Abstract
The impact of T-regulatory cells (Tregs), PD-1 + CD8 T-cells, and their dynamics during treatment with imatinib mesylate remains poorly understood in patients with chronic myeloid leukemia (CML). We conducted a prospective study on newly diagnosed, treatment-naïve adult (> 18 years old) patients with CML in the chronic phase (CP) and age- and sex-matched controls. Peripheral blood samples were collected at diagnosis and after three months of imatinib therapy to assess Tregs and PD-1 + CD8 T-cell levels using flow cytometry. The study comprised 57 patients with a median age of 39 years, including 27 males (47%). At baseline, the mean percentage of Tregs was significantly higher in CML patients (3.6 ± 0.32%) compared to controls (1.58 ± 0.21%) (p < 0.0001) but decreased significantly after three months of imatinib treatment (1.73 ± 0.35%) (p < 0.0001). Baseline Treg% exhibited positive correlations with Sokal (r = 0.29), Hasford (r = 0.33), EUTOS (r = 0.28), and ELTS (r = 0.31) risk scores (p < 0.05), as well as with the BCR-ABL transcript levels at three months (p = 0.03). Furthermore, the mean baseline percentage of PD-1 + CD8 T-cells was significantly elevated in CML patients (7.66 ± 0.36%) compared to controls (2.65 ± 0.32%) (p < 0.0001) and also decreased after treatment (3.44 ± 0.37%) (p < 0.0001). The baseline percentage of PD-1 + T-cells demonstrated positive correlations with Sokal (r = 0.26), Hasford (r = 0.27), and ELTS (r = 0.41) risk scores (p < 0.05). Our findings reveal a significantly higher proportion of Tregs and PD-1 + CD8 T-cells in patients with CML-CP compared to healthy controls, notably diminished following imatinib treatment. These observations suggest the potential for immunotherapy as a promising approach to managing immune exhaustion in CML patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm driven by the oncogenic BCR-ABL1 tyrosine kinase [1]. The immune system has been recognized as playing a crucial role in the initiation and progression of CML [2]. Among the immune cell subsets involved, CD4 + CD25 + FoxP3 + T-regulatory cells (Tregs) are a specific population of T-cells responsible for maintaining immune homeostasis and self-tolerance through the suppression of immune responses [3]. In the tumour microenvironment, the presence of Tregs contributes to the evasion of immune surveillance by malignant cells [4]. Another significant player in immune regulation is the programmed death receptor-1 (PD-1), an inhibitory immune checkpoint receptor expressed on activated cytotoxic CD8 T-cells [5]. Through interaction with its ligand, programmed death receptor-1 ligand (PDL-1), expressed on tumour cells, the PD-1 pathway induces T-cell silencing, leading to immune evasion and disease progression [6, 7].
Imatinib mesylate, a tyrosine kinase inhibitor (TKI), is widely used to treat CML in the chronic phase (CP) [8]. Beyond its primary effects, growing evidence suggests that TKIs may modulate the immunological microenvironment by influencing Tregs and the PD-1-PDL-1 interaction, ultimately contributing to treatment response in CML [9,10,11,12,13]. This study investigated the dynamics of Tregs and PD-1 + CD8 T-cells in newly diagnosed and treatment-naïve CML-CP patients before and after imatinib therapy. Furthermore, we compared their levels with those of age- and sex-matched healthy controls and explored their correlations with disease severity indices.
Methods
Study Population
Consecutive, newly diagnosed patients with CML in the chronic phase (CML-CP) who were initiated on a once-daily dose of 400 mg imatinib were included in this study after obtaining informed consent. The institutional ethics committee approved the study protocol, and it was conducted according to the principles outlined in the Declaration of Helsinki.
Diagnosis and Risk Assessment
CML-CP diagnosis was established based on bone marrow examination and detection of BCR-ABL1 transcript using real-time polymerase chain reaction (RT-PCR). The Sokal risk score, Hasford score, and European Treatment and Outcome Study (EUTOS) score were calculated using online tools to assess disease severity.
Sample Collection and Processing
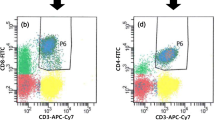
Peripheral blood samples (3 ml) anticoagulated with EDTA were collected from patients at diagnosis and after three months of imatinib treatment. Age- and sex-matched healthy individuals from the community served as controls and underwent similar evaluations. Red blood cell lysis, cellular staining, and flow cytometry analyses were performed. Tregs were identified by staining with fluorochrome-conjugated monoclonal antibodies against human CD3, CD4, CD25, CD127, and FoxP3. PD-1 + CD8 T cells were analysed using anti-CD8 and CD279 antibodies. Flow cytometry data acquisition was performed using a triple laser BD FACS Canto II flow cytometer, and data analysis was conducted using BD FACS DIVA software (Becton Dickinson, USA). The percentage of CD4 + CD25highCD127lowFoxP3 + Tregs was determined relative to CD4 T lymphocytes (Fig. 1), while the percentage of CD8 + CD279high PD-1 + CD8 T cells was calculated relative to CD8 T lymphocytes.
Follow-Up
Patients underwent clinical examinations and regular blood counts every two weeks to monitor clinical and haematological remission. At the three-month follow-up, the percentage of BCR-ABL transcripts was determined using RT-PCR.
Statistical Analysis
Descriptive statistics, including mean (95% CI), median, standard error of the mean, and standard deviation, were used to summarise continuous variables. The Mann–Whitney U test was employed to compare the percentages of Tregs and PD-1 + CD8 T-cells between patients and healthy controls. Changes in the percentages of Tregs and PD-1 + CD8 T-cells before and after treatment in patients were analysed using parametric (paired t-test) or non-parametric (Spearman's rank test), depending on the data distribution. Pearson's test was utilised to assess correlations. Statistical analyses were performed using SPSS software version 22.0. The Chi-square test was employed to analyse qualitative variables. A p-value of less than 0.05 was considered statistically significant. Values were presented as mean ± SD.
Results
The study was conducted at a single centre from January 2017 to June 2018. During this period, a total of 60 cases of CML were screened. Three patients were excluded from the study due to being in the accelerated or blast phases, resulting in a final sample of 57 treatment-naive CML-CP patients. Age- and sex-matched healthy volunteers (n = 30) were included as controls.
The median age of the patients was 39 years, with 27 (47%) being males. The demographic details and disease characteristics are presented in Table 1. The mean Treg% in CML patients (3.61 ± 0.32%) was significantly higher than that in healthy controls (1.58 ± 0.21%, p < 0.0001). Higher-risk groups defined by Sokal, Hasford, EUTOS, and ELTS scores exhibited higher mean Treg% (p < 0.05).
After three months of treatment with imatinib, all 57 patients demonstrated a statistically significant reduction in Treg% (p < 0.0001). Furthermore, all but three patients achieved complete hematologic response (CHR) within three months. Although not statistically significant, patients who achieved CHR at six weeks (n = 34, 59.6%) had a numerically lower Treg% at presentation (3.57%) compared to those who did not achieve CHR (n = 23, 40%; 3.66% Tregs). Patients with an early molecular response (EMR), defined by BCR-ABL transcript levels of < 10% after three months of therapy, exhibited lower baseline Treg% than those with > 10% transcripts [14]. It was also observed that despite a decline with treatment, Treg% levels remained significantly higher than in controls (p = 0.04). Figure 2a is a box plot that suggests a notable difference in the baseline Treg% between the case and control groups, with the case group having higher baseline levels of Treg%. Figure 2b is a scatter plot depicting the significant correlation of baseline Treg% with BCR-ABL transcript levels at three months. Figure 2c is a scatter plot showing no significant correlation between the BCR-ABL transcripts and the percentage of Tregs at the three-month mark, given the flat trend and widening confidence interval. Figure 2d is a clustered bar chart that depicts the Treg% at baseline and after three months in individual patients, along with their Sokal scores. The graph provides a clear visual representation of how Treg% changes over time and across different risk categories.
a Plot representing baseline Treg% in cases and controls. b Plot correlating BCR-ABL transcript level at three months with baseline Treg%. c Plot correlating BCR-ABL transcript level at three months with Treg% at three months. d Treg% at baseline and after three months with Sokal risk score of patients
The mean baseline percentage of PD-1 + CD8 T cells in CML patients (7.66 ± 0.36%) was significantly higher than that in controls (2.65 ± 0.32%) (p < 0.0001). The baseline percentage of PD-1 + T cells in patients correlated with the Sokal (r = 0.26), Hasford (r = 0.27), and ELTS (r = 0.41) scores (p < 0.05). Following three months of therapy, the mean percentage of PD-1 + CD8 T cells in CML patients (3.44 ± 0.36%) was significantly lower than their mean baseline value (7.66 ± 0.36%) (p < 0.0001). The details are presented in Table 2.
Discussion
In this study, we investigated the dynamics of immune cells in chronic myeloid leukaemia in chronic phase (CML-CP) patients undergoing imatinib treatment. Our findings demonstrated that the mean percentage of regulatory T cells (Tregs) at presentation was significantly elevated compared to the healthy population in CML-CP patients. Furthermore, we observed a significant correlation between the level of Tregs and the risk groups of the disease, which is consistent with previous literature [15,16,17]. These results support the hypothesis that Tregs play a role in the initiation and progression of CML by facilitating the evasion of immune surveillance by leukemic stem cells [9, 18].
After three months of imatinib therapy, we observed a significant decrease in the percentage of Tregs in all patients. Imatinib is known to suppress the survival and immunosuppressive function of Tregs by targeting cell surface receptors, signalling pathways, and transcription factors [10]. The previous studies in this regard are presented in Table 3. Thus, suppressing Tregs may represent one of the mechanisms by which tyrosine kinase inhibitors (TKIs) exert control over CML [10, 11, 19]. Notably, patients who achieved complete hematologic response at six weeks and early molecular response at three months had lower Treg percentages at presentation. This finding strengthens the hypothesis that the percentage of Tregs could be a potential biomarker for assessing treatment response and prognosis in CML [20].
Consistent with previous studies, our investigation revealed higher levels of programmed cell death protein 1 (PD-1) positive CD8 T-cells in CML patients compared to healthy individuals [12]. The elevated expression of PD-1 was associated with CD8 T-cell exhaustion and impaired anti-leukemia immune responses in myeloid malignancies [21]. Prior studies have reported a higher percentage of PD-1 positive CD8 T-cells in the bone marrow of CML patients with advanced disease stages and higher risk severity indices [2, 12, 22, 23]. In our study, we focused on peripheral blood samples, which are more easily accessible, and found a significant correlation between the percentage of PD-1 positive CD8 T-cells at presentation and risk severity indices. Consistent with previous findings, patients with a better response to therapy exhibited lower percentages of PD-1 + CD8 T-cells [13]. Longer follow-up is necessary to determine whether baseline PD-1 expression in peripheral blood can predict future outcomes.
Studies have demonstrated that leukemic stem cells persisting in the bone marrow during treatment can acquire BCR-ABL1 kinase domain mutations or accumulate genetic alterations, leading to disease relapse and progression [24, 25]. Preclinical models have shown that blocking PD-1 receptors, with or without adoptive cytotoxic T-cell transfer, can eliminate CML stem cells [7, 26]. Considering the immunomodulatory effects of imatinib, it is worth exploring whether the addition of immunotherapy could enhance the achievement of deeper molecular remission, either as an upfront treatment or in the relapsed-refractory setting. The ongoing Blast MRD CML 1 phase II trial aims to address this question [27].
One of the primary goals in managing CML is discontinuing TKIs, although studies have indicated that only 20–30% of newly diagnosed patients can achieve treatment-free remission [28,29,30,31]. Interestingly, our study revealed that although the percentage of Tregs and PD-1 positive T-cells decreased significantly with treatment, their levels at three months remained significantly higher than those observed in healthy controls. Further research is necessary to investigate whether the kinetics of immune regulatory cells and the normalisation of their levels can serve as predictive markers for the successful discontinuation of TKIs [32, 33].
Our study's limitations include focusing solely on the percentage of Tregs without investigating their functional mechanisms. Additionally, we did not perform RQ-PCR to quantify BCR-ABL transcripts at baseline, which could have provided a more accurate understanding of the proportional decrease in BCR-ABL levels compared to the reduction in Treg percentages. We did not analyse Treg% in healthy controls at three months, which would have provided a more precise illustration of imatinib’s effect on reducing Tregs in cases. Furthermore, more extended follow-up periods would be beneficial to establish a stronger correlation between Treg percentages and late responders.
Conclusion
Our study revealed a significantly higher proportion of Tregs and PD-1 + CD8 T-cells in patients with CML-CP compared to healthy controls, notably diminished following imatinib treatment. The critical role of the immune system in the initiation and progression of CML and the modulation of the immune response through targeted agents and immunotherapy to achieve sustained drug-free remission in CML are subjects of further research.
References
Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM (1999) The biology of chronic myeloid leukemia. N Engl J Med 341:164–172
Brück O, Blom S, Dufva O, Turkki R, Chheda H, Ribeiro A et al (2018) Immune cell contexture in the bone marrow tumor microenvironment impacts therapy response in CML. Leukemia 32:1643–1656
Kondĕlková K, Vokurková D, Krejsek J, Borská L, Fiala Z, Ctirad A (2010) Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Med 53:73–77
D’Arena G, Vitale C, Coscia M, Festa A, Di Minno NMD, De Feo V et al (2017) Regulatory T cells and their prognostic relevance in hematologic malignancies. J Immunol Res 2017:1832968
Topalian SL, Drake CG, Pardoll DM (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24:207–212
Boussiotis VA (2016) Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 375:1767–1778
Mumprecht S, Schürch C, Schwaller J, Solenthaler M, Ochsenbein AF (2009) Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood 114:1528–1536
Goldman JM (2007) How I treat chronic myeloid leukemia in the imatinib era. Blood 110:2828–2837
Bachy E, Bernaud J, Roy P, Rigal D, Nicolini FE (2011) Quantitative and functional analyses of CD4+CD25+FoxP3+ regulatory T cells in chronic phase chronic myeloid leukaemia patients at diagnosis and on imatinib mesylate. Br J Haematol 153:139–143
Larmonier N, Janikashvili N, LaCasse CJ, Larmonier CB, Cantrell J, Situ E et al (1950) Imatinib mesylate inhibits CD4+CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABLnegative tumors. J Immunol Baltim Md 2008(181):6955–6963
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen B, Götz M et al (2009) Dasatinib inhibits the proliferation and function of CD4+CD25+ regulatory T cells. Br J Haematol 144:195–205
Lee MY, Park CJ, Cho YU, You E, Jang S, Seol CA et al (2020) Differences in PD-1 expression on CD8+ T-cells in chronic myeloid leukemia patients according to disease phase and TKI medication. Cancer Immunol Immunother 69:2223–2232
Hughes A, Clarson J, Tang C, Vidovic L, White DL, Hughes TP et al (2017) CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood 129:1166–1176
Chikkodi SV, Malhotra P, Naseem S, Khadwal A, Prakash G, Sahu KK et al (2015) Factors affecting early molecular response in chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk 15(Suppl):S114-119
Zahran AM, Badrawy H, Ibrahim A (2014) Prognostic value of regulatory T cells in newly diagnosed chronic myeloid leukemia patients. Int J Clin Oncol 19:753–760
Rojas JM, Wang L, Owen S, Knight K, Watmough SJ, Clark RE (2010) Naturally occurring CD4+ CD25+ FOXP3+ T-regulatory cells are increased in chronic myeloid leukemia patients not in complete cytogenetic remission and can be immunosuppressive. Exp Hematol 38:1209–1218
Hus I, Tabarkiewicz J, Lewandowska M, Wasiak M, Wdowiak P, Kusz M et al (2011) Evaluation of monocyte-derived dendritic cells, T regulatory and Th17 cells in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors. Folia Histochem Cytobiol 49:153–160
Hinterbrandner M, Rubino V, Stoll C, Forster S, Schnüriger N, Radpour R et al (2021) Tnfrsf4-expressing regulatory T cells promote immune escape of chronic myeloid leukemia stem cells. JCI Insight 6:e151797
Tanaka A, Nishikawa H, Noguchi S, Sugiyama D, Morikawa H, Takeuchi Y et al (2020) Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. J Exp Med 217:e20191009
Fujioka Y, Sugiyama D, Matsumura I, Minami Y, Miura M, Atsuta Y et al (2021) Regulatory T cell as a biomarker of treatment-free remission in patients with chronic myeloid leukemia. Cancers 13:5904
Tan J, Chen S, Lu Y, Yao D, Xu L, Zhang Y et al (2017) Higher PD-1 expression concurrent with exhausted CD8+ T cells in patients with de novo acute myeloid leukemia. Chin J Cancer Res 29:463–470
Lee MY, Park C, Cho YU, You E, Jang S, Seol CA et al (2017) Expression levels of PD-1 on CD8+ T cells in chronic myeloid leukemia with and without BCR-ABL1 kinase mutation. Blood 130:4178–4178
Christiansson L, Söderlund S, Svensson E, Mustjoki S, Bengtsson M, Simonsson B et al (2013) Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/PRogrammed death receptor 1, and soluble CD25 in sokal high risk chronic myeloid leukemia. PLoS ONE 8:e55818
Holyoake TL, Vetrie D (2017) The chronic myeloid leukemia stem cell: stemming the tide of persistence. Blood 129:1595–1606
Sharma P, Sachdeva MUS, Naseem S, Sreedharanunni S, Das R, Malhotra P et al (2022) Identification of peripheral blood CD26+ leukemic stem cells has a potential role in the rapid diagnosis of chronic myeloid leukemia. Int J Lab Hematol 44:518–523
Riether C, Gschwend T, Huguenin AL, Schürch CM, Ochsenbein AF (2015) Blocking programmed cell death 1 in combination with adoptive cytotoxic T-cell transfer eradicates chronic myelogenous leukemia stem cells. Leukemia 29:1781–1785
Zeidan AM, Wang V, Radich JP, Bewersdorf JP, Bhatt VR, Sharon E et al (2020) Blast MRD CML 1 Trial: Blockade of PD-1 added to standard therapy to target measurable residual disease (MRD) in chronic myeloid leukemia (CML)- a phase II study of adding the anti-PD-1 pembrolizumab to tyrosine kinase inhibitors in patients with chronic myeloid leukemia and persistently detectable minimal residual disease: a trial of the ECOG-ACRIN cancer research group (EA9171). Blood 136:1–1
Anand MS, Varma N, Varma S, Rana KS, Malhotra P (2012) Cytogenetic & molecular analyses in adult chronic myelogenous leukaemia patients in north India. Indian J Med Res 135:42–48
Atallah E, Schiffer CA (2020) Discontinuation of tyrosine kinase inhibitors in chronic myeloid leukemia: when and for whom? Haematologica 105:2738–2745
Goni D, Jain A, Singh C, Jindal N, Nampoothiri R, Jandial A et al (2023) Feasibility of treatment-free remission with generic imatinib: Results of generic imatinib-free trial-in-chronic myeloid leukaemia-chronic phase study. Indian J Med Res 157:87–91
Yanamandra U, Malhotra P (2019) CML in India: Are We There Yet? Indian J Hematol Blood Transfus 35:1–2
Stuckey R, López-Rodríguez JF, Sánchez-Sosa S, Segura-Díaz A, Sánchez-Farías N, Bilbao-Sieyro C et al (2020) Predictive indicators of successful tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia. World J Clin Oncol 11:996–1007
Hsieh YC, Kirschner K, Copland M (2021) Improving outcomes in chronic myeloid leukemia through harnessing the immunological landscape. Leukemia 35:1229–1242
Acknowledgements
None.
Funding
No external sources of funding.
Author information
Authors and Affiliations
Contributions
Fen Saj prepared the manuscript; Ram Vasudevan Nampoothiri, Deepesh Lad, and Aditya Jandial provided the concept; Man Updesh Singh Sachdeva, Parveen Bose, and Neelam Varma helped with the flow cytometry work; Alka Khadwal and Gaurav Prakash critically reviewed the manuscript, and Pankaj Malhotra was the Principal Investigator.
Corresponding author
Ethics declarations
Conflict of interest
There are no relevant conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saj, F., Vasudevan Nampoothiri, R., Lad, D. et al. Prognostic Significance of Regulatory T-Cells and PD-1 + CD8 T-Cells in Chronic Myeloid Leukemia Patients Treated with Generic Imatinib. Indian J Hematol Blood Transfus (2024). https://doi.org/10.1007/s12288-024-01843-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12288-024-01843-6