Abstract
Macrophages play a key role in atherosclerosis, cancer, and in the response to implanted medical devices. In each of these situations, the mechanical environment of a macrophage can vary from soft to stiff. However, how stiffness affects macrophage behavior remains uncertain. Using substrates of varying stiffness, we show macrophage phenotype and function depends on substrate stiffness. Notably, the cell area increases slightly from a sphere after 18 h on substrates mimicking healthy arterial stiffness (1–5 kPa), whereas macrophages on stiffer substrates (280 kPa–70 GPa) increased in area by nearly eight-fold. Macrophage migration is random regardless of substrate stiffness. The total average track speed was 7.8 ± 0.5 μm/h, with macrophages traveling fastest on the 280-kPa substrate (12.0 ± 0.5 μm/h) and slowest on the 3-kPa substrate (5.0 ± 0.4 μm/h). In addition F-actin organization in macrophages depends on substrate stiffness. On soft substrates, F-actin is spread uniformly throughout the cytoplasm, whereas on stiff substrates F-actin is functionalized into stress fibers. The proliferation rate of macrophages was faster on stiff substrates. Cells plated on the 280-kPa gel had a significantly shorter doubling time than those plated on the softer substrate. However, the ability of macrophages to phagocytose 1-μm particles did not depend on substrate stiffness. In conclusion, the results herein show macrophages are mechanosensitive; they respond to changes in stiffness by modifying their area, migration speed, actin organization, and proliferation rate. These results are important to understanding how macrophages respond in complex mechanical environments such as an atherosclerotic plaque.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mechanical environment of a cell may influence the properties and behavior of that cell. In general, an artery seeks to return to homeostasis, the mechanical state before a perturbation. For example, a vascular smooth muscle cell exposed to greater cyclic stretch than normal will synthesize platelet-derived growth factor (PDGF) and proliferate (Humphrey 2008). This cellular response is what causes, in part, arterial wall thickening in the case of hypertension. In turn, wall thickening returns the circumferential stress closer to the value before the increase in arterial pressure and cyclic stretch. This relationship can be appreciated through the simple hoop stress equation: \(\sigma_{\theta } = \frac{Pa}{h}\). Where the mean homeostatic circumferential stress, \(\sigma_{\theta }\), in a large artery is around 100 kPa, P is the transmural pressure, a is the radius, and h is the wall thickness. Whereby if the pressure increases, either the radius needs to decrease or the wall thickness needs to increase in order to restore the circumferential stress. In addition to arterial remodeling, perturbations to the mechanical properties can lead to sudden catastrophic events. The material properties of constituents in an atherosclerotic plaque can range in stiffness from 1 to 250 kPa; ranging approximately from lipid (1 kPa), cellular fibrotic (10 kPa), hypocellular fibrotic (60 kPa), elastic (80 kPa) to calcified (250 kPa) areas. Mechanical discontinuities in the material properties of a plaque, particularly microcalcifications near the cap, result in local stress concentrations and often lead to fissures (Rambhia et al. 2012; Vengrenyuk et al. 2006). Yet, it is still unclear how the mechanical perturbations in arterial tissue, as in the case of an advanced atherosclerotic plaque, affect the properties of residing macrophages.
Other types of vascular cells (i.e., endothelial, smooth muscle, and fibroblast) have been shown to respond to the mechanical cues of their environment. Endothelial cells will increase proliferation (Yeh et al. 2012), cell–cell junction width (Huynh et al. 2011) and leukocyte transmigration (Stroka and Aranda-Espinoza 2011; Hayenga and Aranda-Espinoza 2013) on substrates more stiff than the healthy range (i.e., >5 kPa) (Peloquin et al. 2011). Endothelial cells will also increase cell–cell conductivity, cell alignment in the direction of flow, and inhibit smooth muscle cell proliferation, platelet and leukocyte adhesion, and arterial narrowing under physiologic, undisturbed flows (Califano and Reinhart-King 2010). Vascular smooth muscle cells display polarization and durotaxis in response to substrate stiffness gradients (Isenberg et al. 2009). The third primary cell type in arteries, fibroblast cells, have been reported to increase their surface area and form actin stress fibers on substrates above 3 kPa (Yeung et al. 2005). However, not all vascular cells are mechanosensitive. In fact, the spreading area of chemically activated neutrophils does not depend on substrate stiffness. Neutrophils are able to extend an actin-filled protrusion on soft substrates without generating traction forces on the substrate (Yeung et al. 2005; Lee et al. 1994). While limited results have been reported on the mechanosensitivity of murine alveolar (Fereol et al. 2006) and murine tumor (RAW 264.7, U937) macrophages (Patel et al. 2012; Blakney et al. 2012), the mechanosensitive behavior of macrophages derived from a monocyte, a type of leukocyte in the blood, has not been shown.
Indeed discovering how macrophages are affected by their mechanical environment may shed light on mechanical cues that either ameliorate or worsen the progression of atherosclerotic plaques. Studies suggest local proliferation of macrophages residing in a plaque, rather than monocyte recruitment from the blood stream, dominates the progression of atherosclerotic plaques (Robbins et al. 2013; Gordon et al. 1990; Rekhter and Gordon 1995; Katsuda et al. 1993; Lutgens et al. 1999a, b; Aikawa et al. 2001). Macrophages are important to the progression of an atherosclerotic plaque forming fatty streaks in early lesions and the necrotic core in late, unstable plaques. Since macrophages are mechanosensitive, and cells seek to return the environment towards that at homeostasis, we hypothesize the proliferation, migration, phagocytosis, and cytoskeleton of monocyte-derived macrophages will be affected by altered substrate stiffness. Identifying how macrophages are influenced by their mechanical cues will provide insight into how a plaque may progress as well as guide the development of treatment and therapeutic options for atherosclerosis.
Materials and methods
Gel preparation
Fibronectin (0.1 mg/ml) (Sigma, St. Louis, MO, USA) was coated onto polyacrylamide gels of varying stiffnesses. Stiffnesses of the polyacrylamide gels were based on the following concentrations of acrylamide (acyl) and bis: 280 kPa—15 % acryl and 1.2 % bis, 13 kPa—8 % acryl and 0.2 % bis, 5 kPa—8 % acryl and 0.07 % bis, 3 kPa—5 % acryl and 0.05 % bis, 1 kPa—3 % acryl and 0.1 % bis (Bio-Rad laboratories, Hercules, CA, USA) as previously quantified (Stroka and Aranda-Espinoza 2009; Norman and Aranda-Espinoza 2010). Gel substrates of ~80 μm thickness were made by dispensing 30 μl of polyacrylamide onto an amine-activated glass coverslip (22 × 22 mm, No. 1.5, Fisher Scientific, Pittsburg, PA, USA), and then placing a UV-sterilized coverslip on top. The resulting gels were cured for 30 min at room temperature before prying the top coverslip off using the edge of a sterile razor blade.
The uniformity and final concentration of fibronectin on the gels was determined using antibodies targeted to fibronectin (Sigma, St. Louis, MO, USA). A fibronectin coating was chosen because it has been reported to be optimal over collagen-1 and fibrinogen for 2D time-lapse random migration assays of macrophages (Murray et al. 2013).
Migration assay
Forty-thousand human, monocyte-derived macrophages (Celprogen, Torrance, CA, USA) cultured in macrophage media (Celprogen), were plated onto a fibronectin-coated gel. Time-lapse microscopy was completed at 37 °C and 5 % carbon dioxide with an Olympus IX71 microscope and QImaging camera. Phase-contrast images were captured every 5 min for 20 h. Cell area was found using ImageJ software by manually circling the outline of each macrophage. The center of the cell was manually tracked in ImageJ as well, and this data was used to find cell trajectories and speeds. Speed is defined as the displacement of the center of the cell for each 20-min time interval divided by the time interval. The speed was found for each cell then averaged for each stiffness. The trajectory data was used to find the ratio between net translocation and contour length where net translocation is defined as the difference between the coordinates of the cell at 0 and 18 h while contour path is the total distance traveled (Fig. 2a). The ratio gives information about whether a cell is moving randomly; it was found for each cell and then averaged over each stiffness. The cell trajectories were also plotted with each cell beginning at coordinate (0,0).
Phagocytosis assay
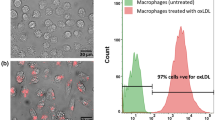
Macrophages were plated onto various substrate stiffnesses (2.5 × 105 cells per 45 × 50 mm gel per 35-mm Petri dish) and incubated for 43 h. Prior to confluency, the macrophages were exposed to 7 ml media with 87.5 μl of 1 μm Nile red fluorescent beads (Life Technologies, Grand Island, NY, USA) for up to 4 h. Afterwards, macrophages were washed with warmed PBS to remove excess beads. Macrophages were then removed from the gels with 1 ml Trypsin–EDTA (Sigma, St. Louis, MO, USA) and fixed with 4 % paraformaldehyde. After centrifugation, macrophages from each condition were counted and resuspended at the same density. Fluorescent flow cytometry (Becton–Dickinson FACScan) was used to count the number of beads internalized by each macrophage (see supplemental Fig. 1). Quantification was confirmed by visualization of the macrophages with optical microscopy (Olympus IX71).
Immunostaining
Single macrophages plated on gels were fixed, permeabilized, and blocked for nonspecific binding. Cells were then stained with phalloidin–tetramethylrhodamine isothiocyanate (Sigma, St. Louis, MO, USA) to label F-actin and 2 μg/ml Hoechst stain (Sigma) to label nuclei. Fluorescence microscopy was done with an Olympus IX71 microscope. ImageJ software was used to determine the fluorescence intensity of each cell.
Proliferation assay
Microcontact printing was used to pattern the surface of the substrates (Thery and Piel 2009). PDMS stamps with 400-μm-diameter circles were cast from a silicon wafer that was etched 40 μm deep with standard lithography. These stamps were inked in a 0.1 % rhodamine fibronectin-solution (Cytoskeleton, Denver, CO, USA) for 30 min, then pressed into a dehydrated gel and allowed to sit for 2 h in order for the fibronectin to transfer to the gel. Then, the gel was rehydrated in PBS for at least 2 h before 4 × 104 cells were plated in macrophage media. Images were captured at 1, 24, 48, and 72 h after plating and cells in each circle were counted using the Cell Counter Plug-in in ImageJ Software. Doubling time (DT) between each of the time points was found according to the following equation: \(DT = \frac{{T { \ln }\left( 2 \right)}}{{{ \ln }\left( {\frac{{X_{e} }}{{X_{b} }}} \right)}}\) where T is the incubation time, X b is the cell number at the start of the incubation time, and X e is the cell number after the incubation time. The calculated doubling time was then averaged to find a doubling time for each stiffness. At least 20 stamped circles were imaged for each stiffness at each time point.
Statistical analysis
Statistical tests were done among groups of data using ANOVA, and between pairs using Student’s t test. Statistical significance is considered for p < 0.05 and results are reported as mean ± standard error mean (SEM).
Results
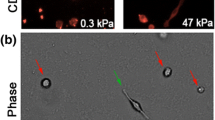
Cell spreading area is dependent on substrate stiffness and time. First, we found that fibronectin density and uniformity on the gel surface is independent of gel stiffness. The fluorescent intensity of fibronectin antibodies to the 100 μg/ml fibronectin-coated gels was not statistically significant (data not shown). This result is consistent with those reported by others (Flanagan et al. 2002; Yeung et al. 2005). Macrophages plated on substrates of each stiffness initially began as a round sphere (Fig. 1b) with an average area of 155 ± 11 μm2. By 1 h, macrophages plated on the stiffer substrate (280 kPa) were already significantly larger than cells plated on soft gels (1, 3, and 5 kPa) (Fig. 1a). This result suggests that macrophages began sensing and responding to the substrate stiffness in less than 1 h after plating. The increased area on stiff substrates was also seen up to 18 h after plating, when cells on the softer substrates had an average area of 318 ± 31 μm2 and macrophages plated on the stiff substrate had a significantly larger area of 988 ± 136 μm2.
The surface area of macrophages is dependent on substrate stiffness. Initially all macrophages have the same area. After 1 h, the cells have nearly reached their maximum area on each substrate stiffness. a The maximum area is statistically different for the soft (1–5 kPa) substrates compared to the stiff (280 kPa—glass) substrates. b On glass, only there is a significant increase in cell area between the 1-h and 18-h time points. Scale bar is 25 μm. *p < 0.05
To understand the migration of single macrophages, the trajectories of their migration were plotted (Fig. 2c). While most cells seemed to cluster around the origin, some cells on substrates of each stiffness displayed more directed migration to locations further out. To more quantitatively analyze whether directed migration was occurring on substrates of a particular stiffness, the ratio of net translocation to contour length was found for each cell. The averages for each stiffness are all under 0.5, suggesting migration is random on all stiffnesses (Fig. 2b). Although migration was random on all stiffnesses, macrophage migration speed is affected by substrate stiffness. The speed was found for each cell, then averaged for each stiffness. Cells were found to move significantly faster on the 280-kPa substrate 12.0 ± 0.5 μm/h, and significantly slower on the 3-kPa substrate, 5.0 ± 0.4 μm/h (Fig. 3). Moreover, on the most soft (1 kPa), intermediate (5 kPa), and most stiff (glass) substrates the speed was around 7.4 μm/h with speeds of 7.4 ± 0.6 μm/h on 1 kPa, 7.3 ± 0.6 μm/h on 5 kPa and 7.5 ± 0.2 μm/h on glass. These differences were not significant if the time interval over which the speed was determined was greater than 20 min.
Macrophage migration paths were random, with minimal dependency on substrate stiffness. a Directionality is determined by dividing the cell’s contour path by the net translocation displacement. A ratio <0.5 suggests the motion of the macrophages is not directed. b Although macrophages on the 3-kPa substrate had a slightly higher ratio, it was still under 0.5. c Plots of the cell tracks, with each cell starting at the origin, illustrates the random motion of the macrophages (n = 10). *p < 0.05
The speed of macrophages was fastest on the 280-kPa gel (12.0 ± 0.5 μm/h) and slowest on the 3-kPa gel (5.0 ± 0.4 μm/h). The other substrates had an average speed of 7.4 ± 0.5 μm/h. However, this difference was not significant if the time interval used to determine the speed was 40 min or greater. *p < 0.05
The total amount of F-actin appears to be a function of substrate stiffness (Fig. 4). Macrophages spread out to a greater extent on stiff substrates (Fig. 1). Therefore, quantifying F-actin per area reveals that the F-actin/area ratio is greatest in macrophages on soft substrates (1 and 3 kPa), slightly less on intermediate substrates (5 and 13 kPa), and the least on the stiff substrate (280 kPa). Moreover, the total F-actin per macrophage did not significantly change on soft and intermediate substrates but was significantly lower on the stiff substrate. In cells, actin moves between pools of monomeric (G-actin) and double helical filaments (F-actin). On soft substrates, actin may be mostly in the monomeric form, as opposed to macrophages on stiff substrates where the actin is primarily organized into filaments (Fig. 4b). Thus, the total F-actin content in macrophages appears lower on stiff substrates due to the actin reorganization into F-actin stress fibers.
F-actin content as determined by fluorescent intensity per macrophage. a The total F-actin content on the basal surface of each macrophage is fairly consistent, regardless of substrate stiffness. However, the F-actin per macrophage area is greatest on the 1-kPa gel and least on the 280-kPa gel. b This difference is perhaps due to actin fibers in macrophages becoming more organized and forming longer stress fibers on stiffer substrates. Scale bar 25 μm. *p < 0.05
The ability of macrophages to phagocytose particles does not depend on substrate stiffness (Fig. 5). Based on results from flow cytometry, macrophages internalized, on average, two 1 μm fluorosphere beads after 1 h. After 3 h, an average of 35 beads were internalized. The number of beads per cell was identified based on mean fluorescence. That is, the mean fluorescence of a single fluorosphere bead was about 4000 a.u., for two beads 8000 a.u., three beads 12,000 a.u., and so on (supplemental Fig. 1A, 1B). Bead distribution was also determined for cells incubated with beads for 1 h (supplemental Fig. 1C). Macrophages only took up an average of two beads and there was no difference between different stiffnesses (Fig. 5c). Together, the results emphasize that macrophage phagocytosis does not depend on substrate stiffness. Moreover, the same results were obtained using different experimental methods (i.e., measuring fluorescence with a plate reader and taking optical images and quantifying beads/cell).
Macrophage phagocytosis of 1-μm particles does not depend on substrate stiffness. a, b On average, macrophages phagocytosed 2.0 ± 0.3 beads after 1 h and 35.0 ± 1.3 beads after 3 h. c Regardless of substrate stiffness, after 1 h on average about 38.0 ± 2.4 %, 43.0 ± 1.1 %, and 19.0 ± 1.7 % of macrophages had consumed no beads, 1–3 beads, and over three beads, respectively. *p < 0.05
Macrophage proliferation in fibronectin-stamped circles was observed over 72 h and the doubling time was calculated. One hour after plating the cells there was an average of 15.0 ± 1.7 and 17.2 ± 2.4 macrophages per circle on the stiff 280-kPa and 13-kPa gels, respectively. After 70 h, there was an average of 259.8 ± 9.9 cells per circle on the stiff substrate and 221.4 ± 18.3 cells per circle on the 13-kPa gel (Fig. 6b). These time points were not included in the doubling time calculation, however, since limited space may have retarded cell proliferation within the circles. Forty-eight hours after plating the cells, there was an average of 121.3 ± 9.3 cells per circle on the 280-kPa gel and 85.72 ± 8.49 cells on the 13-kPa gel and images show there was room for additional cells (Fig. 6c). Therefore, from the cell counts at 1, 24, and 48 h it was found that, on average, cells on the 13-kPa gel doubled in 19.0 ± 0.1 h, while macrophages on the stiffest gel (280 kPa) proliferated faster with a doubling time of 14.6 ± 0.2 h (Fig. 6a).
Macrophages proliferate in less time on stiffer substrates. Initially, the same number of cells were plated on gels with fibronectin-stamped circles. a Cells on stiff substrates (280 kPa) had a significantly smaller doubling time than cells on the substrate of intermediate stiffness (13 kPa). b The number of cells in each 400-μm circle was not statistically different at 1, 24, or 70 h, but at 45 h the stiff substrate (280 kPa) had significantly more cells. c Images captured during a time-lapse video show cells proliferating to fill in patterned circles at 0, 18, 36, and 54 h *p < 0.05
Discussion
Herein we report that human monocyte-derived macrophages are able to sense their mechanical environment and respond via morphological and biophysical changes. In general, on soft matrices, mechanosensitive cells take on a rounded shape with few focal adhesion complexes and no F-actin stress fibers. In contrast, on stiff substrates, cells will flatten, form multiple strong adhesions, and form thick stress fibers (Jacobs et al. 2013).
Previous studies claim stress fibers are not present in macrophages, even on stiff substrates (Fereol et al. 2006; Blakney et al. 2012). They say actin stress fibers spanning the length of a cell are seen in contractile cells with low motility (endothelial cells, fibroblasts, smooth muscle, etc.) and not macrophages. They hypothesize macrophages are not prestressed and use some other mechanism for mechanosensing the environment. However, the cytoskeletal stiffness of macrophages increases as the substrate stiffness increases (Fereol et al. 2006; Patel et al. 2012). Moreover, Roduit et al. showed that if actin is depolymerized in macrophages, with cytochalasin, macrophage stiffness decreased from ~132 to ~28 kPa, supporting the notion that actin fibers contribute to macrophage elasticity (Roduit et al. 2012). Herein, we observed F-actin fibers are present in monocyte-derived macrophages. The F-actin fibers become more pronounced and span a greater length of the cell as the substrate stiffness increases (Fig. 4). These findings challenge the current paradigm and suggest a causal relationship between the formation of F-actin stress fibers (pre-stress) in macrophages and the increased stiffness in macrophages on stiffer substrates, as is seen in contractile tissue cells (Wang et al. 2002).
In addition to increased F-actin fiber formation with substrate stiffness, the morphology of the macrophages was affected by substrate stiffness. On soft substrates (1–5 kPa), the macrophages increased their area twofold (from about 155 to 318 μm2), whereas on stiff substrates (280 kPa and glass) the macrophages formed protrusions (e.g., lamellipodium, filopodium, etc.) resulting in an over six-fold increase in area (to 988 μm2). Murine alveolar macrophages also spread more on stiffer substrates. However, they appear to be smaller, only spreading to about 100 μm2 on soft and 375 μm2 on stiff (glass) substrates (Fereol et al. 2006). There are noticeable differences in morphology and function from different lineages of macrophages. The mechanism for these differences remains to be elucidated.
Proliferation is important in lesions, in late plaques maybe even more so than monocyte recruitment (Robbins et al. 2013). It was shown that blocking macrophage proliferation with 5-FU slows progression of plaque in mice. Herein, we showed for healthy monocyte-derived macrophages the average doubling rate goes from 19.0 to 14.6 h by increasing the substrate stiffness from 13 to 280 kPa. Understanding the cues that lead to increased proliferation could be useful as possible therapeutic targets. Interestingly, in addition to the mechanical environment, the macrophage cell line also determines the proliferation rate. The doubling time of macrophage cell lines from cancer subjects has been reported to be anywhere between 11 h for RAW264.7 cells (Sakagami et al. 2009), 27 h (van Furth et al. 1987) for J774A.1 and 50 h (Ziegler-Heitbrock et al. 1988) for Mono Mac-6 cells. Conceivably, the time required to proliferate is modulated by the mechanical environment for these macrophage cell lines as well. However, further studies are needed to confirm this.
Unlike the relatively quick proliferation rate, the track speed of monocyte-derived macrophages is relatively slow. The average migration speed for monocyte-derived macrophages was between 0.08 and 0.20 μm/min, whereas the average speed is about 0.8 μm/min for murine bone marrow-derived macrophages (Murray et al. 2013), about 4 μm/min for FNLP-stimulated murine alveolar macrophages (Glasgow et al. 1989), and about 12 μm/min in the tailfin of juvenile fish (Grabher et al. 2007). Van Goethem et al. found that the average velocity of human monocyte-derived macrophages was about 0.2 μm/min on soft (0.1 kPa) gels (Matrigel and gelled collagen I), and about 0.7 μm/min on soft (0.02 kPa) fibrillar collagen I substrates (Van Goethem et al. 2010). The investigators attributed the slow (0.2 μm/min) movement to a mesenchymal mode of migration and the 0.7 μm/min migration to an amoeboid mode where the cells stayed more round in shape.
Previously, it has been found that mouse RAW264.7 and human alveolar macrophages phagocytosed fewer 2 μm latex beads when plated on softer substrates compared to stiffer ones (Patel et al. 2012). However, gene expression studies could not predict these results (Patel et al. 2012). The authors also found a significant increase in phagocytosis by adding a stimulant, LPS. Conversely, we did not observe a significant increase in phagocytosis after treating the macrophages with 10 ng/ml LPS for 24 h (data not shown). Further studies are needed to elucidate how posttranslational protein modifications in tumor and alveolar macrophages can modulate phagocytosis sensitivity to substrate stiffness, but not in monocyte-derived macrophages.
The extracellular matrix (ECM) proteins may also influence cell behavior (Uitto and Larjava 1991; Teti 1992). To isolate the effects of mechanical properties alone on macrophage behavior, the ECM protein remained constant in this study. Fibronectin was chosen as the extracellular matrix protein because it is a glycoprotein that connects cells with a variety of components in the ECM (e.g., collagen, fibrin, and heparin sulfate proteoglycans) and it contains an amino acid sequence [arginine-glycine-aspartic acid (RGD)] common to many ECM proteins (including elastin) (Jeon et al. 2012; Ruoslahti 1996; Halper and Kjaer 2014; Dufour et al. 1986). The RGD sequence binds to integrins on the surface of macrophages to facilitate reorganization of the cytoskeleton and cell migration. Thus, fibronectin is an essential protein allowing macrophages to sense the environment, however, further studies are needed to elucidate if specific binding to other ECM proteins affects the behavior of macrophages.
References
Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P (2001) An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation 103:276–283
Blakney AK, Swartzlander MD, Bryant SJ (2012) The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly(ethylene glycol)-based hydrogels. J Biomed Mater Res Part A 100:1375–1386. doi:10.1002/jbm.a.34104
Califano JP, Reinhart-King CA (2010) Exogenous and endogenous force regulation of endothelial cell behavior. J Biomech 43:79–86. doi:10.1016/j.jbiomech.2009.09.012
Dufour S, Duband JL, Thiery JP (1986) Role of a major cell-substratum adhesion system in cell behavior and morphogenesis. Biol Cell 58:1–13
Fereol S, Fodil R, Labat B, Galiacy S, Laurent VM, Louis B, Isabey D, Planus E (2006) Sensitivity of alveolar macrophages to substrate mechanical and adhesive properties. Cell Motil Cytoskelet 63:321–340. doi:10.1002/cm.20130
Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA (2002) Neurite branching on deformable substrates. Neuroreport 13:2411–2415. doi:10.1097/01.wnr.0000048003.96487.97
Glasgow JE, Farrell BE, Fisher ES, Lauffenburger DA, Daniele RP (1989) The motile response of alveolar macrophages. An experimental study using single-cell and cell population approaches. Am Rev Respir Dis 139:320–329. doi:10.1164/ajrccm/139.2.320
Gordon D, Reidy MA, Benditt EP, Schwartz SM (1990) Cell proliferation in human coronary arteries. Proc Natl Acad Sci USA 87:4600–4604
Grabher C, Cliffe A, Miura K, Hayflick J, Pepperkok R, Rorth P, Wittbrodt J (2007) Birth and life of tissue macrophages and their migration in embryogenesis and inflammation in medaka. J Leukoc Biol 81:263–271. doi:10.1189/jlb.0806526
Halper J, Kjaer M (2014) Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol 802:31–47. doi:10.1007/978-94-007-7893-1_3
Hayenga HN, Aranda-Espinoza H (2013) Stiffness increases mononuclear cell transendothelial migration. Cell Mol Bioeng 6:253–265. doi:10.1007/s12195-013-0284-9
Humphrey JD (2008) Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension 52:195–200. doi:10.1161/HYPERTENSIONAHA.107.103440
Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA (2011) Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med 3:112ra122. doi:10.1126/scitranslmed.3002761
Isenberg BC, DiMilla PA, Walker M, Kim S, Wong JY (2009) Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys J 97:1313–1322. doi:10.1016/j.bpj.2009.06.021
Jacobs CR, Huang H, Kwon RY (2013) Introduction to cell mechanics and mechanobiology. Garland Science, New York
Jeon WB, Park BH, Choi SK, Lee KM, Park JK (2012) Functional enhancement of neuronal cell behaviors and differentiation by elastin-mimetic recombinant protein presenting Arg-Gly-Asp peptides. BMC Biotechnol 12:61. doi:10.1186/1472-6750-12-61
Katsuda S, Coltrera MD, Ross R, Gown AM (1993) Human atherosclerosis. IV. Immunocytochemical analysis of cell activation and proliferation in lesions of young adults. Am J Pathol 142:1787–1793
Lee J, Leonard M, Oliver T, Ishihara A, Jacobson K (1994) Traction forces generated by locomoting keratocytes. J Cell Biol 127:1957–1964. doi:10.1083/jcb.127.6.1957
Lutgens E, Daemen M, Kockx M, Doevendans P, Hofker M, Havekes L, Wellens H, de Muinck ED (1999a) Atherosclerosis in APOE*3-Leiden transgenic mice: from proliferative to atheromatous stage. Circulation 99:276–283
Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ (1999b) Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovas Res 41:473–479
Murray MY, Birkland TP, Howe JD, Rowan AD, Fidock M, Parks WC, Gavrilovic J (2013) Macrophage migration and invasion is regulated by MMP10 expression. PloS One 8:e63555. doi:10.1371/journal.pone.0063555
Norman L, Aranda-Espinoza H (2010) Cortical neuron outgrowth is insensitive to substrate stiffness. Cell Mol Bioeng 3:398–414. doi:10.1007/s12195-010-0137-8
Patel NR, Bole M, Chen C, Hardin CC, Kho AT, Mih J, Deng L, Butler J, Tschumperlin D, Fredberg JJ, Krishnan R, Koziel H (2012) Cell elasticity determines macrophage function. PloS One 7:e41024. doi:10.1371/journal.pone.0041024
Peloquin J, Huynh J, Williams RM, Reinhart-King CA (2011) Indentation measurements of the subendothelial matrix in bovine carotid arteries. J Biomech 44:815–821. doi:10.1016/j.jbiomech.2010.12.018
Rambhia SH, Liang X, Xenos M, Alemu Y, Maldonado N, Kelly A, Chakraborti S, Weinbaum S, Cardoso L, Einav S, Bluestein D (2012) Microcalcifications increase coronary vulnerable plaque rupture potential: a patient-based micro-CT fluid-structure interaction study. Ann Biomed Eng 40:1443–1454. doi:10.1007/s10439-012-0511-x
Rekhter MD, Gordon D (1995) Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am J Pathol 147:668–677
Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, Zavitz CC, Shikatani EA, Parsons M, van Rooijen N, Lin HY, Husain M, Libby P, Nahrendorf M, Weissleder R, Swirski FK (2013) Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med 19:1166–1172. doi:10.1038/nm.3258
Roduit C, Longo G, Benmessaoud I, Volterra A, Saha B, Dietler G, Kasas S (2012) Stiffness tomography exploration of living and fixed macrophages. J Mol Recognit 25:241–246. doi:10.1002/jmr.2184
Ruoslahti E (1996) RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12:697–715. doi:10.1146/annurev.cellbio.12.1.697
Sakagami H, Kishino K, Amano O, Kanda Y, Kunii S, Yokote Y, Oizumi H, Oizumi T (2009) Cell death induced by nutritional starvation in mouse macrophage-like RAW264.7 cells. Anticancer Res 29:343–347
Stroka KM, Aranda-Espinoza H (2009) Neutrophils display biphasic relationship between migration and substrate stiffness. Cell Motil Cytoskelet 118:1632–1640. doi:10.1002/cm.20363
Stroka KM, Aranda-Espinoza H (2011) Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood 66:328–341. doi:10.1182/blood-2010-11-321125
Teti A (1992) Regulation of cellular functions by extracellular matrix. J Am Soc Nephrol 2:S83–S87
Thery M, Piel M (2009) Adhesive micropatterns for cells: a microcontact printing protocol. Cold Spring Harb Protoc 7:pdb prot5255. doi:10.1101/pdb.prot5255
Uitto VJ, Larjava H (1991) Extracellular matrix molecules and their receptors: an overview with special emphasis on periodontal tissues. Crit Rev Oral Biol Med 2:323–354
van Furth R, Elzenga-Claasen I, van Schadewijk-Nieuwstad M, Diesselhoff-den Dulk MM, Toivonen H, Rytomaa T (1987) Cell kinetic analysis of a murine macrophage cell line. Eur J Cell Biol 44:93–96
Van Goethem E, Poincloux R, Gauffre F, Maridonneau-Parini I, Le Cabec V (2010) Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J Immunol 184:1049–1061. doi:10.4049/jimmunol.0902223
Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S (2006) A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Pro Natl Acad Sci USA 103:14678–14683. doi:10.1073/pnas.0606310103
Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D (2002) Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol 282:C606–C616. doi:10.1152/ajpcell.00269.2001
Yeh YT, Hur SS, Chang J, Wang KC, Chiu JJ, Li YS, Chien S (2012) Matrix stiffness regulates endothelial cell proliferation through septin 9. PloS One 7:e46889. doi:10.1371/journal.pone.0046889
Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA (2005) Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskelet 60:24–34. doi:10.1002/Cm.20041
Ziegler-Heitbrock HW, Thiel E, Futterer A, Herzog V, Wirtz A, Riethmuller G (1988) Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer J Int du Cancer 41:456–461
Acknowledgments
We gratefully appreciate UMD undergraduates Megan Mathews and Connie Chen for making polyacrylamide gels and analyzing data, as well as UTD undergrad Melanie Maurer for conducting some of the proliferation studies. Funding was provided by the National Science Foundation CMMI-0643783 and Human Frontier Science Project Organization RGP0058/2012 (H.A.E.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Adlerz, K.M., Aranda-Espinoza, H. & Hayenga, H.N. Substrate elasticity regulates the behavior of human monocyte-derived macrophages. Eur Biophys J 45, 301–309 (2016). https://doi.org/10.1007/s00249-015-1096-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-015-1096-8