Abstract
HLA class I (HLA-I) molecules play a crucial role in the presentation of tumor antigenic peptides to CD8+ T cells. Tumor HLA-I loss provides a route of immune escape from T cell-mediated killing. We analyzed HLA-I expression in 98 cryopreserved breast cancer tissues using a broad panel of anti-HLA-I antibodies. Genomic HLA-I typing was performed using DNA obtained from autologous normal breast tissue. Analysis of the loss of heterozygosity (LOH) in the HLA-I region of chromosome 6 (LOH-6) and in the β2-microglobulin (B2M) region of chromosome 15 (LOH-15) was done by microsatellite amplification of DNA isolated from microdissected tumor areas. B2M gene sequencing was done using this DNA form HLA-I-negative tumors. Immunohistological analysis revealed various types of HLA-I alterations in 79 tumors (81%), including total HLA-I loss in 53 cases (54%) and partial loss in 16 samples (14%). In 19 cases (19%), HLA-I expression was positive. Using microsatellite analysis, we detected LOH in 36 cases out of 92 evaluated (39%), including 15 samples with only LOH-6, 14 with LOH-15, and seven tumors with LOH-6 and LOH-15 at the same time. Remarkably, we detected LOH-6 in eight tumors with positive HLA-I immunolabeling. We did not find any B2M mutations in HLA-I-negative breast tumors. In conclusion, LOH at chromosomes 6 and 15 has a high incidence in breast cancer and occurs in tumors with different HLA-I immunophenotypes. This common molecular mechanism of HLA-I alterations may reduce the ability of cytotoxic T lymphocytes to kill tumor cells and negatively influence the clinical success of cancer immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely accepted that one of the principal mechanisms of anti-tumor immunity is T cell-mediated cytotoxicity, which relies on the recognition of tumor antigenic peptides presented by HLA class I (HLA-I) molecules expressed on tumor cell surface (Boesen et al. 2000; Coulie et al. 2014; Wang et al. 2008; Ryschich et al. 2005). The absence of HLA-I expression is a common finding in different tumor tissues (Lopez-Nevot et al. 1989; Garrido et al. 1993; Marincola et al. 2000; Seliger et al. 2002) and is a major mechanism used by tumor cells to escape from T cell-mediated immune surveillance (Romero and Coulie 2014; Boesen et al. 2000; Garrido et al. 1997a, 2017a). In addition, there is a growing evidence demonstrating that HLA-I expression in different types of cancer has a prognostic value and is associated with disease progression, invasiveness, metastatic potential, and resistance to therapy (del Campo et al. 2014; Perea et al. 2017; Sade-Feldman et al. 2017). It has been recently reported that adoptive transfer of tumor-infiltrating lymphocytes specific for neo-antigens detected in the tumor in conjunction with interleukin-2 and checkpoint blockade induced a complete durable regression of metastatic breast cancer (Zacharakis et al. 2018). This neoantigen-specific T cell therapy requires normal expression of HLA-I molecules on tumor cell surface. Therefore, the status of tumor HLA-I expression is important for the success of T cell and peptide-mediated cancer immunotherapy.
It is also well established that the molecular mechanisms responsible for the HLA-I loss plays a crucial role in the ability to recover the HLA expression in different types of cancer immunotherapy. These mechanisms can be subdivided into two major groups: reversible/“soft” or irreversible/“hard” (Garrido et al. 2010a, 2010b). Reversible alterations are associated with transcriptional downregulation of HLA-I and antigen-presenting machinery (APM) genes and can be recovered by different cytokines. The “hard” alterations are caused by mutations/deletions in HLA-I, B2M, and/or IFN-gamma genes and cannot be corrected by cytokines or by immunotherapy. Hence, it is essential to investigate molecular mechanisms responsible for HLA-I alterations in cancer to understand the mechanisms of tumor escape and predict the response to therapy.
There have been several reports describing the frequency of HLA-I alterations in breast cancer, ranging from as high as 90% (Cabrera et al. 1996; Perez et al. 1986) to 30% (Kaneko et al. 2011). This discrepancy could be due to the different methods and anti-HLA antibodies used in various studies. For example, in formalin fixed paraffin embedded (FFPE) tissues, HLA heavy chain/B2M complex on the cell surface loses conformational epitopes and cannot be detected by the commonly used w6/32 antibody. In addition, there is little information about the molecular mechanisms involved in HLA-I altered expression in breast cancer (Madjd et al. 2005; Concha et al. 1991a, b; Pedersen et al. 2017).
In this manuscript, we present the results of immunohistological and molecular analysis of HLA-I and II (HLA-II) expression performed on 98 cryopreserved breast cancer samples. We also evaluated 53 HLA-I-negative samples for the presence of mutation/deletions in B2M gene and investigated the frequency of the loss of heterozygosity at chromosomes 6 (LOH-6, HLA heavy chain genes) and 15 (LOH-15, B2M gene) in 92 samples.
Material and methods
Patients and samples
Ninety-eight patients with breast carcinoma were included in this study. All patients were female and their mean age was 59 (ranging from age 26 to 81). Patient samples (tumor specimens and autologous normal breast tissue samples) were obtained from Virgen de las Nieves University Hospital (Granada, Spain). Demographic, clinical, and histological characteristics of the studied subjects/tumors are summarized in Table 1. Before the study, all medical records and tumor sections were reviewed by an oncologist and a surgical pathologist. Signed informed consent approved by the Ethics Committee of our institution was obtained from all the patients. The specimens included 67 infiltrating ductal carcinoma (IDC), 17 infiltrating lobular carcinoma (ILC), and 14 tumors of various histological type (see Table 1) based on WHO criteria of histopathological classification. Tumors were classified as stages I (n = 24), II (n = 27), III (n = 46), and IV (n = 1) based on the American Joint Committee on Cancer Guidelines, tumor-node-metastasis (TNM) (Sobin et al. 2009). Expression of the estrogen receptor (ER), progesterone receptor (PgR), and HER2 were examined by immunohistochemical staining.
HLA typing
HLA typing of the patients using DNA isolated from the autologous normal breast tissue was performed in our laboratory using low-resolution genomic sequence-specific oligonucleotide analysis (SSO) from Dynal RELI HLA-A, B, C, DR kits (Dynal Biotech Ltd., Wittal, UK).
Immunohistological analysis of HLA-I and HLA-II expression in breast cancer specimens
Tumor samples from primary breast tumors and autologous normal breast tissue samples were obtained by surgical excision and immediately stored at − 80 °C. Four to eight-micrometer-thick cryopreserved tumor tissue sections were allowed to dry at room temperature for 4–18 h, fixed in acetone at 4 °C for 10 min, and stored at − 40 °C until immunohistological analysis using Biotin-Streptavidin System (NovolinkTM Polymer Detection System). Table 2 summarizes all mouse monoclonal antibodies (mAbs) used to analyze HLA-I and HLA-II expression. Total HLA-I loss was considered when less than 25% tumor cells were stained. When between 25 and 75% of tumor cells were labeled positively, tumor was considered to have a heterogeneous pattern. Finally, immunolabeling was considered to be positive when more than 75% of tumor cells labeled with W6/32 and GRH-1 mAbs according to the criteria established by the HLA and cancer component of the 1996 International Histocompatibility Workshop (Garrido et al. 1997b; Cabrera et al. 2003). In negative controls, the primary antibody was replaced with PBS.
Tumor microdissection and DNA isolation
Four to eight-micrometer-thick cryopreserved tumor tissue sections were fixed in 70% ethanol, stained with a 0.05% w/v solution of toluidine blue and microdissected using a laser micromanipulator (PALM Micro Laser Systems, ZEISS). Microdissected tumor fragments were collected in PALM Adhesive Caps and used to isolate DNA with Qiagen DNA isolation kit (QIAamp Tissue Kit, the Netherlands). This DNA was used for microsatellite analysis (LOH studies) and for B2M sequencing (in HLA-I-negative tumors).
Microsatellite analysis to detect LOH at chromosomes 6 and 15
This analysis was done on 92 of the studied tumors. Eight short tandem repeat (STRs) markers (7 in 6p21 y 1 in 6q21) were used for the LOH study at chromosome 6 (HLA heavy chain genes) (D6S291, D6S273, D6S265, D6S105, D6S276, C.1.2.C, C.1.2.5, and D6S311 respectively). Five markers spanning B2M genes were used for the analysis of LOH-15 (D15S126, D15S146, D15S1028, D15S153 in the 15q21 adjacent to the B2M gene and a telomeric marker D15S209) (Maleno et al. 2001; Maleno et al. 2006; Ramal et al. 2000). The amplification reaction was done in 15 μl volume using 1.5 μl of DNA (0.50 μg/μl) and 1 μl of the primer mixture (5 μl of each). The products of the amplification were analyzed by 5% acrylamide gel electrophoresis and sequenced using an automatic sequencer ABI PRISM 377 ADN (PE Applied Biosystems). Data analysis was performed using the software Genotyper program (PE Applied Biosystem). As a reference control, we used DNA obtained from normal autologous breast tissue. LOH was calculated as height of the signal of the tumor allele two/height of area of tumor allele one divided by the height of the signal of normal allele two/height of area of normal allele one. LOH was assigned when more than 25% of signal reduction of one allele was observed in the tumor simples as compared to the normal tissue. Haplotype loss was considered to exist when a tumor exhibited an allelic reduction in three or more STRs markers in chromosome 6. LOH-15 was assigned to the sample when signal was reduced in two or more STRs in chromosome 15 (Ramal et al. 2000).
B2M gene sequencing in HLA-I-negative tumors
The amplification of B2M gene from tumor fragments microdissected from HLA-I-negative tumors was performed using genomic DNA and Illustra PuRe-Taq Ready-To-GoTM PCR Beads (GE Healthcare Europe, Barcelona, Spain) with the following forward primers: 5′-CG ATATTCCTCAGGTACTC C-3′ and 5′-GGTGAATTCAGTG TAGTACAAG-3′, and one reverse primer: 5′-ACACAACTTTCAGCAGCTTAC-3′. The predicted PCR product sizes were 311 and 114 bp, respectively. Sequencing was performed with the Big Dye Terminator v1.1 Cycle Sequencing kit (Applied Biosystems, Warrington, UK) using Centri-Sep Columns (Applied Biosystems) and ABI 3130× = Genetic Analyzer and Sequencing Analysis v5.2 software (Applied Biosystems).
Statistical analysis
All statistical analyses were performed using the Statistical Package for the IBM-SPSS Statistics Ver.21. Variables with normal distribution are expressed as means with standard deviation, minimum, maximum, and range. The categorical variables such as tumor size, nodal invasion, estrogen receptor, progesterone receptor, TNM, histological tumor characteristics, HER2 receptor, and HLA-I expression were coded in two groups and analyzed using the chi-square (χ2) or Fisher exact test in case when the validity criteria were not reached. Differences were considered statistical significant for p < 0.05.
Results
Immunohistological analysis of HLA class I expression in breast cancer tissue samples
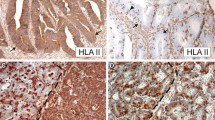
The expression of HLA-I antigens was evaluated in 98 breast cancer tissue using immunohistochemical staining with monoclonal antibodies directed against B2M and against monomorphic, HLA-A and HLA-B locus-specific and HLA-I allelic determinants. We detected various types of HLA-I alterations in 79 out of 98 studied samples (81%) (Tables 3 and 4). Total HLA-I loss, with negative immunolabeling of both Β2Μ (GRH-1 antibody) and HLA-I/Β2Μ complex (W6/32 antibody), was found in 54% (53 out of 98) of the samples. We also included in this group 7 cases with heterogeneous HLA-I staining. Interestingly, in 13 cases out of these 53 HLA-I-negative cases, we observed positive intracellular labeling of free heavy chains (HC-10 antibody). Sequencing of exons 1 and 2 of Β2Μ gene in all breast HLA-I-negative tumors (n = 53) did not reveal any mutations/deletions. In 9 cases out of 98 (9%), we detected a selective loss of HLA-A or HLA-B locus expression and in 5 cases (5%), we observed single HLA-I allelic losses. In 4 tumors, we found a combination of different HLA-I alterations. Only 19 out of 98 (19%) tumors showed “normal” expression of HLA-I antigens. We also studied HLA-II expression in 71 tumors using monoclonal antibody against HLA-DR (GRB-1), 54 of which (76%) were negative and 17 (24%) were positive (Table 3). Figure 1 depicts representative images of HLA-ABC-positive and HLA-ABC-negative tumors (W6/32 antibody). Table 1 summarizes a correlation between different clinicopathologic parameters (age, tumor size, nodal invasion, estrogen and progesterone receptor, metastasis, TNM, histology, and HER2 receptor) and tumor HLA expression. The only statistically significant correlation (p < 0, 05) was found between HLA-I expression and tumor size demonstrating that tumor with larger size have less HLA-I expression.
Genomic HLA typing of normal breast tissue
Table 3 shows the results of HLA-A, B, and C locus genomic typing performed on normal autologous tissue of 98 breast cancer patients. Only patient BC15 was homozygous for HLA-I. Loss of single HLA-I alleles was determined by lack of immunostaining of tumor tissue with specific antibodies and confirmed by its presence in patient’s HLA haplotype. In Table 4, HLA-I alleles lost in tumors are in italics.
Molecular mechanisms involved in the alterations of HLA class I expression: analysis of LOH and B2M sequencing
One of the objectives of our work was to investigate the molecular mechanisms involved in HLA-I alterations in breast cancer. Of the 92 cases studied for LOH-6 and LOH-15 (Table 4), 36 cases (39%) showed LOH at one or both chromosomes. Using microsatellite analysis, loss of heterozygosity was detected in 36 cases (39%). Among them, 15 cases had only LOH-6, 14 tumors only LOH-15, and in 7 samples, we detected LOH at chromosomes 6 and 15 (Table 4). Interestingly, some tumors positive for HLA-I expression by immunohistochemistry (w6/32 MoAbs) had LOH in chromosomes 6 or 15 (Table 4). Figure 2 illustrates a localization of STR markers at chromosome 6 and demonstrates representative results of the LOH-6 analysis in some breast cancer samples, including samples with haplotype loss.
Discussion
Tumor HLA-I expression is one of the fundamental factors responsible for the efficacy of adaptive anti-tumor immune response, while the high frequency of HLA-I loss suggests that this is a key route of cancer immune escape from T cell-mediated lysis (Garrido et al. 1997b; Garrido and Algarra 2001; Aptsiauri et al. 2007).
In this work, using immunohistochemistry with a large panel of monoclonal antibodies against monomorphic, locus and allelic specific HLA-I and HLA-II determinants, we found that loss and downregulation of HLA-I expression is a frequent event in breast cancer. Around 81% of the studied tumors demonstrated various types of HLA-I alterations. Molecular analysis of the microdissected tumor samples revealed that 39% of the tumor samples have loss of heterozygosity (LOH) at chromosomes 6 and/or 15, which harbor the HLA-I region (6p21) and B2M gene (6p15), respectively. Previous studies demonstrated that HLA-I expression is often downregulated in different types of malignancy, including breast cancer (de Kruijf et al. 2010; Aptsiauri et al. 2007). There have been several reports showing different results of the immunohistological analysis of HLA expression in breast cancer in correlation with clinical parameters. The discrepancy could be associated with the type of tissue and antibodies used.
On the other hand, there are only few publications on molecular analysis of genetic defects in HLA-I genes responsible for HLA alterations in breast cancer. These alterations could be due to structural defects in HLA-I genes (“hard” lesions) or regulatory aberrations (“soft” lesions) reversible by pro-inflammatory cytokines in the tumor microenvironment (Garrido et al. 2010b; Garrido et al. 2016; Aptsiauri et al. 2014). HLA-I expression in tumors can potentially predict the response to immunotherapy in cancer patients, and it depends on the nature of the alteration. It is believed that if the defect is genetic, it is unlikely that immunostimulation of T cells in tumor microenvironment induced by immunotherapy can upregulate normal HLA-I expression and antigen presentation. In this case, the escape mechanisms could prevail and lead to the generation of dangerous HLA-I-negative tumor escape variants providing the basis for tumor heterogeneity. Therefore, the success or failure of immunotherapy depends on the nature of preexisting HLA-I alterations (Garrido et al. 2016).
Among the structural (“hard”) HLA-I alterations, LOH-6 is an important mechanism that generates HLA-I haplotype loss in various human tumors with high incidence (Maleno et al. 2004; Maleno et al. 2006). Mutations in B2M gene together with the loss of another gene copy caused by LOH-15 are also responsible for the irreversible total HLA-I and have been described in various types of malignancy, both in cell lines and in tumor tissues, including a proportion of HLA-I-deficient melanoma and MSI-H colorectal cancers (Bernal et al. 2012). In the present study, we did not find mutations/deletions in B2M gene. LOH-15 may be unnoticed in tumor cells with “normal” HLA-I immunolabeling pattern and could represent one of the early events in malignant transformation driving pre-committed tumors to become HLA escape variants. Most tumors derive from HLA-I-positive normal epithelia and constantly acquire new HLA-I alterations during cancer progression and dissemination. It creates variability within primary tumors as well as between the primary carcinoma and metastases and may negatively impact survival and treatment efficacy.
In our study, we found a significant correlation between tumor size and HLA-I expression (when tumors were divided into two groups: T1 versus T2+T3+T4), demonstrating that tumors with larger size have less HLA-I expression. It can be explained by a gradual elimination of HLA-positive tumor cells by CTLs and escape of HLA-I-negative cells during tumor growth.
Intratumor heterogeneity among cancer cells is promoted by reversible or irreversible genetic alterations, by different microenvironmental factors and also by immunotherapy. In patients with bladder carcinoma, recurrent tumors after BCG therapy have a higher percentage of LOH-6 and LOH-15 and increased incidence of other HLA-I alterations (Carretero et al. 2011). Deletion of HLA genes may enable the clonal expansion of HLA-negative tumor cells and this selective pressure could explain the increased frequency of LOH within the HLA genes after immunotherapy. In melanoma, we have previously observed that LOH-6 and LOH-15 were the earliest HLA-I alterations occurring in a primary tumor, followed by an emergence of a Β2Μ mutation and complete loss of HLA-I in successive metastatic lesions (del Campo et al. 2014). The role of LOH in HLA genes during cancer evolution has been also recently described by us in a lung cancer study (Perea et al. 2017). In this context, another group reported that LOH affecting HLA-I genes in lung cancer is one of the immune escape mechanisms that is subject to strong immune selection pressure during tumor evolution (McGranaham et al. 2017). Selective allelic HLA-I losses caused by LOH-6 (HLA haplotype loss) could also potentially compromise T cell cytotoxicity directed against tumor antigenic peptide presented by a particular HLA-I allele.
Loss of HLA class I expression should render tumor tissues prone to destruction by NK cells. However, as it has been reported in different types of solid tumors, NK cells are rarely detected in the tumor infiltrate or even in the tumor margin (del Campo et al. 2014; Garrido et al. 2017b). In addition, tumor area in lung cancer has been reported to be enriched with the NK cell subpopulation with non-ctytotoxic CD56bright CD16− phenotype (Del Mar Valenzuela-Membrives et al. 2016). It is probably induced by the tumor microenvironment, which could locally impair NK homing and differentiation rendering these cells less cytotoxic and favoring the immune escape of HLA-I-negative tumor cells.
The results reported in this work suggest that a combination of multiple molecular mechanisms is responsible for HLA-I loss in breast cancer. It has been previously reported that HER2/neu oncogene expression in breast cancer has a correlation with HLA-I downregulation (Vertuani et al. 2009; Herrmann et al. 2004; Seliger and Kiessling 2013). However, in this study, we did not find any association between HLA-I and HER2/neu receptor expression (see Table 1).
Overall, the findings presented in this article indicate that LOH has a high frequency in breast cancer, even in tumor samples with positive HLA-I expression. These tumors were classified as positive with the anti-HLA-I antibody directed against HLA-I monomorphic determinants (w6/32). Nevertheless, these tumors harbor molecular HLA-I alterations, such as LOH-6 causing loss of HLA-I haplotype. However, we could not define the missing HLA-I alleles in each case. LOH, as a mechanism of HLA-I alteration, is definitely underestimated and may negatively influence T cell-mediated tumor rejection and clinical success of cancer eradiation by immunotherapy. We believe that the high frequency of LOH-6 and/or LOH-15 in breast cancer (approximately in 39% of the samples) and especially the coincidence of the LOH at chromosomes 6 and 15 might have a strong impact on tumor immunogenicity and on the efficacy of cancer immunotherapy, since the loss of any given HLA-I locus or of a single allele could result in the lack of CD8+T cell stimulation by a potentially important tumor-associated neo-antigen.
It is becoming increasingly evident that immune checkpoint blocking therapies are associated with recurrent metastatic tumor lesions harboring mutations in B2M and IFN genes (Zaretsky et al. 2016), which are interfering with tumor antigen presentation to T lymphocytes (Sade-Feldman et al. 2017). However, the massive sequencing techniques used in these modern studies do not take into account loss of genetic material in chromosomes 6 and 15, which represent structural/“hard” HLA-I lesions described by us and other groups in different types of cancer.
With the recent development of the field of cancer immunotherapy, the focus has shifted to the investigation of how tumors acquire resistance to treatment and to the discovery of novel predictive markers of the efficacy of therapy. Although anti-“immune checkpoint” immunotherapies have produced dramatic results in a subset of some malignancies, the percentage of non-responders, mixed responders, and post-treatment recurrences is rather high. Based on the existing scientific evidence, it could be, at least partially, explained by the loss of tumor HLA-I expression caused by structural genetic lesions in HLA-I/B2M genes. Therefore, investigation of genetic aberrations underlying altered tumor HLA-I expression is necessary for developing effective therapies.
Conclusions
Based on immunohistological and molecular analysis of breast tumor tissues, we discovered that partial and total HLA-I loss is a frequent finding in breast carcinoma. Here, we demonstrated that the leading molecular mechanism responsible for HLA-I altered expression is a loss of heterozygosity in the HLA-I region of chromosome 6 and in the B2M region of chromosome 15, which was detected in about 39% of studied tumors. It can be potentially overlooked and underestimated in tumors analyzed only by immunohistochemistry, when apparently HLA-I-positive tumors (labeled with antibodies against HLA monomorphic determinants) harbor potentially dangerous structural/irreversible genetic aberrations. These alterations may reduce the ability of tumor cells to present antigens to T lymphocytes in the context of HLA-I molecules and, consequently, could lead to immune escape and resistance to immunotherapy. Nevertheless, mechanisms responsible for total HLA-I loss (observed in 54% of the studied breast tumors) still remain to be defined.
Abbreviations
- HLA:
-
Human leukocite antigens
- MHC:
-
Major histocompatibility antigens
- LOH:
-
Loss of heterozygosity
- Β2Μ:
-
Beta-2-microglobilin
- FFPE:
-
Formalin fixed paraffin embedded
- IDC:
-
Infiltrating ductal carcinoma
- ILC:
-
Infiltrating lobular carcinoma
- TNM:
-
Tumor-node-metastasis
- ER:
-
Estrogen receptor
- PgR:
-
Progesterone receptor
- SSO:
-
Sequence-specific oligonucleotide analysis
- STR:
-
Short tandem repeat
References
Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F (2007) Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol 601:123–131
Aptsiauri N, Garcia-Lora A, Garrido F (2014) ‘Hard’ and ‘soft’ loss of MHC class I expression in cancer cells. In Tumor Immunology and Immunotherapy. Edited by Rees RC. Oxford University Press, 63–78
Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A (1978) Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell 14(1):9–20
Bernal M, Ruiz-Cabello F, Concha A, Paschen A, Garrido F (2012) Implication of the β2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother 61(9):1359–1371
Boesen M, Svane IM, Engel AM, Rygaard J, Thomsen AR, Werdelin O (2000) CD8+ T cells are crucial for the ability of congenic normal mice to reject highly immunogenic sarcomas induced in nude mice with 3-methylcholantrene. Clin Exp Immunol 121:210–215
Burrone OR, Kefford RF, Gilmore D, Milstein C (1985) Stimulation of HLA-A,B,C by IFN-alpha. The derivation of Molt 4 variants and the differential expression of HLA-A,B,C subsets. EMBO J 4(11):2855–2860
Cabrera T, Ruiz-Cabello F, Lopez MA, de la Higuera B, Sanchez M, Garrido F (1986) Characterization of monoclonal antibodies directed against HLA class II molecules. Hybridoma 5(3):191–197
Cabrera T, Fernandez MA, Sierra A, Garrido A, Herruzo A, Escobedo A, Fabra A, Garrido F (1996) High frequency of altered HLA class I phenotypes in invasive breast carcinomas. Hum Immunol 50:127–134
Cabrera T, Lopez-Nevot MA, Gaforio JJ, Ruiz-Cabello F, Garrido F (2003) Analysis of HLA expression in human tumor tissues. Cancer Immunol Immunother 52:1–9
Carretero R, Cabrera T, Sáenz-López P, Maleno I, Aptsiauri N, Cózar JM, Garrido F (2011) Bacillus Calmette-Guerin immunotherapy of bladder cancer induces selection of human leukocyte antigen class I-deficient tumor cells. Int J Cancer 129(4):839–846
Concha A, Cabrera T, Ruiz-Cabello F, and Garrido F. Can the HLA phenotype be used as a prognostic factor in breast carcinomas? Int J Cancer, 1991a; Suppl 6, 146–54
Concha A, Esteban F, Cabrera T, Ruiz-Cabello F, Garrido F (1991b) Tumor aggressiveness and MHC class I and II antigens in laryngeal and breast cancer. Semin Cancer Biol 2:47–54
Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T (2014) Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 14(2):135–146
Del Mar Valenzuela-Membrives M, Perea-García F, Sanchez-Palencia A, Ruiz-Cabello F, Gómez-Morales M, Miranda-León MT, Galindo-Angel I, Fárez-Vidal ME (2016) Progressive changes in composition of lymphocytes in lung tissues from patients with non-small-cell lung cancer. Oncotarget 7:71608–71619
Garrido F, Algarra I (2001) MHC antigens and tumor escape from immune surveillance. Adv Cancer Res 83:117–158
Garrido F, Cabrera T, Concha A, Glew S, Ruiz-Cabello F, Stern PL (1993) Natural history of HLA expression during tumour development. Immunol Today 14:491–499
Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL (1997a) Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today 18:89–95
Garrido, T. Cabrera, R.S. Accola, J.C. Bensa, W. Wodmer, G. Dohr, B. Drenou, M. Drouet, R. Fauchet, G.B. Ferrara, S. Ferrone, P. Giacomini, T. Kageshita, L. Koopman, M. Maio, F. Marincola, C. Mazzilli, P.A. Morell, A. Murray, Crh. Papasteriades, L. Salvaneschi, P.L. Stern, A. Ziegler. HLA and cancer: 12th International Histocompatibility workshop study. In: Genetic diversity of HLA. Functional and Medical Implications. EDK (Ed. by D. Charron) vol. I, 1997b; 445–452
Garrido F, Algarra I, García-Lora AM (2010a) The escape of cancer from T lymphocytes: immunoselection of MHC class I loss variants harboring structural-irreversible "hard" lesions. Cancer Immunol Immunother 59(10):1601–1606
Garrido F, Cabrera T, Aptsiauri N (2010b) ‘Hard’ and ‘soft’ lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer 127:249–256
Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T (2016) The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol 39:44–51
Garrido F, Ruiz-Cabello F, Aptsiauri N (2017a) Rejection versus escape: the tumor MHC dilemma. Cancer Immunol Immunother 66:259–271
Garrido F, Perea F, Bernal M, Sánchez-Palencia A, Aptsiauri N, Ruiz-Cabello F. The escape of cancer from T cell-mediated immune surveillance: HLA class I loss and tumor tissue architecture. Vaccines (Basel). 2017b Feb 27;5(1)
Herrmann F, Lehr HA, Drexler I, Sutter G, Hengstler J, Wollscheid U, Seliger B (2004) HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res 64:215–220
Kaneko K, Isihigami S, Kijima Y, Funasako Y, Hirata M, Okumura H, Shinchi H, Koriyama C, Ueno S, Yoshinaka H, Natsugoe S (2011) Clinical implication of HLA class I expression in breast cancer. BMC Cancer 11:454–459
de Kruijf EM, van Nes JG, Sajet A, Tummers QR, Putter H, Osanto S, Speetjens FM, Smit VT, Liefers GJ, van de Velde CJ, Kuppen PJ (2010) The predictive value of HLA class I tumor cell expression and presence of intratumoral Tregs for chemotherapy in patients with early breast cancer. Clin Cancer Res 16:1272–1280
del Campo AB, Kyte JA, Carretero J, Zinchencko S, Mendez R, Gonzalez-Aseguinolaza G, Ruiz-Cabello F, Aamdal S, Gaudernack G, Garrido F, Aptsiauri N (2014) Immune escape of cancer cells with beta2- microglobulin loss over the course of metastatic melanoma. Int J Cancer 134:102–113
López Nevot MA, Ruiz-Cabello F, Huelin C, Cabrera A, Garrido F (1986) A monoclonal antibody produced against the surface immunoglobulin of B-prolymphocytic leukemia. Sangre (Barc) 31(6):751–758
Lopez-Nevot MA, Esteban F, Ferron A, Gutierrez J, Oliva MR, Romero C, Huelin C, Ruiz-Cabello F, Garrido F (1989) HLA class I gene expression on human primary tumours and autologous metastases: demonstration of selective losses of HLA antigens on colorectal, gastric and laryngeal carcinomas. Br J Cancer 59:221–226
Madjd Z, Spendlove I, Pinder SE, Ellis IO, Durant L (2005) Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer 117:248–255
Maleno I, Aptsiauri N, Cabrera T, Gallego A, Paschen A, López-Nevot MA, Garrido F (2001) Frequent loss of heterozygosity in the □2-microglobulin region of chromosome 15 in primary human tumors. Immunogenetics 63(2):65–71
Maleno I, Cabrera CM, Cabrera T, Paco L, Lopez-Nevot MA, Collado A, Ferron A, Garrido F (2004) Distribution of HLA class I altered phenotypes in colorectal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Immunogenetics 56:244–253
Maleno I, Romero JM, Cabrera T, Paco L, Aptsiauri N, Cozar JM, Tallada M, Lopez-Nevot MA, Garrido F (2006) LOH at 6p21.3 region and HLA class altered phenotypes in bladder carcinomas. Immunogenetics 58(7):503–510
Marincola FM, Jafee EM, Hicklin DJ, Ferrone S (2000) Escape of human solid tumors from T cell recognition: molecular mechanisms and functional significance. Adv Immunol 74:181–273
McGranaham N, Rosenthal R, Hiley C, Rowan AJ, Watkins T, Wilson G, Birkbak N, Veeriah S, Van Loo P, Herrero J, Swanton C, TRACERx Consortium (2017) Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 171(6):1259–1271
Neefjes JJ, Ploegh HL (1988) Allele and locus-specific differences in cell surface expression and the association of HLA class I heavy chain with beta 2-microglobulin: differential effects of inhibition of glycosylation on class I subunit association. Eur J Immunol 18(5):801–810
Pedersen MH, Hood BL, Beck HC, Conrads TP, Ditzel HJ, Leth-Larsen R (2017) Downregulation of antigen presentation-associated pathway proteins is linked to poor outcome in triple-negative breast cancer patient tumors. 6(5):e1305531. https://doi.org/10.1080/2162402X.2017.1305531 eCollection 2017
Perea F, Bernal M, Sanchez-Palencia A, Carretero J, Torres C, Bayarri C, Gomez-Morales GF, Ruiz-Cabello F (2017) The absence of HLA class I expression in non-small cell lung cancer correlates with the tumor tissue structure and the pattern of T cell infiltration. Int J Cancer 140:888–899
Perez M, Cabrera T, Lopez Nevot MA, Gomez M, Peran F, Ruiz-Cabello F, Garrido F (1986) Heterogeneity of the expression of class I and II HLA antigens in human breast carcinomas. J Immunogenet 13:247–253
Ramal LM, Feenstra M, van der Zwan AW, Collado A, Lopez-Nevot MA, Tilanus M, Garrido F (2000) Criteria to define HLA haplotype loss in human solid tumors. Tissue Antigens 55:443–448
Romero P, Coulie P (2014) Adaptive T-cell immunity and tumor antigen recognition. Tumor Immunology and Immunotherapy. Edited by Rees RC. Oxford University Press, In, pp 1–14
Ryschich E, Notzel T, Hinz U, Autschbach F, Ferguson J, Simon I, Weitz J, Frohlich B, Klar E, Buchler MW, Schmidt J (2005) Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res 11(Pt 1):498–504
Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, Bjorgaard SL, Hammond MR, Vitzthum H, Blackmon SM, Frederick DT, Hazar-Rethinam M, Nadres BA, Van Seventer EE, Shukla SA, Yizhak K, Ray JP, Rosebrock D, Livitz D, Adalsteinsson V, Getz G, Duncan LM, Li B, Corcoran RB, Lawrence DP, Stemmer-Rachamimov A, Boland GM, Landau DA, Flaherty KT, Sullivan RJ, Hacohen N (2017) Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nature Communications 8(1):1136–1143
Seliger B, Kiessling R (2013) The two sides of HER2/neu: immune escape versus surveillance. Trends Mol Med 19(11):677–684
Seliger B, Cabrera T, Garrido F, Ferrone S (2002) HLA class I antigen abnormalities and immune escape by malignant cells. Semin Cancer Biol 12:3–13
Sobin L, Gospodarowiaz M, Wittekind CH. TNM classification of malignant tumours. UICC 2009, 7th edn. Chichester: Wiley, 2010; 310p
Spear BT, Kornbluth J, Strominger JL, Wilson DB (1985) Evidence for a shared HLA-A intralocus determinant defined by monoclonal antibody 131. J Exp Med 162(6):1802–1810
Vertuani S, Triulzi C, Roos AK, Charo J, Norell H, Lemonnier F, Pisa P, Seliger B, Kiessling R (2009) HER-2/neu mediated down-regulation of MHC class I antigens processing prevents CTL-mediated tumor recognition upon DNA vaccination in HLA-A2 transgenic mice. Cancer Immunol Immunother 58:653–654
Wang E, Worschech A, Marincola FM (2008) The immunological constant of rejection. Trends Immunol 29:256–262
Zacharakis N, Chinnasamy H, Black M, Xu H, Lu YC, Zheng Z, Pasetto A, Langhan M, Shelton T, Prickett T, Gartner J, Jia L, Trebska-McGowan K, Somerville RP, Robbins PF, Rosenberg SA, Goff SL, Feldman SA (2018 Jun) Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med 24(6):724–730
Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly et al (2016) Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 375(9):819–829
Acknowledgements
We would like to thank Antonia Martín Casares for the technical support in HLA typing and Amanda Rocío González-Ramírez for the statistical analysis of the obtained results.
Funding
This work was supported by the grants from Spanish Institute of Health Carlos III (ISCIII, Instituto Carlos III) co-financed by European Union (FEDER-Fondo Europeo de Desarrollo Regional) (PI12/02031, PI08/1265, PI11/01022, PI11/01386, RETIC RD 06/020, RD09/0076/00165, PT13/0010/0039, PI14/01978, PI16/00752, PI17/00197), the Junta de Andalucía in Spain (Groups CTS-143), and Beckman-Coulter. This study is part of the doctoral thesis of Maria A. Garrido.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study protocol was approved by the ethical committee of the Virgen de las Nieves University Hospital and Instituto de Investigación Biosanitaria “ibs. Granada” (Comité de Ética de la Investigación de Centro de Granada (CEI Granada), number 2014-22/12). Signed informed consent approved by the Ethics Committee of our institution was obtained from all the patients.
Competing interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Garrido, M.A., Rodriguez, T., Zinchenko, S. et al. HLA class I alterations in breast carcinoma are associated with a high frequency of the loss of heterozygosity at chromosomes 6 and 15. Immunogenetics 70, 647–659 (2018). https://doi.org/10.1007/s00251-018-1074-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-018-1074-2