Abstract
The ability to reduce selenite (SeO3 2−) ions with the formation of selenium nanoparticles was demonstrated in Azospirillum brasilense for the first time. The influence of selenite ions on the growth of A. brasilense Sp7 and Sp245, two widely studied wild-type strains, was investigated. Growth of cultures on both liquid and solid (2 % agar) media in the presence of SeO3 2− was found to be accompanied by the appearance of the typical red colouration. By means of transmission electron microscopy (TEM), electron energy loss spectroscopy (EELS) and X-ray fluorescence analysis (XFA), intracellular accumulation of elementary selenium in the form of nanoparticles (50 to 400 nm in diameter) was demonstrated for both strains. The proposed mechanism of selenite-to-selenium (0) reduction could involve SeO3 2− in the denitrification process, which has been well studied in azospirilla, rather than a selenite detoxification strategy. The results obtained point to the possibility of using Azospirillum strains as endophytic or rhizospheric bacteria to assist phytoremediation of, and cereal cultivation on, selenium-contaminated soils. The ability of A. brasilense to synthesise selenium nanoparticles may be of interest to nanobiotechnology for “green synthesis” of bioavailable amorphous red selenium nanostructures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium is a trace element essential in nature in small amounts but toxic at high concentrations [28, 30, 32, 47, 51]. Its relatively well-soluble and, therefore, most toxic forms are represented by the selenate (SeVIO4 2−) and selenite (SeIVO3 2−) oxyanions.
The ability to reduce selenates and selenites to elementary selenium (Se0) is widespread in microorganisms. These include bacteria isolated from areas contaminated with various pollutants, including selenium: Stenotrophomonas maltophilia [12], Bacillus sp. [23], Ralstonia metallidurans [44], Dechloromonas sp. and Thauera sp. [52]; anaerobes that can utilise selenites and selenates in their respiratory chain as electron acceptors, often along with sulphates and sulphites: Desulfomicrobium sp. [17], Desulfurispirillum indicum [40], and hyperthermophilic archaea [20]; as well as certain rhizosphere microorganisms: Rhizobium [2, 21] and Pseudomonas sp. [22]. All these microorganisms can transform the toxic selenite and/or selenate anions to the insoluble (hence non-toxic) elementary selenium [12, 21–23, 44, 49]. Most published studies have reported the transformation of selenates or selenites to the amorphous red modification, a form of elementary selenium.
Bacteria of the genus Azospirillum belong to the plant-growth-promoting rhizobacteria that have received the most study [3, 13]. They have positive effects on the development of their plant hosts through phytohormone production, nitrogen fixation and several other processes [3, 4, 13]. Because azospirilla, ubiquitous in virtually all climatic zones, inhabit the plant rhizosphere, they possess a high potential to adapt to negative external influences, including salinity, aridity and a dearth of nutrients. Compared to other rhizosphere microorganisms, azospirilla exhibit fairly high resistance to heavy metals [25, 27] and other stress factors [26]. Recent work has shown that the use of rhizobacteria, including azospirilla, can improve bioremediation (phytoremediation) and enable a better extraction of harmful substances [4, 5, 8, 10, 19, 34]. However, there is still no information about the toxicity of selenium compounds to azospirilla or about any effects of selenium on them. In view of this, we sought in this study to examine the effect of selenite (SeO3 2−, a SeIV compound) on two widely studied wild-type strains, Azospirillum brasilense Sp7 and Sp245, which can occupy different ecological niches in the rhizosphere [45] and have been reported to respond differently to various stresses or behave differently under similar conditions [3, 4, 25, 27, 36, 39]. The hypothesis tested by this study is that the two wild-type strains of A. brasilense might be capable of reducing SeO3 2− to red elementary selenium (in the form of nanosized particles) as a result of their cellular metabolic activity.
Material and Methods
Bacterial Strains and Growth Media
A. brasilense strains Sp245 and Sp7 (from The Collection of Rhizosphere Microorganisms, [WDCM 1021] according to the World Federation of Culture Collections (http://www.wfcc.info/), Institute of Biochemistry and Physiology of Plants and Microorganisms, Russian Academy of Sciences, Saratov, Russia) were used in this work. These strains were cultivated in a liquid or on a solid (2 % agar) modified malate salt medium (MSM [7]) containing the following (grams per litre): K2HPO4, 3.0; KH2PO4, 2.0; NH4Cl, 0.5; NaCl, 0.1; FeSO4·7H2O, 0.02 (added as chelate with nitrilotriacetic acid); CaCl2, 0.02; MgSO4·7H2O, 0.2; Na2MoO4·2H2O, 0.002; and sodium malate, 5.0 (obtained by mixing 3.76 g of malic acid with 2.24 g NaOH per litre), pH 6.8–7.0. Inoculation cultures were grown with shaking (180 rpm) at 31–32 °C for 18–22 h. The culture volume was 100 or 25 ml in 250- or 50-ml Erlenmeyer flasks, respectively. Cell growth was monitored at OD600 (Specol 221, Germany).
In selenite toxicity tests in the liquid medium, cultures of A. brasilense Sp7 and Sp245 were grown for 18 h under aerobic conditions (corresponding to the end of the logarithmic and the beginning of the stationary growth phase for azospirilla in the MSM under these conditions) without (control) or with Na2SeO3 concentrations ranging from 0.005 to 10 mM (experiment). The initial culture density in all variants was 2·107 cells ml−1. Growth inhibition was determined using optical density measurements. In each variant, parallel experiments were repeated a minimum of five times.
According to the literature, selenites and selenates may be reduced in the stationary, not the logarithmic, phase of growth [12, 44]. For this reason, the period of bacterial growth was extended to 7 days. The Na2SeO3 concentrations used were 0.2, 0.3 and 0.4 mM. After cultivation, the numbers of viable cells (colony-forming units, CFU) were determined as follows. A series of consecutive tenfold dilutions of a bacterial suspension was prepared using physiological saline solution (0.87 % NaCl). CFU numbers were determined by spreading 100 μl of the corresponding diluted samples on agar plates and incubating at 32 °C in a thermostat for 4–5 days. For determining dry cell biomass, cells were collected by centrifugation (7,000×g, 10 min) and dried at 105 °C down to a constant weight. The pH of the medium was also measured after cultivation (pH-meter Multitest IPL-301, Russia).
For selenite toxicity tests during cultivation on the solid medium, the strains were grown without selenite (control) and in the presence of 0.3, 1.0 or 10 mM Na2SeO3 (experiment; stock Na2SeO3 solution was added to the final concentrations after autoclaving the medium; the pre-culture had been grown for 20 h on the liquid MSM up to (4.5–5.0)·109 CFU ml−1). CFU analyses were performed as described above, with a minimum of three parallel dishes for each concentration.
For X-ray fluorescence analysis (XFA), transmission electron microscopy (TEM) and electron energy loss spectroscopy (EELS), cells were grown for 7 days under static conditions (without shaking) at 31 °C in the liquid MSM (control) and in the presence of 0.3 mM Na2SeO3.
For location of the selenite-reducing enzymes, A. brasilense Sp7 and Sp245 were grown in the Na2SeO3-free MSM for 18 h under aerobic conditions (the onset of the stationary phase under the given conditions for azospirilla). Both cultures were grown up to an absorbance (A 600) of 0.97 (1-cm optical path cuvettes).
X-ray Fluorescence Analysis (XFA)
The cells (grown as described in “Bacterial Strains and Growth Media”) were collected by centrifugation at 7,000×g for 10 min and air-dried at room temperature. Elemental XFA of the dried biomass samples was carried out using an energy-dispersive X-ray spectrometer ED2000 (Oxford Instruments, UK). The measurements were carried out under the following conditions: range of detected elements from Na to U, Х-ray tube with a silver anode, voltage 35 kV (“Medium elements”), a thin Ag filter for primary X-ray radiation, exposure 600 s and air as the medium. The elements were detected using the method of fundamental parameters implemented in the software package provided with the instrument.
Transmission Electron Microscopy (TEM) and Electron Energy Loss Spectroscopy (EELS)
The cells were grown as described in “Bacterial Strains and Growth Media”, collected by centrifugation, washed twice with phosphate-buffered saline (PBS, pH 7) and finally washed twice with purified distilled water. Next, the bacterial cells were resuspended in a minimal volume of distilled water and placed onto nickel grids coated with formvar (1 % formvar in dichloroethane). For EELS, after drying the sample, the cells were contrasted with 1 % aqueous uranyl acetate.
Taking TEM photographs, selenium mapping in the electron spectroscopic imaging (ESI) mode and registering electron energy loss spectra for selenium were performed using a Libra 120 electron microscope (Carl Zeiss, Germany) at 120 kV.
Location of the Selenite-Reducing Enzymes
The cells were grown as described in “Bacterial Strains and Growth Media”. All further work was done under sterile conditions. The grown cells (2 ml of suspension) were sedimented by centrifugation at 7,000×g for 15 min, washed three times with physiological saline solution (0.87 % NaCl) and resuspended in the same volume of solution. After this treatment, cells remain viable but become depleted of nutrients. Na2SeO3 was added to the supernatant liquid and to the cells in the physiological saline solution up to a final concentration of 0.3 mM Na2SeO3. As a control (dead cells), the culture was thermally treated at 95 °C for 5 min. The samples were incubated in a thermostat at 31 °C for 7 days.
Results
Effect of Selenite on A. brasilense
We first tested the sensitivity of A. brasilense Sp7 and Sp245 to Na2SeO3, which was used at concentrations ranging from 0.005 to 10 mM. The cultures were grown for 18 h under aerobic conditions, corresponding to the end of the logarithmic and the beginning of the stationary phase (for azospirilla in the MSM under these conditions). Our efforts to determine exactly the minimal growth-inhibiting concentration (i.e. that at which the absorbance of the culture decreased as compared with the control) failed. In various experiments, this concentration varied from 0.08 to 0.3 mM. Neither could we observe under these conditions any change in the medium’s colour in the presence of selenite. The culture growth was completely inhibited at 0.5 to 1 mM Na2SeO3.
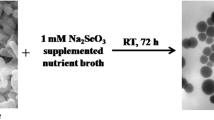
The change in the colour of microbial colonies growing in the presence of selenium-containing compounds to various hues of pink and red is known to be the first sign of reduction of these compounds to the red modification of Se0 [22, 42]. As mentioned above, selenites and selenates may be reduced to Se0 in the stationary, not the logarithmic, phase of growth [12, 44]. For this reason, the period of bacterial growth was extended. The Na2SeO3 concentrations used were at levels that caused incomplete inhibition of bacterial growth after 18 h of cultivation (0.2, 0.3 and 0.4 mM). After 42 h, growth was observed in all flasks, and a weak red colouration of the medium in the flasks appeared. After 66 h, active growth was recorded for both strains at all selenite concentrations used, and the colour of the growth medium changed to red. On day 4 of growth of both strains, the intensity of the red colouration of the growth medium increased with the selenite concentration (Fig. 1, upper row; the data for strain A. brasilense Sp245 are shown as an example). A similar effect was observed when the strains were grown at selenite concentrations of up to 1 mM, as well as in static conditions of cultivation (data not shown). At 10 mM Na2SeO3, no growth and no red colour were observed throughout the experiment (up to 30 days).
A. brasilense Sp245 culture grown for 4 days under aerobic conditions in the liquid MSM (upper row) without selenite (control, 1) and in the presence of 0.2 mM (2), 0.3 mM (3) and 0.4 mM Na2SeO3 (4). Bacterial colonies of strains A. brasilense Sp7 (middle row) and Sp245 (bottom row) grown on solid MSM for 5 days. Control without selenite (a, d), with 0.3 mM (b, e) and 1 mM Na2SeO3 (c, f). For interpretation of the references to colour in the text, the reader is referred to the web version of this article
The pH values of the cultivation culture after cell growth were as follows: for A. brasilense Sp7, in the control and in the medium with 0.2 mM Na2SeO3—9.3 and for higher selenite concentrations (0.3 and 0.4 mM)—8.6. For A. brasilense Sp245, the pH values were 9.4 in the control; for 0.2 mM Na2SeO3, 9.1; and for 0.3 and 0.4 mM Na2SeO3, 8.9. The weight of dry biomass of the cultures grown in the presence of selenite decreased as compared with that of the control by 11.7–28.6 % for strain A. brasilense Sp7 and by 23.0–34.4 % for strain A. brasilense Sp245. The CFU values for both strains were found to be comparable in the controls and in the presence of selenite: for A. brasilense Sp7, 5.0·108 CFU ml−1 (control) and (2.0–7.5)·108 CFU ml−1 (with selenite) and for A. brasilense Sp245, 5.1·108 CFU ml−1 (control) and 3.6·108 CFU ml−1 (with 0.2 mM selenite). For higher selenite concentrations, the type and number of A. brasilense Sp245 colonies changed, they became smaller and their number significantly increased, which precluded exact CFU calculations.
When the coloured cultures were sedimented by centrifugation, the supernatant liquid remained uncoloured and the bacterial cell biomasses were bright red. This also suggests that the colouration of the growth medium was associated predominantly with the cells.
We next cultured the strains in Petri dishes. With 0.3 mM Na2SeO3, growth remained unchanged as compared with the control; with 1 mM, it was somewhat inhibited; and with 10 mM, it was absent. With 0.3 mM, only some of the colonies were red; with 1 mM, all the growing colonies were red, and some of them increased in diameter (two- to threefold as compared with those in the control). The colonies of both strains were bright red, with no colouration of the adjacent areas. This result also indicates that the red colouration was associated with the bacterial cells and that the enzymes reducing Na2SeO3 to elementary selenium were possibly localised inside the cells (Fig. 1, middle and bottom rows).
X-ray Fluorescence Analysis
XFA of the bacterial cells (Fig. 2) showed a considerable accumulation of selenium in the biomass growing with Na2SeO3, which was reflected in the spectra as the appearance of intense dominating emission lines corresponding to selenium (Fig. 2b, d; note that this XFA methodology, besides the qualitative detection of elements, is semiquantitative and only allows significantly differing amounts of elements to be compared). The presence of selenium was determined by its two main lines, at 11.22 keV (K α) and 12.49 keV (K β). The spectra taken for the control cultures showed only a very small amount of selenium as a microcomponent (Fig. 2a, c), along with a few lines (more or comparably intense) corresponding to the following (from left to right): Ca (3.69 keV, K α, and 4.01 keV, K β), Fe (6.40 keV, K α1, and 7.06 keV, K β), Cu (8.04 keV, K α1) and Zn (8.64 keV, K α1, and 9.57 keV, K β) as main typical trace elements found in bacterial cells, in particular, in A. brasilense by means of analytical methods [24, 25, 27].
X-ray fluorescence analysis of dried bacterial cells of A. brasilense strains Sp7 (a, b) and Sp245 (c, d) grown in liquid MSM in the absence (control) (a, c) and in the presence of 0.3 mM Na2SeO3 (b, d) for 7 days under static conditions. Emission lines at 11.22 and 12.49 keV correspond to selenium (K α and K β lines, respectively)
Transmission Electron Microscopy (TEM) and Electron Energy Loss Spectroscopy (EELS) Analysis
We performed TEM and EELS of A. brasilense strains Sp245 and Sp7 grown under the same conditions in the presence of 0.3 mM Na2SeO3. As seen in Fig. 3c, the cells contained spherical electron-dense formations, which were absent in the control. Most of these formations were localised inside the bacterial cells. The size of these particles ranged from 50 to 400 nm, with 200-nm-diameter particles being predominant. Some isolated spherical particles could be observed outside the cells.
Transmission electron microscopy (TEM) images of A. brasilense Sp245 (a, c) after reduction of selenite. Cells were grown on MSM for 7 days in the presence 0.3 mM Na2SeO3. Electron energy loss spectroscopy (EELS) was used to create an elemental map of selenium (red colour) (b). The electron-dense precipitates generally inside the cell are reduced selenium, including the electron-dense globules in the cytoplasm. Bars: a, b—2 μm; c—1 μm. For interpretation of the references to colour in the text and in this caption, the reader is referred to the web version of this article
A map of selenium distribution was obtained by ESI, showing considerable accumulation of elementary selenium inside the cells: the more intensive the red colour, the more selenium is contained at the given spot (Fig. 3a—TEM, Fig. 3b—the same sample in the ESI mode). The spectra obtained for spherical particles by EELS demonstrated that they consisted of selenium (Fig. 4).
Selenite Reduction to Elementary Selenium
To unravel the mechanism responsible for the reduction of selenite to elementary selenium, both the supernatant liquid and the cells were incubated with 1 mM Na2SeO3 (experiment). After 18 h, the colouration of the cell samples changed from opaque to orange. Throughout the experiment (7 days), we did not observe any changes in the colour of the medium and, consequently, any reduction of Na2SeO3 to red elementary selenium in either the cell-free supernatant liquid or the thermally treated cells. These results show that the selenite transformation to elementary selenium was associated with the activity of live bacterial cells, not with the cell-free medium remaining after removing the cells.
Discussion
When the A. brasilense strains were grown aerobically for 18 h, corresponding to logarithmic and early stationary growth for Azospirillum, their growth was partly inhibited at selenite concentrations above 0.08–0.2 mM. Complete inhibition was observed at 0.5 to 1 mM. (Under the latter conditions, the growth medium did not change its colour.) Some microorganisms have about the same resistance: Bacillus subtilis (>1 mM), Rhodospirillum rubrum DSM 467 (>2 mM), Wolinella succinogenes DSM 1740 (1 mM) and R. metallidurans (6.0 mM), whereas others are much more resistant: Pseudomonas sp. strain CA5 (>150 mM), Rhizobium leguminosarum bv. viceae (25–200 mM) [22]. Note for comparison that growth rates and biomass of natural freshwater phytoplankton cultures (dominated by an Ankistrodesmus sp., a Dictyosphaerium sp. and an unidentified monad) were found to be significantly reduced by selenate (SeO4 2−) at concentrations as low as 0.32 μM (and higher), while they were not affected by SeO3 2− at concentrations of up to 2.5 μM [41]; it was concluded that natural communities seemed to be more sensitive to SeO4 2− than cultured algae.
The A. brasilense strains were able to reduce selenite several days after beginning to grow in liquid culture, under both aerobic and static growth conditions, as evidenced by a colour change in the medium to red. Under aerobic conditions, the colour intensity increased with the selenite concentration in the medium (see Fig. 1, upper row). We note a considerable alkalisation of the culture medium, caused by cell growth, in the control and in the presence of 0.2 mM SeO3 2− up to pH 9.1–9.4; in the presence of 0.3 and 0.4 mM SeO3 2−, the alkalisation was lower, up to pH 8.6–8.9.
Sedimentation of liquid cultures and Azospirillum growth on solid medium indicated that the appearance of colouration was associated with the cells, as shown also for R. metallidurans [44] and R. rubrum [29]. In Pseudomonas fluorescens and B. subtilis, elementary selenium was similarly found to be deposited as granules throughout the cell and between the cell wall and the plasma membrane, respectively [16]. In other microorganisms, either extracellular or intracellular selenite reduction followed by release to the outside was described [33].
As mentioned above, the change in the colour in the presence of selenium-containing compounds to various hues of pink and red is the first sign of reduction of these compounds to the red modification of Se0. Compounds of SeIV and SeVI are not red (i.e. are colourless), and the presence of this colour can be considered to be a clear evidence for selenite reduction to the red modification of Se0. However, one cannot rule out the possibility of parallel formation of any other Se compounds, e.g. organoselenium compounds or Se in (an)other oxidation state(s). This possibility was not examined in the current study, but it may be of interest in future research.
Elemental XFA showed considerable accumulation of Se in the strains growing with selenite (see Fig. 2), while ТЕМ demonstrated the presence of round electron-dense particles, most of which were found inside and some outside the cells (see Fig. 3c). Most probably, the presence of such particles outside the cells resulted from occasional cell lysis. The map of Se distribution obtained by ESI analysis showed that Se was localised inside the cells (the red colouration corresponds to a high Se content, see Fig. 3b). The EEL spectra for the nanoparticles confirmed that they consisted of Se (Fig. 4a).
The reduction of selenite (SeIV), which has a low redox potential [11], is a fairly easy process; accordingly, possible mechanisms of this phenomenon may include both simple chemical reduction, induced by specific medium components [11, 28], and microbiological reduction, induced by the action of enzymes (see below). To rule out the possibility that selenite was reduced by the components of the growth medium, we ran an additional control: a flask that contained the azospirilla-free MSM with 0.3 mM Na2SeO3. The flask was incubated at 31 °C for 30 days. After 30 days, the medium with selenite did not change its colour and had the same transparency as at the start of the experiment.
The experiment to locate the selenite-reducing enzymes (see “Results”, the subsection “Selenite Reduction to Elementary Selenium”) showed that it was the cells, not the supernatant liquid, which contained the factor(s) such as enzymes that were responsible for reducing selenite. After 18 h, an intense red colouration was observed in treatments containing washed cells. This conclusion was corroborated by the results of TEM (see above, Fig. 3), showing that reduced Se accumulated as nanospherical particles inside bacterial cells. In addition, thermally treated cells were not capable of reducing selenite to Se0, which is consistent with the participation of bacterial enzymes in this process. Thus, we have shown that in A. brasilense, a biochemical reduction occurs that is directly associated with the metabolism of the bacteria and that does not depend on either the extracellular synthetic products or a change in pH of the medium (as shown above, after 7 days of cultivation, a considerable alkalisation of the medium was observed).
Azospirilla can fix and assimilate nitrogen, and one of the mechanisms by which these bacteria benefit plants is the provision of nitrogen for them [4]. For azospirilla, genes involved in nitrogen metabolism have been isolated and described [4]. The relevant enzymes include nitrogenase, which fixes atmospheric nitrogen, as well as nitrite and nitrate reductases. On the other hand, several authors have shown the possibility of selenite and selenate reduction under the effect of nitrite and nitrate reductases. Specifically, the reduction of selenite to elementary Se may involve nitrate reductase in Escherichia coli [1] and nitrite reductase in Rhizobium sullae [2] and in Thauera selenatis [9]. The ability to reduce selenites, selenates and tellurites is often associated with denitrification [43], which azospirilla are also able to perform. The metabolism of Se in bacteria has been reviewed by Stolz et al. [48], who analysed the genes and proteins responsible for selenate and selenite reduction and showed that there is some similarity between the selenate reductase of T. selenatis and respiratory nitrate reductases of E. coli, Aeropyrum pernix and Thermus thermophilus.
In addition, it has to be mentioned that the nitrate ion (NO3 −) is structurally similar to SeO3 2−, permitting the assumption that the former gets replaced by the latter in the biochemical process of denitrification by azospirilla. While this is feasible, note that the structure of the selenate ion (SeO4 2−) is significantly different from that of the nitrate ion. Indeed, we found that replacing selenite with selenate did not give rise to a red colouration (data not shown), a finding that serves as an indirect proof of the suggested mechanisms of selenite reduction.
We suggest that in Azospirillum, selenite reduction most probably occurs under the effect of either nitrite or nitrate reductase. It should be noted that these enzymes are localised inside the cell which is exactly where our research shows that selenite reduction occurs.
In summary, one can speculate that selenite reduction by azospirilla is more than just a protective mechanism serving to detoxify selenite to less toxic insoluble Se: it may be the inclusion of this toxic compound in the metabolic nitrogen cycle, with its transformation occurring under the effect of the enzyme(s) responsible for denitrification. However, other mechanisms of selenite transformation (e.g. to some organoselenium compounds) are also possible. To determine exactly the mechanism responsible for the transformation of SeIV compounds in the bacteria of the genus Azospirillum is an exciting subject for further study.
Our study is the first to have shown for bacteria of the genus Azospirillum that A. brasilense can reduce selenite and synthesise selenium nanoparticles. It should be pointed out that, as reported in articles on the reduction of selenites and selenates to elementary Se, not all microorganisms can reduce selenium to a nanoparticulate form. A recent review on microbial nanoparticle synthesis [37] summarises what is currently known about microorganisms, including Gram-negative bacteria, that can produce nanoparticles of gold, silver, selenium and other elements. This ability of azospirilla may be of nanobiotechnological interest and represent a direction in nanoparticle synthesis that is called “green chemistry” [37], considering the specific properties of Se nanoparticles [6] (as well as the recently reported ability of A. brasilense to reduce gold(III) ([AuCl4]−) with the formation of gold nanoparticles [50]). Of particular importance is the documented bioavailability of Se nanoparticles (in contrast to micrometer-sized elementary selenium which is biologically inert) accompanied by their lower toxicity as compared with Se oxyanions (see, e.g. [15, 18, 31, 35, 38, 46] and references reported therein). In addition, azospirilla as plant-stimulating rhizobacteria may be useful as micropartners to assist phytoremediation of, and cereal cultivation on, selenium-contaminated soils [47]. This is another fascinating direction for further studies on the ecology and geomicrobiology of these ubiquitous bacteria [14], including also their possible role in selenium biogeochemistry.
References
Avazeri C, Turner RJ, Pommier J, Weiner JH, Giordano G, Vermeglio A (1997) Tellurite and selenate reductase activity of nitrate reductases from Escherichia coli: correlation with tellurite resistance. Microbiology 143:1181–1189
Basaglia M, Toffanin A, Baldan E, Bottegal M, Shapleigh JP, Casella S (2007) Selenite-reducing capacity of the copper-containing nitrite reductase of Rhizobium sullae. FEMS Microbiol Lett 269(1):124–130
Bashan Y, de-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv Agron 107:77–136
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003). Can J Microbiol 50(8):521–577
Belimov AA, Dietz K (2000) Effect of associative bacteria on element composition of barley seedlings grown in solution culture at toxic cadmium concentrations. Microbiol Res 155(2):113–121
Buchs B, Evangelou MW, Winkel LH, Lenz M (2013) Colloidal properties of nanoparticular biogenic selenium govern environmental fate and bioremediation effectiveness. Environ Sci Technol 47(5):2401–2407
Day JM, Döbereiner J (1976) Physiological aspects of N2-fixation by a Spirillum from Digitaria roots. Soil Biol Biochem 8:45–50
de-Bashan LE, Hernandez JP, Morey T, Bashan Y (2004) Microalgae growth-promoting bacteria as “helpers” for microalgae: a novel approach for removing ammonium and phosphorus from municipal wastewater. Water Res 38(2):466–474
DeMoll-Decker H, Macy JM (1993) The periplasmic nitrite reductase of Thauera selenatis may catalyze the reduction of selenite to elemental selenium. Arch Microbiol 160(3):241–247. doi:10.1007/BF00249131
de Souza MP, Huang CPA, Chee N, Terry N (2009) Rhizosphere bacteria enhance the accumulation of selenium and mercury in wetland plants. Planta 209:259–263
Doran JW (1982) Microorganisms and the biological cycling of selenium. In: Marshall KC (ed) Advances in microbial ecology. Plenum, New York, pp 1–32. http://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/978-1-4615-8318-9_1
Dungan RS, Yates SR, Frankenberger WT (2003) Transformations of selenate and selenite by Stenotrophomonas maltophilia isolated from a seleniferous agricultural drainage pond sediment. Environ Microbiol 5(4):287–295
Fibach-Paldi S, Burdman S, Okon Y (2012) Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol Lett 326(2):99–108
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156(3):609–643
Gao X (2012) Selenium nanoparticles with improved biological effects. US Patent Application 13/206,542 (filed Aug. 10, 2011). Publ. no. 2012/0202062 A1 (publ. date: August 9, 2012). http://www.faqs.org/patents/app/20120202062
Garbisu C, Ishii T, Leighton T, Buchanan BB (1996) Bacterial reduction of selenite to elemental selenium. Chem Geol 132(1):199–204
Hockin S, Gadd GM (2006) Removal of selenate from sulfate-containing media by sulfate-reducing bacterial biofilms. Environ Microbiol 5:816–826
Hu CH, Li YL, Xiong L, Zhang HM, Song J, Xia MS (2012) Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol 177:204–210
Huang XD, El-Alawi Y, Penrose DM, Glick BR, Greenberg BM (2004) A multi-process phytoremediation system for removal of polycyclic aromatic hydrocarbons from contaminated soils. Environ Pollut 130(3):465–476
Huber R, Sacher M, Vollmann A, Huber H, Rose D (2000) Respiration of arsenate and selenate by hyperthermophilic archaea. Syst Appl Microbiol 23(3):305–314
Hunter WJ, Kuykendall LD (2007) Reduction of selenite to elemental red selenium by Rhizobium sp. strain B1. Curr Microbiol 55(4):344–349
Hunter WJ, Manter DK (2009) Reduction of selenite to elemental red selenium by Pseudomonas sp. strain CA5. Curr Microbiol 58:493–498
Ikram M, Faisal M (2010) Comparative assessment of selenite (SeIV) detoxification to elemental selenium (Se0) by Bacillus sp. Biotechnol Lett 32:1255–1259
Kamnev AA, Renou-Gonnord M-F, Antonyuk LP, Colina M, Chernyshev AV, Frolov I, Ignatov VV (1997) Spectroscopic characterization of the uptake of essential and xenobiotic metal cations in cells of the soil bacterium Azospirillum brasilense. Biochem Mol Biol Int 41(1):123–130
Kamnev AA, Tugarova AV, Antonyuk LP, Tarantilis PA, Kulikov LA, Perfiliev YuD, Polissiou MG, Gardiner PHE (2006) Instrumental analysis of bacterial cells using vibrational and emission Mössbauer spectroscopic techniques. Anal Chim Acta 573–574:445–452
Kamnev AA, Sadovnikova JN, Tarantilis PA, Polissiou MG, Antonyuk LP (2008) Responses of Azospirillum brasilense to nitrogen deficiency and to wheat lectin: a diffuse reflectance infrared Fourier transform (DRIFT) spectroscopic study. Microb Ecol 56:615–624
Kamnev AA, Tugarova AV, Tarantilis PA, Gardiner PHE, Polissiou MG (2012) Comparing poly-3-hydroxybutyrate accumulation in Azospirillum brasilense strains Sp7 and Sp245: the effects of copper(II). Appl Soil Ecol 61:213–216
Kessi J, Hanselmann KW (2004) Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirillum rubrum and Escherichia coli. J Biol Chem 279(49):50662–50669
Kessi J, Ramuz M, Wehrli E, Spycher M, Bachofen R (1999) Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum. Appl Environ Microbiol 65:4734–4740
Kramer GF, Ames BN (1988) Mechanisms of mutagenicity and toxicity of sodium selenite (Na2SeO3) in Salmonella typhimurium. Mutat Res Fundam Mol Mechan Mutagen 201(1):169–180. doi:10.1016/0027-5107(88)90123-6
Kuzmin PG, Shafeev GA, Voronov VV, Raspopov RV, Arianova EA, Trushina EN, Gmoshinskii IV, Khotimchenko SA (2012) Bioavailable nanoparticles obtained in laser ablation of a selenium target in water. Quant Electron 42(11):1042–1044. doi:10.1070/QE2012v042n11ABEH014754
Lenz M, Lens PNL (2009) The essential toxin: the changing perception of selenium in environmental sciences. Sci Total Environ 407(12):3620–3633
Losi ME, Frankenberger WT Jr (1997) Reduction of selenium oxyanions by Enterobacter cloacae SLD1a-1: isolation and growth of the bacterium and its expulsion of selenium particles. Appl Environ Microbiol 63(8):3079–3084
Lucy M, Reed E, Glick BR (2004) Applications of free living plant growth-promoting rhizobacteria. Antonie Van Leeuwenhoek 86(1):1–25
Mennini T (2012) Elemental selenium nanoparticles with reduced toxicity. Nutrafoods 11: N25-N26. http://www.nutrafoods.eu/pubs/pdf/Nanotech-2012(2).pdf
Mulyukin AL, Suzina NE, Pogorelova AYu, Antonyuk LP, Duda VI, El’-Registan GI (2009) Diverse morphological types of dormant cells and conditions for their formation in Azospirillum brasilense. Microbiology (Moscow) 78:33–41
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interf Sci 156:1–13
Peng D, Zhang J, Liu Q, Taylor EW (2007) Size effect of elemental selenium nanoparticles (nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J Inorg Biochem 101:1457–1463
Pogorelova AYu, Mulyukin AL, Antonyuk LP, Galchenko VF, El’-Registan GI (2009) Phenotypic variability in Azospirillum brasilense strains Sp7 and Sp245: association with dormancy and characteristics of the variants. Microbiology (Moscow) 78(5):559–568
Rauschenbach I, Yee N, Häggblom MM, Bini E (2011) Energy metabolism and multiple respiratory pathways revealed by genome sequencing of Desulfurispirillum indicum strain S5. Environ Microbiol 13(6):1611–1621
Riedel GF, Sanders JG, Gilmour CC (1996) Uptake, transformation, and impact of selenium in freshwater phytoplankton and bacterioplankton communities. Aquat Microb Ecol 11(1):43–51
Roux M, Sarret G, Pignot-Paintrand I, Fontecave M, Coves J (2001) Mobilization of selenite by Ralstonia metallidurans CH34. Appl Environ Microbiol 67(2):769–773
Sabaty M, Avazeri C, Pignol D, Vermeglio A (2001) Characterization of the reduction of selenate and tellurite by nitrate reductases. Appl Environ Microbiol 67(11):5122–5126
Sarret G, Avoscan L, Carrière M, Collins R, Geoffroy N, Carrot F, Covès J, Gouget B (2005) Chemical forms of selenium in the metal-resistant bacterium Ralstonia metallidurans CH34 exposed to selenite and selenate. Appl Environ Microbiol 71(5):2331–2337
Schloter M, Hartmann A (1998) Endophytic and surface colonization of wheat roots (Triticum aestivum) by different Azospirillum brasilense strains studied with strain-specific monoclonal antibodies. Symbiosis 25:159–179
Shi L, Xun W, Yue W, Zhang C, Ren Y, Shi L, Wang Q, Yang R, Lei F (2011) Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin Res 96(1):49–52
Simon L, Biró B, Balázsy S, Győri Z (2008) Rhizosphere processes in the selenium accumulation of fodder radish. Sereal Res Commun 36(Suppl):1811–1814. http://www.akademiai.com/content/074301116x42787w/?p=f0fbc94f56e940ceb997872545ea741b&pi=2
Stolz JF, Basu P, Santini JM, Oremland RS (2006) Arsenic and selenium in microbial metabolism. Annu Rev Microbiol 60:107–130
Stolz JF, Oremland RS (1999) Bacterial respiration of selenium and arsenic. FEMS Microbiol Rev 23:615–627
Tugarova AV, Burov AM, Burashnikova MM, Kamnev AA (2014) Gold(III) reduction by the rhizobacterium Azospirillum brasilense with the formation of gold nanoparticles. Microb Ecol 67(1):155–160. doi:10.1007/s00248-013-0329-6
Weiss KF, Ayres JC, Kraft AA (1965) Inhibitory action of selenite on Escherichia coli, Proteus vulgaris, and Salmonella thompson. J Bacteriol 90(4):857–862
Williams KH, Wilkins MJ, N’Guessan AL, Arey B, Dodova E, Dohnalkova A, Holmes D, Lovley DR, Long PE (2013) Field evidence of selenium bioreduction in a uranium‐contaminated aquifer. Environ Microbiol Rep 5(3):444–452. doi:10.1111/1758-2229.12032
Acknowledgements
The authors are grateful to Dr. A.G. Shchelochkov and V.F. Kurskii (Saratov, Russia) for their help in carrying out the X-ray fluorescence analyses. This work was supported in part under the Agreement on Scientific Cooperation between the Russian and Hungarian Academies of Sciences for 2011–2013 (Project 29).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tugarova, A.V., Vetchinkina, E.P., Loshchinina, E.A. et al. Reduction of Selenite by Azospirillum brasilense with the Formation of Selenium Nanoparticles. Microb Ecol 68, 495–503 (2014). https://doi.org/10.1007/s00248-014-0429-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-014-0429-y