteristic increase in Tregs and demethylation level of FOXP3-TSDR, which may reflect the suppression degree of immune effector cells response to the increased leukemic clone cells, and this may be through contribution of Tregs to inhibit the immune response to the disease activity (Hinterbrandner et al. 2021). Conversely, the optimal response CML patients with MMoR status showed lower Tregs and demethylation level of FOXP3 TSDR, indicating the efficacy of treatment that directly or indirectly reduced the Tregs counts and reconstitute the immune function (Tanaka et al. 2020).
Here, one of the mechanisms of immune system impairment to control the proliferation of mutated leukemic cloned cells is the ability of these cells to escape the immune surveillance process by involving with a large number of immunosuppressive mechanisms to alleviate anti-tumor immune responses, causing progressive growing to the leukemic cells (Tarafdar et al. 2017). The over-expression of Tregs and FOXP3 TSDR demethylation level through increasing numbers of the leukemic cells, increase in the inhibitory surface markers on leukemic cells, and the increase in immune suppressive cytokines like IL-10, TGF-β, and IL-35(Koyama and Nishikawa 2021) thereby may enhancement the suppresser function of Treg as an escape mechanism for proliferation of leukemic cells, which lead to loss functions of tumor reactive T cells and other immune cells to control the disease (Koyama and Nishikawa 2021).
In this study, an excellent prediction of AUC value results was seen in both Tregs level and FOXP3-TSDR demethylation level, the higher sensitivity and specificity (90%) are determined in measurement of Tregs level by flowcytometry at cutoff value > 3.3% which revealed this way of measurement is considered better than of FOXP3-TSDR demethylation assessment with an excellent accuracy to discriminate between the optimal from failure response CML patients with (P ≤ 0.001), whereas both Tregs and the FOXP3-TSDR demethylation were equal in their expressions levels and could not be able to discriminate among both the healthy peoples and the optimal response, making the treatment response in CML patients a crucial in remodulating the immune system response to near normal as the healthy control group. Up to our knowledge, no previous cut-off value established for Tregs percentage in different response types of CML to TKI, in this study the level of Tregs in CD4 + T cells higher than 3.3 making it a good marker to distinguish the failure response CML patients.
Among the failure responding CML patients, there was a significant direct relationship between CD4 + CD25 + FOXP3 + Treg cells and FOXP3-TSDR demethylation levels (r = 0.634; P < 0.001), and in the control group was r = 0.638, P < 0.001. A similar correlation between flow cytometry and methylation-specific qPCR to quantify Tregs in blood has been reported by (Liu et al. 2010) (r = 0.49) and (Sehouli et al. 2011) (r = 0.74), making of the measurement of Treg cells are valuable and comparable in failure response patients by the flow cytometry and qMSP.
This relationship was lost in optimal response CML patients (r = 0.036, P = 0.852), and this may be explained by the presence of transient expression of FOXP3 in activated T cells (Saito et al. 2016), due to the persistent residual disease among the optimal responders CML patients.
In a present study, we tried to assess and compare the CD4 + CD25 + FOXP3 + Tregs and FOXP3-TSDR demethylation percentage through different levels of BCR-ABL1 transcripts (> 0.1%, 0.1–0.0032%, and < 0.0032%) of CML patients groups. Significant higher CD4 + CD25 + FOXP3 + Tregs and FOXP3-TSDR demethylation levels in failure response group (BCR-ABL1 level > 0.1%) compared to optimal response CML patient with BCR-ABL1 levels (0.1–0.0032% and < 0.0032%, P < 0.001). But this significant difference was lost between the optimal CML patients with different molecular responses (P = 0.9), making the immune response status of the optimal response patients at log3, log4, log4.5, and log5 BCR-ABL reduction levels had no difference regarding the Tregs status. Current data suggest the ability of imatinib therapy to reduce the Treg cell levels, thereby reducing their inhibitory effect on immune response, and this in turn contributes to strong activation in vivo immune response regardless of the molecular reduction levels.
Labib and Haggag (2015) found significant positive correlations between the percentage of Tregs with the percentage of BCR-ABL1 which indicates that it has an immune modulating effect that may be significant in the progression of CML; also, Tregs percent is positively correlated with bone marrow blast cells percent, which may indicate that higher Tregs levels are associated with poor disease course or transformation in to advance stage.
Conclusions
Significant increase in Treg cells in failure response while decreased in optimal response CML patients indicating that might play an important role in the pathogenesis of CML progression and in the disease control for a stable deep molecular response in patients who achieved log 3 BCR-ABL reduction levels and deeper molecular response. Also, there is no variance in the status of the immune behavior regardless the log scale of the optimal response, making a MMoR (0.1% IS) enough to restore the immune escape defect and the immunosuppressive properties of regulatory T cells, and seems to be particularly important in the disease control status.
Data availability
All data used in current study are available from the corresponding author on reasonable request.
References
Ali MD, Badi AI, Al-Zebari SSM, Al-Allawi NAS (2014) Response to tyrosine kinase inhibitors in chronic myeloid leukemia: experience from a west Asian developing country. Int J Hematol 100(3):274–280. https://doi.org/10.1007/s12185-014-1627-6
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S et al (2013) European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Blood 122(6):872–884. https://doi.org/10.1182/blood-2013-05-501569
Branford S, Yeung DT, Ross DM, Prime JA, Field CR (2013) Early molecular response and female sex strongly predict stable undetectable BCR- ABL1, the criteria for imatinib discontinuation in patients with CML. Blood 121(19):3818–3824
Cheng Y, Yang G, Sun M, Chang K, Long R et al (2017) The expression and clinical significance of Treg cells in chronic myelocytic leukemia. Biomed Res 28(21):9188–9192
Cottrell S, Jung K, Kristiansen G, Eltze E, Semjonow A et al (2007) Discovery and validation of 3 novel DNA methylation markers of prostate cancer prognosis. J Urol 177(5):1753–1758. https://doi.org/10.1016/j.juro.2007.01.010
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4 + CD25+ regulatory T cells. Nat Immunol 4(4):330–336. https://doi.org/10.1038/ni904. (Epub 2003 Mar 3)
Hinterbrandner M, Rubino V, Stoll C, Forster S, Schnüriger N et al (2021) Tnfrsf4-expressing regulatory T cells promote immune escape of chronic myeloid leukemia stem cells. JCI Insight 6(23):e151797. https://doi.org/10.1172/jci.insight.151797
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF et al (2020) European Leukemia Net 2020 recommendations for treating chronic myeloid leukemia. Leukemia 34:966–984. https://doi.org/10.1038/s41375-020-0776-2
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S et al (2017) Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med 376(10):917–927. https://doi.org/10.1056/NEJMoa1609324
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299(5609):1057–1061. https://doi.org/10.1126/science.1079490
Hsieh YC, Kirschner K, Copland M (2021) Improving outcomes in chronic myeloid leukemia through harnessing the immunological landscape. Leukemia 35:1229–1242. https://doi.org/10.1038/s41375-021-01238-w
Hughes A, Clarson J, Tang C, Vidovic L, White DL et al (2017) CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood 129(9):1166–1176. https://doi.org/10.1182/blood-2016-10-745992
Hughes A, Yong ASM (2017) Immune effector recovery in chronic myeloid leukemia and treatment-free remission. Front Immunol 8(469):1–12. https://doi.org/10.3389/fimmu.2017.00469
Ilander M, Mustjoki S (2017) Immune control in chronic myeloid leukemia. Oncotarget 8(61):102763–102764
Jabbour E, Kantarjian H (2016) Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am J Hematol 91(2):252–265. https://doi.org/10.1002/ajh.24275
Kennedy JA, Hobbs G (2018) Tyrosine kinase inhibitors in the treatment of chronic phase CML: strategies for frontline decision-making. Curr Hematol Malig Rep 13(3):202–211. https://doi.org/10.1007/s11899-018-0449-7
Klumper N, Ralser DJ, Bawden EG, Landsberg J, Zarbl R et al (2020) LAG3 (LAG-3, CD223) DNA methylation correlates with LAG3 expression by tumor and immune cells, immune cell infiltration, and overall survival in clear cell renal cell carcinoma. J Immunother Cancer 8(1):e000552. https://doi.org/10.1136/jitc-2020-000552
Koyama S, Nishikawa H (2021) Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer 9:e002591. https://doi.org/10.1136/jitc-2021-002591
Labib HA, Haggag R (2015) CD4 + CD25 + high FoxP3 regulatory T-Cells in typical and atypical Philadelphia+ve CML as regards response to imatinib therapy. J Am Sci 11(5):34–41
Liu J, Lluis A, Illi S, Layland L, Olek S et al (2010) T regulatory cells in cord blood–FOXP3 demethylation as reliable quantitative marker. PLoS One 5(10):e13267. https://doi.org/10.1371/journal.pone.0013267
Matti BF, Naji AS, Alwan AF (2013) Evaluation of the safety of imatinîb mesylate in 200 iraqi patients with chronic myeloid leukemia in the chronie phase:single-center study. Turk J Hematol 30:387–393. https://doi.org/10.4274/Tjh.2Oi2.oi35
Nachi M, Kihel I, Guella D, Dali-Ali A, Abed A et al (2019) Sex and major molecular response to imatinib treatment for patients with chronic myeloid leukemia. Biochem Pharmacol 8:263. https://doi.org/10.4172/2167-0501.1000263
Rojas JM, Wang L, Owen S, Knight K, Watmough SJ et al (2010) Naturally occurring CD4 + CD25 + FOXP3+ T-regulatory cells are increased in chronic myeloid leukemia patients not in complete cytogenetic remission and can be immunosuppressive. Exp Hematol 38:1209–1218. https://doi.org/10.1016/j.exphem.2010.09.004
Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D et al (2016) Two FOXP3 + CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med 22(6):679–684. https://doi.org/10.1038/nm.4086
Saubele S, Richter J, Hochhaus A, Mahon FX (2016) The concept of treatment-free remission in chronic myeloid leukemia. Leukemia 30:1638–1647. https://doi.org/10.1038/leu.2016.115
Sehouli J, Loddenkemper C, Cornu T, Schwachula T, Hoffmuller U et al (2011) Epigenetic quantification of tumor-infiltrating T-lymphocytes. Epigenetics 6(2):236–246. https://doi.org/10.4161/epi.6.2.13755
Shang B, Liu Y, Jiang SJ, Liu Y (2015) Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 5(15179):1–9. https://doi.org/10.1038/srep15179
Stephens MA (1974) EDF statistics for Good ness of fit and some comparisons. J Am Stat Assoc 69:730–737
Tanaka A, Nishikawa H, Noguchi S et al (2020) Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. J Exp Med 217(2):e20191009. https://doi.org/10.1084/jem.20191009
Tarafdar A, Hopcroft LE, Gallipoli P, Pellicano F, Cassels J et al (2017) CML cells actively evade host immune surveillance through cytokine-mediated downregulation of MHC-II expression. Blood 129(2):199–208. https://doi.org/10.1182/blood-2016-09-742049
Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S et al (2009) Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res 69(2):599–608. https://doi.org/10.1158/0008-5472.CAN-08-2361
Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M et al (2012) Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med 209(10):1713–1722. https://doi.org/10.1084/jem.20120822
Zafari P, Yari K, Mostafaei S, Iranshahi N, Assar S et al (2018) Analysis of Helios gene expression and Foxp3 TSDR methylation in the newly diagnosed rheumatoid arthritis patients. Immunol Invest 47(6):632–642. https://doi.org/10.1080/08820139.2018.1480029
Zahran AM, Badrawy H, Ibrahim A (2014) Prognostic value of regulatory T cells in newly diagnosed chronic myeloid leukemia patients. Int J Clin Oncol 19(4):753–760. https://doi.org/10.1007/s10147-013-0615-9
Zahran AM, Elsayh KI (2012) CD4+ CD25 + High Foxp3+ regulatory T cells, B lymphocytes, and T lymphocytes in patients with acute ITP in Assiut Children Hospital. Clin Appl Thromb Hemost 20(1):61–67. https://doi.org/10.1177/1076029612454937
Zhuo C, Li Z, Xu Y, Wang Y, Li Q et al (2014) Higher FOXP3-TSDR demethylation rates in adjacent normal tissues in patients with colon cancer were associated with worse survival. Mol Cancer 13(153):1–13. https://doi.org/10.1186/1476-4598-13-153
Author information
Authors and Affiliations
Contributions
Shahla’a Fadhil Sabir: methodology, conducted the laboratory work, data curation, writing—original draft, preparation, visualization, investigation, and writing—review and editing. Bassam Francis Matti: had the idea for this research, cases follow-up, supervision, and project administration. Wifaq Mahmood Ali Alwatar: supervision and project administration. All authors contributed to reviewing and revising the paper. Final approval of manuscript was done by all the authors.
Corresponding author
Ethics declarations
Ethics approval
Ethics approval is sought from the scientific ethical committee of the college of medicine /Baghdad University (Date 27/2/2019 /No.365) and this study was performed in line with the principles of the Declaration of Helsinki.
Consent to participate
Informed consent is obtained from each participant prior to enrollment in this study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sabir, S.F., Matti, B.F. & Alwatar, W.M.A. Assessment of regulatory T cells (Tregs) and Foxp3 methylation level in chronic myeloid leukemia patients on tyrosine kinase inhibitor therapy. Immunogenetics 75, 145–153 (2023). https://doi.org/10.1007/s00251-022-01291-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-022-01291-4