Abstract
Biogeographical studies considering the entire bacterial community may underestimate mechanisms of bacterial assemblages at lower taxonomic levels. In this context, the study aimed to identify factors affecting the spatial and temporal dynamic of the Mycobacterium, a genus widespread in aquatic ecosystems. Nontuberculous mycobacteria (NTM) density variations were quantified in the water column of freshwater lakes at the regional scale (annual monitoring of 49 lakes in the Paris area) and at the local scale (2-year monthly monitoring in Créteil Lake) by real-time quantitative PCR targeting the atpE gene. At the regional scale, mycobacteria densities in water samples ranged from 6.7 × 103 to 1.9 × 108 genome units per liter. Density variations were primarily explained by water pH, labile iron, and dispersal processes through the connection of the lakes to a river. In Créteil Lake, no spatial variation of mycobacterial densities was noticed over the 2-year monthly survey, except after large rainfall events. Indeed, storm sewer effluents locally and temporarily increased NTM densities in the water column. The temporal dynamic of the NTM densities in Créteil Lake was associated with suspended solid concentrations. No clear seasonal variation was noticed despite a shift in NTM densities observed over the 2012–2013 winter. Temporal NTM densities fluctuations were well predicted by the neutral community model, suggesting a random balance between loss and gain of mycobacterial taxa within Créteil Lake. This study highlights the importance of considering multiple spatial scales for understanding the spatio-temporal dynamic of bacterial populations in natural environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Mycobacterium genus is composed of about 170 species, most belonging to the group of the nontuberculous mycobacteria (NTM) [1]. Numbers of NTM species are considered essential to degrade polycyclic aromatic hydrocarbon [2]. However, several NTM species are infamous for being opportunistic pathogens in humans and animals [3, 4]. Pathogenic species are mainly transmitted by environmental sources including surface water ecosystems [5]. Historically, most environmental studies focused on the drinking water distribution systems [6,7,8] or hospital water supply networks [9,10,11] due to the prevalence of mycobacteriosis contracted after water contact [1]. However, recent molecular studies suggest that mycobacteria are also widespread in various freshwaters [12,13,14], and may constitute a significant part of the bacterial assemblage [15, 16].
While NTM can be considered as common inhabitants of natural freshwater environments, their habitat preferences remain poorly documented. Part of the knowledge gap is due to methodological issues, such as the difficulties in isolating NTM from the environment using culture methods [17] or a bias in DNA extraction due to the toughness of NTM cell walls [18], but also from the fact that natural ecosystems do not represent the main infection routes in most mycobacteriosis cases compared to the water distribution system [5]. Yet, freshwater habitats, and especially lacustrine ecosystems, may constitute a potential infection route as they attract a high number of visitors due to the numerous cultural, provisional, and recreational services that they provide. Thus, considering the human population exposure to natural surface water ecosystems, a better comprehension of the spatio-temporal dynamic of mycobacterial assemblage is needed.
Like any other component of the bacterial community [19], NTM assemblages are likely subject to ecological processes shaping their spatial and temporal distribution, including dispersal, drift, selection, and speciation. Some ecological processes are difficult to assess using observational studies (e.g., ecological drift [20]). Thus, drivers of the spatio-temporal distribution are often determined by evaluating the relative contribution of local environmental conditions (species sorting), processes affecting species dispersal (dispersal limitation) and stochastic processes (neutral processes), i.e., stochastic balance between dispersal, immigration, and species losses [21, 22]. The importance of these processes may vary with spatial scale [22]. Indeed, some processes relevant at smaller spatial scales may minimize their significance at larger scales and vice versa. For example, the environmental conditions appear to be more substantial at small scales, for which dispersal processes seem to be more negligible [22]. While neutral processes have been indiscriminately observed to shape bacterial structure at local and regional scales [23, 24]. Moreover, even if mechanisms structuring bacterial patterns at regional scales may also influence patterns observed at local scales [25], separating the relative influence of regional and local factors is a prerequisite for understanding the processes causing temporal and spatial variations in local assemblage structure of ecological communities [26].
So far, limited data are available on the processes that impact the distribution and densities of NTM species. Most studies exploring aquatic habitats focused on one specific or cultivable species [27,28,29,30,31,32,33]. Nevertheless, temporal surveys in freshwater ecosystems revealed important shifts in mycobacterial diversity or in particular species densities [29, 32], especially after rainfall events [29, 30]. Evidence from experimental studies and environmental monitoring indicate the prevalence of mycobacteria in extreme environments, i.e., habitats with low dissolved oxygen or high metal concentrations and acid brown water [34, 35]. However, there are little data available on the factors determining the occurrence of NTM in more common freshwater environments, such as urban and peri-urban waterbodies. Recently, we demonstrated at the local scale that the spatial distribution of mycobacteria varied among water, sediment, and biofilms in two urban lakes [13]. However, this study did not include a temporal survey nor did test the influence of dispersal and stochastic processes. To the best of our knowledge, no published study focusing on entire NTM assemblages in lacustrine ecosystems has been performed using molecular methods.
The aim of this study was to determine the factors and processes that modulate the spatio-temporal dynamic of NTM densities in freshwater lakes at the regional and at the local scales. At the regional scale, the water column of 49 freshwater lakes located in the Paris area (France) was sampled once a year for two consecutive years (2012–2013). This region is characterized by a strong gradient in the intensity of anthropogenic pressures and in the degree of connectivity of individual waterbodies to the hydrological network [36, 37]. At the local scale, the water column of Créteil Lake was collected along a horizontal and a vertical transects over a 2-year period (2011–2013). This study also investigated the impact of six important rainfall events responsible for stormwater discharges into Créteil Lake. This dataset was used to characterize the spatio-temporal dynamic of NTM densities and to identify the impact of local and environmental factors (e.g., physicochemical parameters and stormwater effluent flux). Finally, we evaluated to what extent neutral processes could explain temporal dynamic of the NTM densities at the local scale. NTM densities were quantified using real-time quantitative PCR [38].
Material and Methods
Study Area and Sampling
Regional Scale Sampling: The Paris Area
This study was conducted in the Île-de-France region around Paris (c.a. 12,000 km2 concentrating 18% of the French population). Among the 248 waterbodies larger than 5 ha and referenced in the hydrological database Carthage 3.0® (IGN, Paris, France), 49 lakes were selected (Fig. 1 and Supplementary data 1) to obtain a representative and unbiased set of waterbodies reflecting the whole range of environmental conditions found in the Paris area [36].
Lakes were sampled in late July in 2012 and late July 2013. Both sampling campaigns were conducted within less than 15 days to reduce the temporal variability in meteorological conditions and nutrient inputs. For each lake, three sampling stations equidistant from one another to the siwe of the lake were defined. At each station, water samples were collected from surface, middle, and bottom waters column using a Niskin bottle (General Oceanics Inc., USA). All samples from the same lake were then pooled together to obtain an average sample. Immediately after collection, samples were kept at 4 °C for a maximum of 24 h.
Local Scale Sampling: Créteil Lake
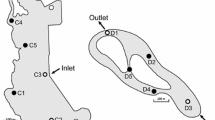
In Créteil Lake (Fig. 1), three sampling stations (Fig. 2) were monthly monitored from December 2011 to December 2013 (25 sampling dates). This mesotrophic lake (Table 1) covers an area of 40 ha and is located in a densely urbanized suburb of Paris (Créteil, Val-de-Marne, France). The lake is mainly supplied by alluvial groundwater, but it also receives effluents from a storm sewer that collects stormwater from a 100-ha urban catchment. To study the horizontal heterogeneity in NTM densities, sub-surface samples were obtained at three stations (Fig. 2) along the inlet-outlet axis of the lake (storm sewer outlet (S1), center of the lake (C1), and the lake outlet (O1)). Vertical heterogeneity was assessed at the center of the lake (station C), by sampling the water column at three discrete depths (C1, C2, and C3). At each sampling campaign, an average sample (M) was also obtained by mixing samples collected at the three stations at the three depths (S1, S2, S3, C1, C2, C3, O1, O2, and O3).
Sampling strategies in Créteil Lake. a Location of the three stations S, C, and O. b Representation of the sampling depths over the 2-year monthly monitoring (solid circles) and supplementary integrative samples over the rainfall monitoring (open rectangles). During the 2-year and rainfall monitoring, the average sample (M) was obtained by mixing samples from the three stations at three depths (dashed circles)
To evaluate the impact of rainfall events on mycobacterial densities in Créteil Lake, the storm sewer effluents generated by six high rainfall events (> 4 mm of precipitation) occurring in the late afternoon were sampled between June to November 2013. For that purpose, the storm sewer was equipped with a flowmeter (Mainstream IV, Hydreka Inc., France) above the lake high-water line to avoid back flow. One representative sample of the effluent was collected using an automatic sampler triggered to the flow volume and to the water level inside the stormwater sewer (Sigma sd900, Hach Inc., USA). Early in the morning the following day, effluent samples were collected and a sampling campaign was conducted on Créteil Lake. At each of the three stations (S, C, and O), a specific average sample integrating the three depths was collected, in addition to the average sample (M) (described above) (Fig. 2).
All samples were stored at 4 °C up to 4 h prior to processing.
Sample Pretreatment
For samples collected from the regional scale campaigns, three successive centrifugations (1 L at 7500×g for 2 h at 4 °C; 50 mL at 10,000×g for 50 min at 4 °C, and 2 mL at 16,000×g for 20 min) were performed to concentrate 1 L into a 2-mL sterile tube. In order to facilitate pellet resuspension, 1 mL of the surfactant Tween 80 (final concentration, 0.01% vol/vol) was added before centrifugation to each sample.
For samples from local scale campaigns, 1 L of Créteil lake samples and from 100 to 800 mL of stormwater effluent samples were filtered through a 0.22-μm pore-size Sterivex GP-filter (Millipore, USA) after prefiltration through a 50-μm pore-size nylon mesh. Filtered samples were kept at 4 °C during transport and then stored at − 20 °C. Prior to DNA extraction, Sterivex GP-filter membranes were extracted under sterile conditions and cut into small pieces of approximately 1 mm2. All membrane pieces were pooled in a 2-mL sterile tube. All samples obtained using centrifugation and filtration were stored at − 20 °C until DNA extraction.
The choice of Sterivex filtration was taken as it allows a fast concentration of the water samples. Indeed, it appeared logistically difficult to centrifuge all Créteil samples in a short time after each sampling campaign. However, we assume that the difference of protocol used to concentrate the bacteria between the 49 lakes (centrifugation) and Créteil Lake (filtration) did not introduce a considerable bias in NTM densities, since no significant difference of DNA recovery was observed between these two protocols over preliminary experiments and during simultaneous campaigns (Wilcoxon test, n = 8, W = 27, P = 0.208).
DNA Extraction and Quantification of Mycobacterial Densities
Total DNA was extracted using the FastDNA® SPIN Kit (QBiogene, USA) according to the manufacturer’s instructions. Two modifications to this protocol were applied: cells were lysed in a FastPrep bead beater three times for 30 s at 4.0 ms−1 and the SPIN filters were washed twice. All samples were eluted in 50 μL of deionized sterile water. The quality and quantity of extracted DNA were analyzed at 230, 260, and 280 nm by spectrophotometry before storage at − 20 °C. In 2012, five samples were not considered due to a technical issue.
To quantify the abundance of Mycobacterium spp. in the water samples, TaqMan® real-time quantitative PCR assays targeting the atpE gene were carried out in duplicate as previously described by Radomski and colleagues [38]. The atpE copy numbers concentration was estimated from crude extracts using a Mycobacterium chelonae standard curve from 1.0 × 101 to 1.0 × 106 copies μL−1. Contamination of the PCR mix was checked using negative controls. Presence of PCR inhibitors in DNA templates was verified using a non-competitive exogenous internal control that was included in the PCR buffer at 1000 copies μL−1. This internal control corresponded to a partial sequence of the human β-actin gene cloned in pGEM-T-easy vector (Promega, USA) [39]. Absence of significant PCR inhibition of the atpE assay was checked by comparing results with the average quantitation cycle (C q) value (+/− standard deviation) obtained from 100 repeated PCR reactions containing 1000 copies of the human β-actin gene fragment.
PCR Assay Performance
Real-time quantitative PCR efficiency was estimated to be 94.6% using a standard curve of M. chelonae (R 2 = 0.999). Considering that the atpE gene is found in a single copy in mycobacterial genomes [38], results were expressed as genome unit per liter based on the M. chelonae standard curve.
The performance of the modified DNA extraction protocol was evaluated by centrifuging and extracting in triplicate 1 L of raw water from Créteil Lake and 1 L of water from Créteil Lake spiked with a known concentration of M. chelonae. The difference between the number of atpE gene copies recovered in the spiked and raw waters was compared with the expected concentration of M. chelonae (estimated using culture-based method) to determine the performance of NTM cells recovery from freshwater samples.
Physicochemical and Meteorological Parameters
For both regional and local scales sampling, vertical profiles of physicochemical parameters were performed at the three sampling stations previously described. Chlorophyll a (Chl a) concentration was determined using an in situ fluorometer (FluoroProbe, BBE-Moldaenke GmbH, Germany). Conductivity, temperature, pH, and oxygen profiles were measured using a submersible profiler (SBE 19 Seacat, Sea-Bird Electronics Inc., USA). Total suspended solids (TSS) concentration was quantified after filtration of 1 L of water on precombusted tarred Whatman GF/F filter. Dissolved organic carbon (DOC) concentrations were measured using a TOC-VCSN carbon analyzer (Shimadzu, USA). Water transparency was measured with the use of a Secchi disc.
Additional measurements were obtained for the 49 lakes. Total nitrogen (TN) and phosphorus (TP) concentrations were measured by colorimetry using a spectrophotometer (Cary 50 Scan, Varian Inc., USA) according to Rogora and colleagues [40] and the French standard (AFNOR NF T 90-023), respectively.
Polycyclic aromatic hydrocarbons (PAHs) were quantified both in dissolved and particulate fractions in the water samples. Deuterated PAHs were used as internal standards for gas chromatography coupled with mass spectrometry (GC/MS Focus DSQ, Thermo Fisher Scientific, USA) [41]. The sum of 13 PAHs (fluorene, phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1–3]pyrene, di-benzo[a,h]anthracene, and benzo[g,h,i]perylene) where then calculated.
Finally, in 2012, the concentrations corresponding to the labile fraction of trace metals (i.e., Ti, Mn, Fe, Co, Ni, Cu, Zn, Cd, Ba, and Pb) were estimated by ICP-MS at the LSCE laboratory (Gif-sur-Yvette, France) after water filtration on chelating disk cartridge [42].
Precipitation on Créteil Lake was measured continuously with a weather real-time transmitter (WXT520, Vaisala Inc., USA) placed on a measuring buoy (LakeESP, PME Inc., USA).
Data Analysis
All statistical analyzes were conducted using the statistical environment R version 3.3.2 [43].
Regional Scale Variation in NTM Densities
For each lake and for both sampling campaigns, a single value was used for the local explaining variables, i.e., conductivity, water temperature and transparency, pH, oxygen, Chl a, TSS, DOC, TN, TP, PAHs, and labile metals concentrations. This value is the average of the measurements realized at different depths or vertical profiles over the three stations. The trophic state index (TSI) of the lakes was determined by averaging the four TSI indexes calculated from the Chl a, TN, TP concentrations, and Secchi depth [44, 45]. Variables used for the TSI calculation were not included in the statistical analysis. All environmental variables except pH, oxygen, water temperature, and DOC concentration were log transformed to meet normality assumptions. In addition to the environmental parameters, two dummy variables reflecting the dispersal of bacteria taxa were included, i.e., the presence of storm sewer outlet discharging into lakes (SSO) and the presence of a connection between the lakes and a stream or a river (C).
To evaluate the effect of the environmental and watershed variables on logarithm-transformed NTM densities, a multiple regression analysis was performed. Statistical significance of regression models was tested by an analysis of variance (ANOVA) based on Type III sums of squares because of the unbalanced nature of the data. Since this analysis is sensitive to highly correlated variables, only variables with a variance inflation factor less than 5 were included [46]. Prior to the analysis, a forward selection was performed using the function “stepAIC.” We also tested the significance of the year of sampling as mixed effect by comparing the mixed effect model with a non-mixed effect model using an ANOVA according to Zuur and colleagues [47]. Since no significant difference between both models was observed (data not shown), the non-mixed effect model was preferred. Furthermore, for the year 2012 only, the labile fraction of trace metals was added to the environmental factors.
Spatio-Temporal Dynamics of NTM Densities in Créteil Lake
Spatial NTM density heterogeneity along the horizontal and the vertical transects were assessed using a linear mixed effect models (LMM) from the “nlme” package [48] with sampling dates as mixed effect. The absence of temporal auto-correlation was verified using the auto-correlation function described by Zuur and colleagues [47]. Multiple regression analysis was performed to identify the variables affecting the temporal dynamic of the mycobacteria over the 2-year survey on the average sample M. Variables used in the analysis included (i) environmental variables presenting important seasonal fluctuations (water temperature, TSS, and DOC concentrations), (ii) cumulative precipitation for the 1 to 5 days preceding the sampling campaigns to evaluate the importance of stormwater effluents on NTM densities within Créteil Lake, and (iii) the year of sampling to identify if the mycobacterial densities were constant over the 2-year survey (the Dec. 2011 campaign was considered as part of the 2012 campaigns). Variance inflation factor values were checked and a forward selection retained six variables, i.e., water temperature, TSS concentration, and cumulative precipitation for the 1 to 3 days preceding sampling and the year of sampling.
We also evaluated if the neutral model could explain the temporal variations of the NTM densities as previously described by Ofiţeru and colleagues [49]. Prior to analysis, non-log data densities were transformed into relative abundance by dividing them by the highest abundance. The goodness of fit was evaluated using the adjusted R–squared of the model.
Impact of Rainfall Events on NTM Spatial Heterogeneity in Créteil Lake
The impact of storm sewer effluents on the horizontal distribution of NTM from the sewer outlet (S in Fig. 2) to the lake outlet (O in Fig. 2) was performed using a linear mixed effect model with the sampling dates as mixed effect. A post hoc test using the glht function from the “multcomp” package [50] was performed to identify in which sampling point NTM densities differed from the other points. P values were adjusted using a Bonferroni correction to reveal significant differences. To identify if the NTM densities in the average sample (M) differed significantly between the six rainfall events and the 2-year monitoring, a Mann-Whitney test was performed. Model validation (normality, homoscedasticity, and independence of residuals) was assessed according to Zuur and colleagues [47].
Results
Assay Performances
Following centrifugation and nucleic acid extraction, the recovery of atpE gene copies from freshwater samples was 124 ± 29% (standard deviation; n = 3). The atpE gene was successfully amplified in all the samples (regional scale survey and Créteil Lake monitoring). No inhibition was identified in any sample.
Mycobacterial Distribution at the Regional Scale
The monitoring of 49 lakes revealed that NTM densities per lake were similar, with a significant correlation between the 2012 and 2013 campaigns (non-parametric Spearman’s correlation, n = 44, r s = 0.662, P < 0.001). However, important NTM densities variations were observed among the 49 lakes with values ranging from 6.7 × 103 to 1.9 × 108 genome units per liter (Fig. 1). A multiple regression analysis (F 3,89 = 18.45, R 2 adj = 0.363, P < 0.001) was conducted to identify the factors influencing the mycobacterial density at the regional scale. Table 2 shows that lakes connected to the hydrological network (river or stream) had significantly higher numbers of genome units per liter compared to non-connected lakes (P < 0.001). Water pH as well as conductivity were also identified to be negatively correlated with the genome unit concentration (P = 0.019 and P = 0.005, respectively). No significant correlation was found between NTM density and dissolved oxygen, polycyclic aromatic hydrocarbon, total suspended solids concentrations, trophic status, or the presence of a storm sewer outlet. Furthermore, in 2012 for which trace metal concentrations were available, a multiple regression analysis (F 3,38 = 14.44, R 2 adj = 0.496, P < 0.001, Supplementary data 2) revealed that the labile iron fraction positively correlated with genome unit densities (β = 1.352, P = 0.003) in addition to the connection to the hydrological network (β = 0.793, P < 0.001).
Spatio-Temporal Variations of the Mycobacterial Density in Créteil Lake
No significant spatial heterogeneity of NTM densities along the vertical profile in point C was observed (LMM, P = 0.561). Similarly, no significant impact of the storm sewer was identified along the sub-surface horizontal transect between points S1, C1, and O1 (LMM, P = 0.079). Since no spatial variability was observed within Créteil Lake and all sampling points followed similar temporal trends in mycobacterial density variation (data not shown), a statistical analysis of the temporal dynamic was carried out using the average samples (M).
The 2-year monthly monitoring of the average samples (M) highlighted low NTM density variation, with values ranging from 3.2 × 104 to 4.0 × 105 genome units per liter (Fig. 3). Further, rejecting the extreme value in August 2012 (5.5 × 103 genome units per liter), a multiple regression analysis (F 3,20 = 12.31, R 2 adj = 0.596, P < 0.001, Table 3) did not reveal any significant effect of the water temperature variation on the genome unit densities (β = −0.004, P = 0.563). However, a significant inter-annual variation was noticed between 2012 and 2013 (β = −0.353, P < 0.001), with NTM concentration higher in 2012 compared to 2013. A significant positive relationship between NTM concentration and TSS concentration was also observed (β = 0.088, P = 0.005). Additionally, the temporal dynamic of NTM densities was well predicted by the neutral time-series model (R 2 adj = 87%, P < 0.001) proposed by Ofiţeru and colleagues [49].
Rainfall Impact on the Mycobacterial Densities in Créteil Lake
Regarding the six rainfall events that were monitored, the concentrations of NTM in the storm sewer effluent ranged from 2.2 × 107 to 2.2 × 108 genome units per liter, which represented an average input of 1.7 × 1014 ± 1.5 × 1014 genome units in the lake per rainfall event. This input significantly impacted the atpE gene densities into Créteil Lake within the 5 to 20 h following the rainfall (Fig. 4), time corresponding to the longest duration between the rainfall events and the sampling campaigns on Créteil Lake. Indeed, shortly after the rain events, NTM concentrations were significantly higher at the station (S) in front of the storm sewer outlet compared to the two other stations (post hoc test, stations S-C: P = 0.037; S-O: P = 0.001). Inversely, the mycobacterial densities in the average sample (M) immediately after the six rain events were similar to the densities observed during the 2-year monitoring (Mann-Whitney, P = 0.155, Fig. 4). Besides, in this average sample, the NTM concentrations during the 2-year monitoring were not correlated with the cumulative precipitation for the 1 to 3 days preceding the sampling campaigns, suggesting the absence of significant impact of the storm sewer effluent on the average sample.
Distribution of the mycobacterial genome units per liter in Créteil Lake a for each of the three stations after the six rain events, and b on the average sample (M) after the six rain events and over dry-weather periods (values observed during the 2-year monitoring and campaigns occurring after at least 3 days without rain). Significance for the inter-station comparisons was calculated using post hoc tests adjusted with Bonferroni correction. Significance between wet- and dry-weather periods on the average sample (M) was estimated using a Mann-Whitney rank sum test. Significances excluding outliers are given in brackets. Bars represent the median of observations. Significance levels: NS, P > 0.050; *, P < 0.050; **, P < 0.010; ***, P < 0.001
Discussion
The primary aim of this study was to determine the relative influence of environmental, spatial, and stochastic processes on spatial and temporal dynamics of the abundance and distribution of nontuberculous mycobacteria (NTM), not only at the regional scale but also at the local scale. Indeed, although NTM can represent more than 5% of the bacterial community in freshwater habitats [15], little is known about the processes affecting their dynamics. Such an original investigation, using a recently developed real-time quantitative PCR, provides a new insight of NTM dynamic in freshwater ecosystems that may be extrapolated to other bacterial groups.
Methodological Considerations
Quantitative PCR approach offers the advantage of avoiding the culture biases that prevent the estimation of slow-growing mycobacteria in environmental samples [51, 52]. However, PCR-based methods detect DNA from both live and dead cells. Therefore, the abundance of the live NTM cells acquired in this study may have been overestimated, due to the potential persistence of bacterial DNA after cell death for several weeks [53, 54]. In this assay, the atpE gene recovery from freshwater samples was close to 100% using centrifugation and the extraction protocol. This high efficiency was probably due to the extraction protocol improvements. Indeed, Guo and Zhang noticed the high extraction yields obtained for actinobacterial DNA using the FastDNA® SPIN Kit (Qbiogene), especially due to the beat-beating step [55].
Widespread of NTM in Freshwater Lakes
Previous studies exploring the ecological preferences of mycobacteria usually found higher abundances of mycobacteria in a restricted range of aquatic habitats, especially in humic-rich waters [34, 35, 56]. Nonetheless, in this study, NTM were detected in all the 49 lakes for both years at relatively high and similar abundances between both sampling campaigns (with values ranging from 8.6 × 103 to 9.4 × 106 genome units per liter). In spite of a relatively high variability, the estimated mycobacterial densities are in agreement with the values reported in diverse freshwater or coastal habitats [13, 56, 57]. All together, these findings highlight the ubiquity of NTM in the water column and their ability to persist and/or grow in ecosystems with non-extreme conditions of pH, oxygen, nutrients, or PAH concentrations. The successful recovery of NTM in all the collected water samples could potentially result from the use of a more efficient DNA extraction protocol, a lower limit of detection of the quantitative PCR method, and/or the processing of larger volume of water compared to previous studies [14, 56]. These new findings suggest that Mycobacterium may be considered as a cosmopolitan genus in aquatic environments and could be a candidate for the group of the “pigeons” of the microbial world as named by Hanson and colleagues [20]. However, the high prevalence of a microbial taxon in an environment does not necessarily give clues if it is an active, dormant, or transient member of the bacterial assemblage.
Factors Affecting NTM Densities at the Regional Scale
Although NTM were encountered in all the investigated lakes with similar densities within each lake, relevant environmental parameters and dispersal processes were identified as affecting their distribution. The flowing of rivers into lakes appeared to strongly increase the NTM densities (by 0.8 log unit), suggesting that rivers could represent an important potential reservoir and source of NTM. Indeed, a survey performed in the Paris area reported high densities of NTM in the Marne River with a mean of 2.2 × 105 ± 2.4 × 105 16S rRNA gene copy numbers per liter [58]. As NTM are hydrophobic, these particle associate bacteria may ride into lakes through suspended solids carried by rivers. Alternatively, this relationship could be indirect, through the modification of the water properties and nutrient availability within lakes by the river inflow. Such changes could improve the persistence and/or the competitiveness of NTM in lakes connected to the hydrological network. However, this relationship could also be explained by the dependency between the connection to a river and the trophic status. Indeed, dispersal of aquatic communities is often accompanied by a change in the environmental conditions, making it very difficult to statistically disentangle direct effects of dispersal processes from indirect effects via changes in local conditions [59]. For instance, isolated lakes were mainly oligotrophic or mesotrophic while connected lakes, where NTM were more abundant, were rather eutrophic or hypertrophic.
The significant negative relationship, even weak, between the NTM densities and the water pH values that was found in the 49 lakes dataset supports previous observations in freshwater habitats about the importance of water pH for mycobacteria [34, 35]. Moreover, as previously reported in various terrestrial or aquatic habitats [35, 60], NTM were positively correlated with the labile iron concentration. Iron is an important biocatalyzer of redox reaction in many living cells. Its deficiency in lacustrine ecosystems [61] could drastically impact the biomass of mycobacteria that is dependent upon the production of iron-binding siderophores to acquire this compound [62, 63]. This dependence to siderophore is particularly important in waters with high pH values, where iron is insoluble, and thus, not bioavailable [64].
Although NTM are hydrophobic and are therefore preferentially associated to surfaces (e.g., suspended particles) [65,66,67], no relationship between NTM abundances and total suspended solid concentrations was observed at the regional scale. The absence of correlation can be a direct consequence of the sampling design since the surface and bottom of the water column have been pooled, leading to a loss of discrimination of some physicochemical parameters including total suspended solids or dissolved oxygen between lakes. Moreover, more substantial processes occurring at the regional scale can mask this relationship, which could be significant at smaller scales. The finer survey performed in Créteil Lake showed that the yearly variation of TSS was positively correlated with the variation of NTM densities. No direct relationship between the NTM densities and the PAH concentrations was noticed in this survey, despite the large range of PAH concentrations among the lakes and the fact that some NTM species, dominant in those ecosystems [13], play a role as polycyclic aromatic hydrocarbon degraders [65,66,67]. Similarly, no significant influence of the nutrient levels (total nitrogen, total phosphorus, or DOC concentrations) or the lake trophic status was observed in this study. Yet, previous experiments reported positive correlations between bacterial growth and nutrient concentrations [68, 69]. This result raises different hypotheses: (i) mycobacteria were not limited by the supply of nutrients in the monitored lakes; (ii) relevant organic matter variables were not measured, e.g., acid humic or fulvic content [34, 35]; (iii) small bacterial densities fluctuations in response to nutrient availability were not detectable using our methods; (iv) at the genus level, it is impossible to identify significant density variations occurring at the species level; and/or (v) as slow growers, NTM responds slowly to environmental changes, therefore the concurrent measurement of physicochemical parameters and NTM densities may miss the environmental “trigger” to population dynamics. The absence of a significant influence of the nutrients and organic matter concentrations on NTM densities could also imply that mycobacterial assemblages are inactive or dead, suggesting that the persistence or the increase of NTM densities in these lakes could be mainly explained by exogenous supply, i.e., dispersal. However, some physicochemical water properties (including water pH level and iron concentration) were identified as structuring the NTM densities, suggesting that mycobacteria may not be fully inactive or dead.
Factors Affecting Local Variation in Créteil Lake, the Limited Impact of Rainfall Events
Only a few studies investigated the impact of rain events on mycobacterial densities and no consensus emerged from these studies. Pickup and colleagues did not detect a positive correlation between the precipitation and the Mycobacterium avium subsp. paratuberculosis recovery after 9 days [30]. Inversely, Iivanainen and colleagues observed a positive relationship between the abundance of Mycobacterium spp. and the cumulative precipitation in 53 brook waters, but only the second and the third weeks after the rainfalls [35]. In our study, the large densities of mycobacteria in the stormwater inflow appeared to locally enhance the NTM abundance in Créteil Lake in front of the storm sewer outlet the day following important rainfalls. However, this impact did not last longer than 24 h, as revealed by the absence of relevant horizontal spatial heterogeneity in the surface water of Créteil Lake over the 2-year monitoring. Moreover, discharge plumes may also be rapidly diluted into the lake. Indeed, the water column from the polymictic Créteil Lake may be sufficiently well mixed to avoid significant spatial heterogeneity as suggested by the absence of spatial variability along the horizontal and vertical transects during the 2-year survey. Similar short-term effect of rainfall may also happen in the other 48 lakes, explaining the absence of significant effect of the presence of storm sewer at the regional scale. Besides, the average sample (M) that was measured for the regional scale may mask any local effect of a storm sewer, which could explain the absence of any significant long-term impact of the presence of a storm sewer outlet among the likely polymictic 49 shallow lakes. Yet, the absence of long-term effect on the water column does not imply the absence of any long-term local effect on the sediment compartment. Indeed, in a previous study [13], we showed that in Creteil lake the sediment close to the sewer outlet presented significantly higher levels of mycobacteria compared to other location on the lake. In the present study, the stormwater collected during the six rain events carried large numbers of mycobacteria that may result from the erosion of sedimented matters and biofilms in the storm sewer pipes. In fact, biofilms are well-known reservoirs of mycobacteria in diverse habitats including pipes, such as drinking water systems [70, 71]. Mycobacteria carried by particles may rapidly sediment at the station S and encounter favorable conditions to persist and/or grow.
Temporal Variation in Créteil Lake
Mycobacterial densities appeared to be roughly stable over the year in Créteil Lake, although a significant inter-annual variation was observed in winter 2012/2013. However, it is difficult to draw a general conclusion from the monitoring of a single entire winter season. Temporal dynamic was particularly well predicted by the neutral community model, suggesting that the variations of the Mycobacterium densities were governed by a stochastic balance between loss and gain of taxa [72] as previously demonstrated for many other bacterial groups [59, 73]. Although deterministic parameters such as the temperature fluctuation did not appear to significantly affect the Mycobacterium densities, we cannot exclude a temporal dynamic of the mycobacterial species. Indeed, in the Rio Grande, Bland and colleagues observed important shifts in the NTM assemblage composition over a 1-year monthly monitoring [32]. A similar tendency was also noticed with the non-systematic recovery of M. avium over a 1-year survey in the river Taff [29, 30].
This study emphasizes the ubiquity of mycobacteria in non-extreme freshwater habitats, suggesting that lacustrine ecosystems appeared to be a relevant niche of NTM in an urban area. Environmental factors, dispersal, and neutral processes (at the local scale) were identified to affect spatial and/or temporal NTM density variations in those habitats. However, depending on the spatial scale studied, density variations were explained by different parameters. For instance, no significant relationship was observed between NTM densities and TSS concentrations to explain the spatial distribution at the regional scale, while a significant relation was observed to explain the temporal dynamic of NTM at the local scale. Similarly, no significant impact of the stormwater inflow was observed on NTM densities at both regional and local scales (over the monthly monitoring), while a finer survey performed on Créteil Lake after important rainfall events allowed to identify a local and temporary increase of NTM densities in surface water. These results highlight the importance of considering multiple spatial scales for understanding the spatio-temporal dynamic of bacterial assemblages.
The monitoring of water physicochemical parameters alone does not appear to be sufficient to predict mycobacterial densities. Nevertheless, an additional study assessing the species composition of the NTM communities should help to reveal the existence of a biogeographical structure in mycobacterial communities at both local and regional scales. Especially, it would be interesting to assess the influence of neutral processes on the spatial variations of the NTM density and diversity.
References
Hruska K, Kaevska M (2012) Mycobacteria in water, soil, plants and air: a review. Vet Med (Praha) 57:623–679
Seo J-S, Keum Y-S, Li QX (2009) Bacterial degradation of aromatic compounds. Int J Environ Res Public Health 6:278–309. https://doi.org/10.3390/ijerph6010278
Falkinham III JO (1996) Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev 9:177–215
Mrlik V, Slany M, Kubecka J, et al. (2012) A low prevalence of mycobacteria in freshwater fish from water reservoirs, ponds and farms. J Fish Dis 35:497–504. https://doi.org/10.1111/j.1365-2761.2012.01369.x
Prevots DR, Marras TK (2015) Epidemiology of human pulmonary infection with nontuberculous mycobacteria a review. Clin Chest Med 36:13–34. https://doi.org/10.1016/j.ccm.2014.10.002
Covert TC, Rodgers MR, Reyes AL, Stelma GN (1999) Occurrence of nontuberculous mycobacteria in environmental samples. Appl Environ Microbiol 65:2492–2496
Falkinham III JO, Norton CD, LeChevalier MW (2001) Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl Environ Microbiol 67:1225–1231. https://doi.org/10.1128/AEM.67.3.1225
Vaerewijck MJM, Huys G, Palomino JC, et al. (2005) Mycobacteria in drinking water distribution systems: ecology and significance for human health. FEMS Microbiol Rev 29:911–934. https://doi.org/10.1016/j.femsre.2005.02.001
du Moulin G, Stottmeier K, Pelletier P, et al. (1988) Concentration of Mycobacterium avium by hospital hot water systems. JAMA 260:1599–1601
Fox GE, Wisotzkey JD, Jurtshuk P (1992) How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int J Syst Bacteriol 42:166–170. https://doi.org/10.1099/00207713-42-1-166
Fujita J, Nanki N, Negayama K, et al. (2002) Nosocomial contamination by Mycobacterium gordonae in hospital water supply and super-oxidized water. J Hosp Infect 51:65–68. https://doi.org/10.1053/jhin.2002.1197
Niva M, Hernesmaa A, Haahtela K, et al. (2006) Actinobacterial communities of boreal forest soil and lake water are rich in mycobacteria. Boreal Environ Res 11:45–53
Roguet A, Therial C, Saad M, et al. (2016) High mycobacterial diversity in recreational lakes. Antonie Van Leeuwenhoek 109:1–13. https://doi.org/10.1007/s10482-016-0665-x
Khera T (2012) The diversity and distribution of Mycobacterium species in varying ecological and climatic environments. Doctoral dissertation, University of Warwick
Lee CS, Kim M, Lee C, et al. (2016) The microbiota of recreational freshwaters and the implications for environmental and public health. Front Microbiol 7:1826. https://doi.org/10.3389/fmicb.2016.01826
Unno T, Kim J, Kim Y, et al. (2015) Influence of seawater intrusion on microbial communities in groundwater. Sci Total Environ 532:337–343. https://doi.org/10.1016/j.scitotenv.2015.05.111
Falkinham III JO (2002) Nontuberculous mycobacteria in the environment. Clin Chest Med 23:529–551
Amaro A, Duarte E, Amado A, et al. (2008) Comparison of three DNA extraction methods for Mycobacterium bovis, Mycobacterium tuberculosis and Mycobacterium avium subsp. avium. Lett Appl Microbiol 47:8–11. https://doi.org/10.1111/j.1472-765X.2008.02372.x
Vellend M (2010) Conceptual synthesis in community ecology. Q Rev Biol 85:183–206. https://doi.org/10.1086/652373
Hanson CA, Fuhrman JA, Horner-Devine MC, Martiny JBH (2012) Beyond biogeographic patterns: processes shaping the microbial landscape. Nat Rev Microbiol 10:1–10. https://doi.org/10.1038/nrmicro2795
Leibold MA, Holyoak M, Mouquet N, et al. (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x
Martiny JBH, Bohannan BJM, Brown JH, et al. (2006) Microbial biogeography: putting microorganisms on the map. Nat Rev Microbiol 4:102–112. https://doi.org/10.1038/nrmicro1341
Östman Ö, Drakare S, Kritzberg ES, et al. (2010) Regional invariance among microbial communities. Ecol Lett 13:118–127. https://doi.org/10.1111/j.1461-0248.2009.01413.x
Roguet A, Laigle GS, Therial C, et al. (2015) Neutral community model explains the bacterial community assembly in freshwater lakes. FEMS Microbiol Ecol 91:1–11. https://doi.org/10.1093/femsec/fiv125
Gaston KJ (2000) Global patterns in biodiversity. Nature 405:220–227. https://doi.org/10.1038/35012228
Ilmonen J, Paasivirta L, Virtanen R, Muotka T (2009) Regional and local drivers of macroinvertebrate assemblages in boreal springs. J Biogeogr 36:822–834. https://doi.org/10.1111/j.1365-2699.2008.02045.x
Viallier J, Viallier G (1973) Inventaire des mycobacteries de la nature. Ann Soc Belg Med Trop (1920) 53:361–371
Stinear T, Davies JK, Jenkin GA, et al. (2000) Identification of Mycobacterium ulcerans in the environment from regions in Southeast Australia in which it is endemic with sequence capture-PCR. Appl Environ Microbiol 66:3206–3213
Pickup RW, Rhodes G, Arnott S, et al. (2005) Mycobacterium avium subsp. paratuberculosis in the catchment area and water of the River Taff in South Wales, United Kingdom, and its potential relationship to clustering of Crohn’s disease cases in the city of Cardiff. Appl Environ Microbiol 71:2130–2139. https://doi.org/10.1128/AEM.71.4.2130
Pickup RW, Rhodes G, Bull TJ, et al. (2006) Mycobacterium avium subsp. paratuberculosis in lake catchments, in river water abstracted for domestic use, and in effluent from domestic sewage treatment works: diverse opportunities for environmental cycling and human exposure. Appl Environ Microbiol 72:4067–4077. https://doi.org/10.1128/AEM.02490-05
Gauthier DT, Reece KS, Xiao J, et al. (2010) Quantitative PCR assay for Mycobacterium pseudoshottsii and Mycobacterium shottsii and application to environmental samples and fishes from the Chesapeake Bay. Appl Environ Microbiol 76:6171–6179. https://doi.org/10.1128/AEM.01091-10
Bland CS, Ireland JM, Lozano E, et al. (2005) Mycobacterial ecology of the Rio Grande. Appl Environ Microbiol 71:5719–5727. https://doi.org/10.1128/AEM.71.10.5719
Rahbar M, Lamei A, Babazadeh H, Yavari SA (2010) Isolation of rapid growing mycobacteria from soil and water in Iran. Afr J Biotechnol 9:3618–3621
Kirschner RA, Parker BC, Falkinham III JO (1999) Humic and fulvic acids stimulate the growth of Mycobacterium avium. FEMS Microbiol Ecol 30:327–332
Iivanainen EK, Martikainen PJ, Väänänen PK, Katila ML (1993) Environmental factors affecting the occurrence of mycobacteria in brook waters. Appl Environ Microbiol 59:398–404
Catherine A, Troussellier M, Bernard C (2008) Design and application of a stratified sampling strategy to study the regional distribution of cyanobacteria (Ile-de-France, France). Water Res 42:4989–5001. https://doi.org/10.1016/j.watres.2008.09.028
Catherine A, Mouillot D, Escoffier N, et al. (2010) Cost effective prediction of the eutrophication status of lakes and reservoirs. Freshw Biol 55:2425–2435. https://doi.org/10.1111/j.1365-2427.2010.02452.x
Radomski N, Roguet A, Lucas FS, et al. (2013) atpE gene as a new useful specific molecular target to quantify Mycobacterium in environmental samples. BMC Microbiol 13:277. https://doi.org/10.1186/1471-2180-13-277
Wurtzer S, Prevost B, Lucas FS, Moulin L (2014) Detection of enterovirus in environmental waters: a new optimized method compared to commercial real-time RT-qPCR kits. J Virol Methods 209:47–54. https://doi.org/10.1016/j.jviromet.2014.08.016
Rogora M, Minella M, Orrù A, Tartari GA (2006) A comparison between high-temperature catalytic oxidation and persulphate oxidation for the determination of total nitrogen in freshwater. Int J Environ Anal Chem 86:1065–1078. https://doi.org/10.1080/03067310600739632
Bressy A, Gromaire MC, Lorgeoux C, Chebbo G (2011) Alkylphenols in atmospheric depositions and urban runoff. Water Sci Technol 63(4):671–679. https://doi.org/10.2166/wst.2011.121
Chaminda GGT, Nakajima F, Furumai H (2008) Heavy metal (Zn and Cu) complexation and molecular size distribution in wastewater treatment plant effluent. Water Sci Technol 58:1207–1213
R Core Team (2016) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, http://www.R-project.org/
Kratzer CR, Brezonik PL (1981) A Carlson-type trophic state index for nitrogen in Florida lakes. Water Resour Bull Am Water Resour Assoc 17:713–715. https://doi.org/10.1111/j.1752-1688.1981.tb01282.x
Carlson RE (1977) A trophic state index for lakes. Limnol Oceanogr 22:361–369. https://doi.org/10.4319/lo.1977.22.2.0361
Rogerson PA (2001) Statistical methods for geography. Sage Publications, London,
Zuur A, Ieno EN, Walker N, et al. (2009) Mixed effects models and extensions in ecology with R. Springer Science and Business Media, New-York,
Pinheiro J, Bates D, DebRoy S, Sarkar D (2015) Package “nlme”. Fit and compare Gaussian linear and nonlinear mixed-effects models, version 3.1–120
Ofiţeru ID, Lunn M, Curtis TP, et al. (2010) Combined niche and neutral effects in a microbial wastewater treatment community. Proc Natl Acad Sci USA 107:15345–15350. https://doi.org/10.1073/pnas.1000604107
Hothorn T, Bretz F, Westfall P, Heiberger RM, Schuetzenmeister A, Scheibe S (2015) Package “multcomp”. Simultaneous inference in general parametric models, version 1.4–0
Radomski N, Cambau E, Moulin L, et al. (2010) Comparison of culture methods for isolation of nontuberculous mycobacteria from surface waters. Appl Environ Microbiol 76:3514–3520. https://doi.org/10.1128/AEM.02659-09
Radomski N, Lucas FS, Moilleron R, et al. (2010) Development of a real-time qPCR method for detection and enumeration of Mycobacterium spp. in surface water. Appl Environ Microbiol 76:7348–7351. https://doi.org/10.1128/AEM.00942-10
Josephon KL, Gerba CP, Pepper IL (1993) Polymerase chain reaction detection of nonviable. Appl Environ Microbiol 59:3513–3515
Masters CI, Shallcross JA, Mackey BM (1994) Effect of stress treatments on the detection of Listeria monocytogenes and enterotoxigenic Escherichia coli by the polymerase chain reaction. J Appl Bacteriol 77:73–79
Guo F, Zhang T (2013) Biases during DNA extraction of activated sludge samples revealed by high throughput sequencing. Appl Microbiol Biotechnol 97:4607–4616. https://doi.org/10.1007/s00253-012-4244-4
Jacobs J, Rhodes M, Sturgis B, Wood B (2009) Influence of environmental gradients on the abundance and distribution of Mycobacterium spp. in a coastal lagoon estuary. Appl Environ Microbiol 75:7378–7384. https://doi.org/10.1128/AEM.01900-09
Slana I, Kralik P, Kralova A, Pavlik I (2008) On-farm spread of Mycobacterium avium subsp. paratuberculosis in raw milk studied by IS900 and F57 competitive real time quantitative PCR and culture examination. Int J Food Microbiol 128:250–257. https://doi.org/10.1016/j.ijfoodmicro.2008.08.013
Radomski N (2011) Sources of nontuberculous mycobacteria in watersheds. Doctoral dissertation, Université Paris-Est École des Ponts ParisTech
Lindström ES, Langenheder S (2012) Local and regional factors influencing bacterial community assembly. Environ Microbiol Rep 4:1–9. https://doi.org/10.1111/j.1758-2229.2011.00257.x
Norby B, Fosgate GT, Manning EJB, et al. (2007) Environmental mycobacteria in soil and water on beef ranches: association between presence of cultivable mycobacteria and soil and water physicochemical characteristics. Vet Microbiol 124:153–159. https://doi.org/10.1016/j.vetmic.2007.04.015
Mckay RML, Bullerjahn GS, Porta D, et al. (2004) Consideration of the bioavailability of iron in the north American Great Lakes: development of novel approaches toward understanding iron biogeochemistry. Aquat Ecosyst Heal Manag 7:475–490. https://doi.org/10.1080/14634980490513364
Barclay R, Ratledge C (1983) Iron-binding compounds of Mycobacterium avium, M. intracellulare, M. scrofulaceum, and mycobactin-dependent M. paratuberculosis and M. avium. J Bacteriol 153:1138–1146
Chan K (2009) Exochelin production in Mycobacterium neoaurum. Int. J. Mol. Sci. 10:345–353. https://doi.org/10.3390/ijms10010345
Emmenegger L, Sigg L, Sulzberger B (2001) Light-induced redox cycling of iron in circumneutral lakes. Limnol Oceanogr 46:49–61
Uyttebroek M, Breugelmans P, Janssen M, et al. (2006) Distribution of the Mycobacterium community and polycyclic aromatic hydrocarbons (PAHs) among different size fractions of a long-term PAH-contaminated soil. Environ Microbiol 8:836–847. https://doi.org/10.1111/j.1462-2920.2005.00970.x
Uyttebroek M, Spoden A, Ortega-Calvo J-J, et al. (2007) Differential responses of eubacterial, Mycobacterium, and Sphingomonas communities in polycyclic aromatic hydrocarbon (PAH)-contaminated soil to artificially induced changes in PAH profile. Appl Environ Microbiol 36:1403–1411. https://doi.org/10.2134/jeq2006.0471
Debruyn JM, Mead TJ, Wilhelm SW, Sayler GS (2009) PAH biodegradative genotypes in Lake Erie sediments: evidence for broad geographical distribution of pyrene-degrading mycobacteria. Environ Sci Technol 43:3467–3473
Elser JJ, Stabler LB, Hassett RP (1995) Nutrient limitation of bacterial growth and rates of bacterivory in lakes and oceans: a comparative study. Aquat Microb Ecol 9:105–110. https://doi.org/10.3354/ame009105
Eiler A, Langenheder S, Bertisson S, Tranvik LJ (2003) Heterotrophic bacterial growth efficiency and community structure at different natural organic carbon concentrations. Appl Environ Microbiol 69:3701–3709. https://doi.org/10.1128/AEM.69.7.3701
Dailloux M, Albert M, Laurain C, et al. (2003) Mycobacterium xenopi and drinking water biofilms. Appl Environ Microbiol 69:6946–6948. https://doi.org/10.1128/AEM.69.11.6946-6948.2003
Williams MM, Yakrus MA, Arduino MJ, et al. (2009) Structural analysis of biofilm formation by rapidly and slowly growing nontuberculous mycobacteria. Appl Environ Microbiol 75:2091–2098. https://doi.org/10.1128/AEM.00166-09
Rosindell J, Hubbell SP, Etienne RS (2011) The unified neutral theory of biodiversity and biogeography at age ten. Trends Ecol Evol 26:340–348. https://doi.org/10.1016/j.tree.2011.03.024
Sloan WT, Lunn M, Woodcock S, et al. (2006) Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ Microbiol 8:732–740. https://doi.org/10.1111/j.1462-2920.2005.00956.x
Acknowledgements
This work was supported by the French National Research Agency through the PULSE (Peri-Urban Lakes, Society and Environment) research project (ANR-10-CEPL-010). Eau de Paris financed the mycobacterial analysis. The DSEA (Direction des Services de l’Environnement et de l’Assainissement) and the DGST (Direction Générale des Services Techniques), which belongs to the Departmental Council of the Val-de-Marne district, financed the equipment and monitoring of the storm sewer of Créteil Lake with a flowmeter. The DGST of Créteil City provided the authorizations and the street signalization equipment that were necessary to conduct the storm sewer study in Créteil Lake. We like to thank more specifically Mrs. Chamayou, Mrs. Vernin, Mrs. Berdoulay, and Mrs. Butel-Gomis. We are grateful to the stakeholders, owners, and municipalities for their authorizations to collect samples in each lake, and we especially thank the city of Créteil for their support. We also thank the leisure base of Créteil Lake for lending us their boats over the 2 years of sampling. Finally, we warmly thank all the colleagues who helped in sampling and quantifying the ecological parameters in the 49 lakes and Créteil Lake during these 2 years and the two anonymous reviewers whose comments improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roguet, A., Therial, C., Catherine, A. et al. Importance of Local and Regional Scales in Shaping Mycobacterial Abundance in Freshwater Lakes. Microb Ecol 75, 834–846 (2018). https://doi.org/10.1007/s00248-017-1088-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-1088-6