Abstract
A bacterium that reduces the soluble and toxic selenite anion to insoluble elemental red selenium (Se0) was isolated from a laboratory bioreactor. Biochemical, morphological, and 16S rRNA gene sequence alignment identified the isolate as a Rhizobium sp. that is related to but is genetically divergent from R. radiobacter (syn. Agrobacterium tumefaciens) or R. rubi (syn. A. rubi). The isolate was capable of denitrification and reduced selenite to Se0 under aerobic and denitrifying conditions. It did not reduce selenate and did not use selenite or selenate as terminal e− donors. Native gel electrophoresis revealed two bands, corresponding to molecular weights of ∼100 and ∼45 kDa, that reduced selenite. Tungsten inhibited in vivo selenite reduction, suggesting that a molybdenum-containing protein is involved in selenite reduction. This organism, or its enzymes or DNA, might be useful in bioreactors designed to remove selenite from water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selenium is both an essential trace element and a toxin. In humans, consumption of 50–200 µg Se/day is beneficial, but consumption of 850 µg Se/day can be injurious [23]. In the environment, selenium also plays a dual role by being beneficial in low amounts but damaging at larger amounts. Selenite (SeO 2−3 ), common in environs that are moderately acidic and oxidizing [17], is the most toxic of the Se oxyanions [3]. Natural, agricultural, and industrial activities can result in the contamination of groundwater with SeO 2−3 . Refinery wastewaters, a consequence of the refining of crude oil, contain large amounts of SeO 2−3 and are a significant source of SeO 2−3 contamination [14]. Microorganisms play a key role in selenium transformations. Transformations might involve the conversion of the highly soluble and biologically available SeO 2−3 anion to insoluble and relatively nonavailable elemental selenium (Se0). Also, some microorganisms can lessen the toxicity [5] of selenium oxyanions by reduction and methylation, a process that frequently yields volatile gases such as dimethylselenide (DMSe) or dimethyldiselenide (DMDSe). This article describes the characterization and identification of a bacterial strain, the B1 strain, that was isolated from a laboratory bioreactor used to reduce selenate to Se0 [7].
Materials and Methods
Media, Isolation, Characterization, and Identification
Media were National Water Research Institute (NWRI) media [6], tryptone-yeast extract (TY) media (8 g/l tryptone, 5 g/l yeast extract, and 2.5 g/l NaCl) [11]; and a N-2-hydroxyethylpiperazine-N′-ethanesulfonic acid and 2-(N-morpholino) ethanesulfonic acid (HM) salts media with 30 mM glycerol, 0.1% yeast extract and trace elements [11]. Incubations were at 28°C and, if liquid, at 100 rpm. The B1 strain was obtained from a selenium-reducing bioreactor [7]. The strain’s 16S rRNA gene sequence (GenBank Accession No. EF440185) was determined and analyzed by MIDI Labs (Newark, DE).
Nitrate, Selenite and Selenate as Electron (e−) Acceptors
The setup was as described previously [11] with 30 mM glycerol as the substrate. Electron acceptors were none (control), Na2SeO4, Na2SeO3, NaNO3, Na2SeO4 + NaNO3, or Na2SeO3 + NaNO3. Na2SeO4, and NaNO3 were supplied at 10 mM and Na2SeO3 at 4 mM. Growth (turbidly) was estimated visually.
Effect of NO −3 on Se0 Formation
Culture vessels were 125-mL flasks containing 50 mL of HM media supplemented with 5 mM Na2SeO3 and, where indicated, 10 mM NaNO3. Cultures (five replicates of each treatment) were inoculated with 1 mL of a log-phase culture of cells. At 24-h intervals, samples were collected from the flasks and analyzed for Biuret protein and for SeO 2−3 , NO −3 , and NO −2 as described below.
Influence of Dissolved Oxygen on NO −3 Uptake and N2O Formation
Incubation bottles (three replicates) were 500-mL serum bottles sealed with rubber septa and shaken at 150 rpm. Each contained 100 mL of HM media supplemented with 4.6 mM NaNO3 and an ∼ 400-mL headspace consisting of air and 15 mL of acetylene. Acetylene was added to the headspace to block the conversion of N2O to N2 , thereby allowing N2O to accumulate [21]. Headspace samples (7 mL) were collected through the septa with a gas-tight syringe and analyzed for O2 and N2O. Liquid samples (10 mL) for dissolved oxygen (DO), NO −3 , and NO −2 analysis were collected through a 20-cm-long syringe needle that remained inserted through the septa throughout the study. The needle was connected to a valve and the valve to a PVC transfer line (3.2 mm outer diameter, 1.4 mm inner diameter, 35 cm long) that led to a syringe located inside of a helium (He)-filled glove bag. Analyses were carried out as described below.
Minimal Inhibitory Concentration
The effect of selenate and selenite on the cells was determined by growth on NWRI agar containing Na2SeO4 or Na2SeO3 [11].
Analysis and Statistical Comparisons
Dinitrogen oxide (N2O) was measured by gas chromatography [9]. Atmospheric O2 was measured by injection of a 2-mL headspace sample into an Ametek oxygen meter (Paoli, PA, USA). DO was determined using CHEMets® ampoules (CHEMetrics, Calverton, VA, USA). To prevent atmospheric O2 from interfering with the DO assay, samples were collected and DO was measured in a He-filled glove bag. Selenite was measured by ion chromatography [7]. Nitrate and nitrite were measured by ion chromatography [7] or with an Agri-LAB™ (Spectrum Technologies Inc., Plainfield, IL, USA) analyzer. Protein was estimated by the Biuret procedure [16]. Mean, standard error (SEM), and P-value determinations made using the Instat® computer program (GraphPad Software Inc., San Diego, CA)
Preparation of Cell-Free Extract and Electrophoresis
Cells were grown and harvested, a cell-free preparation was prepared, and native gel electrophoresis was preformed as described previously [8]. NaNO2 reductase activity was visualized by the formation of a clear zone in gels stained deep blue with reduced methyl viologen (MV), and SeO 2−3 reductase activity was visualized as Se0 formed in gels incubated with 100 mM Na2SeO3 and 1 mM NADH [8].
Effect of Tungsten on Selenite Reduction
Cells were incubated under air in sealed 125-mL bottles containing 40 mL of HM media supplemented with 4 mM Na2SeO3 or with 4 mM Na2SeO3 plus 10 mM Na2O4W·2H2O. Incubations were for 11 days; at the end of incubation, precipitated Se0 was harvested and assayed as previously described [8].
DMSe and DMDSe Production Assay
Culture conditions [8] and analyses [10] were as described previously except that cells were incubated for 7 days before analyses.
Results and Discussion
16S rRNA Gene Sequence
The 16S rRNA gene (1482 bp) of the B1 strain was sequenced and analyzed in the Applied Biosystem’s MicroSeq™ database. Sequence analyses showed that the strain belongs to the genus Rhizobium and is related to but differed by more than 2.7% from that of R. radiobacter (syn. Agrobacterium tumefaciens) or R. rubi (syn. A. rubi) (Fig. 1).
Nitrate, Selenite, and Selenate as e− Accepters
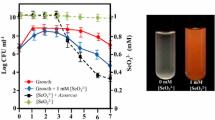
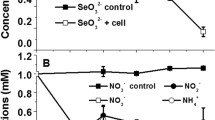
Cells grew well when incubated under He with glycerol as a nonfermentable carbon substrate and NO −3 as an e− acceptor (Table 1). Also, when grown in sealed bottles under air containing acetylene, N2O accumulated and nitrate disappeared when DO levels dropped below 1 ppm, indicating denitrification as a respiratory process (Fig. 2). Cultures incubated anaerobically with SeO 2−4 and SeO 2−3 , as e− acceptors, showed neither growth nor Se0 formation. Under anoxic conditions, growth depended on the presence of NO −3 , and SeO 2−3 was reduced to Se0 only when NO −3 was present (Table 1). The strain used O2 or NO −3 as e− acceptors for growth but not the Se oxyanions.
When incubated aerobically, the strain grew well in HM broth and heavy growth was evident in media containing NO −3 , SeO 2−4 , or SeO 2−3 . A red color developed in flasks containing SeO 2−3 and in flasks containing SeO 2−3 and NO −3 , showing that SeO 2−3 was reduced to Se0 (Table 1). NO −3 was not required for the reduction of SeO 2−3 but did increase the rate at which SeO 2−3 is removed from the medium (Table 2). Cells cultured aerobically without nitrate in the medium removed SeO 2−3 at a maximum rate of 0.08 µmol/mg protein/day. When nitrate was present in the medium, the maximum rate of SeO 2−3 removal was 0.20 µmol/mg protein/day.
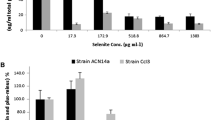
Minimal Inhibitory Concentration for SeO 2−3 and SeO 2−4
The lowest concentrations of SeO 2−3 or SeO 2−4 that prevented the growth of B1 fell between 8 and 16 mM for SeO 2−3 and exceeded 64 mM for SeO 2−4 (Fig. 3). This MIC for SeO −23 is higher than that observed for the “SeO −23 resistant” bacteria Bacillus subtilis [4], Ralstonia metallidurans [19], Rhodobacter spheroides [22], and Rhodospirillum rubrum [13] where the MIC values ranged from 2.0 to 6.0 mM. The minimal inhibitory concentration (MIC) for the B1 strain was lower than the MICs observed with Aeromonas salmonicida, Azospira oryzae, and Stenotrophomonas maltophilia, which were resistant to SeO 2−3 concentrations of 16–50 mM [2, 7, 10]. SeO 2−3 is typically more toxic to bacteria than SeO 2−4 and that is what was observed with the B1 strain.
Heavy metals, such as SeO 2−3 or SeO 2−4 , can cause changes in cell morphology [18]. Stenotrophomonas sp. and Salmonella heidelberg cells were elongate when exposed to ∼0.5 mM selenite [15] and cells of strain B1 grown in HM media (for 3 days) containing 4 mM SeO 2−3 were about 40% longer (3.8 ± 0.2 µm) than cells grown in media that contained no SeO 2−3 .
Selenite Reductase
Native gel electrophoresis of cell-free extracts showed two areas, Rf’s of 0.32 and 0.49, with SeO 2−3 reductase activity (Fig. 4). The Rf 0.32 band corresponded with a band that reduced NO −2 , suggesting that NO −2 reductase might function in the reduction of SeO 2−3 . Also, cells incubated with tungsten showed ∼90% less in vivo reduction of SeO 2−3 than cell incubated without tungsten. Tungsten inhibition suggests that a molybdenum-containing protein is involved in SeO 2−3 reduction [20], but known NO −2 reductases contain iron or copper at their active centers, not molybdenum. Thus, we must conclude that the SeO 2−3 reductase of this strain is a protein with a molecular weight of about 100 kDa or of about 45 kDa and that it is either a molybdenum-containing protein or that its activity is dependent on a molybdenum-containing protein. In a recent article, Basaglia et al. [1] described the presence of an enzymatic activity in R. sullae that reduced both NO −2 and SeO 2−3 and they attributed the activity to a copper-containing NO −2 reductase. Because the B1 strain is also a Rhizobium sp. it is likely that the activity that Basaglia et al. detected and ours are the same, although additional study would be needed to demonstrate a role for a copper NO −2 reductase in the reduction of SeO 2−3 by the B1 strain.
Native polyacrylamide gel electrophoresis of Rhizobium sp. B1 SeO 2−3 reductase activity. Lane A: selenite reductase (Rf’s of 0.32 and 0.49); lane B: nitrite reductase (Rf of 0.34); lane C: molecular weight markers. Selenite reductase activity appears as a dark spot of precipitated Se against a lighter background. Nitrite reductase activity appears as a light spot of oxidized methyl viologen against a darker background of reduced methyl viologen. Proteins appear as dark bands against a lighter background
DMSe and DMDSe Production
Microbial reduction of Se oxyanions might result in the formation of volatile compounds such as DMSe and DMDSe [12, 24]; however, neither was detected over cultures of the B1 strain.
Conclusions
The B1 strain is a Gram-negative motile rod that grows under aerobic and denitrifying conditions. The strain is a α-Proteobacteria belonging to the family Rhizobiaceae and the genus Rhizobium. It is related to R. radiobacter and R. rubi but does not belong to either species (Fig. 1). At O2 levels below 1 ppm DO, the strain utilized NO −3 as a respiratory e− donor. Neither SeO 2−3 nor SeO 2−4 served as e− donors for growth. Under oxic growth conditions and under denitrifying conditions, the strain reduced SeO 2−3 to Se0 but was unable to reduce SeO 2−4 to Se0. The maximum rate at which SeO 2−3 was removed from the medium, 0.20 µmol/mg protein/day, required that nitrate be present. The strain’s MIC for SeO 2−3 was between 8 and 16 mM, a level of resistance that exceeds that of several other “SeO 2−3 -resistant” bacteria but one that is lower than the MIC seen with several other “SeO 2−3 -resistant” bacteria. Electrophoresis of the strain’s cell-free extracts revealed two protein bands with SeO 2−3 reductase activity: one with a molecular weight of ∼100 kDa and the other with molecular weight of about 45 kDa. SeO 2−3 reduction appears to depend, directly or indirectly, on a molybdenum-containing protein. There was no evidence that the strain detoxified SeO 2−3 by reducing it to DMSe or DMDSe.
References
Basaglia M, Toffanin A, Baldan E, Bottegal M, Shapleigh JP, Casella S (2007) Selenite-reducing capacity of the copper-containing nitrite reductase of Rhizobium sullae. FEMS Microbiol Lett 269:124–130
Di Gregorio S, Lampis S, Vallini G (2005) Selenite precipitation by a rhizospheric strain of Stenotrophomonas sp. isolated from the root system of Astragalus bisulcatus: a biotechnological perspective. Environ Int 31:233–241
Doran JW (1982) Microorganisms and the biological cycling of selenium. Adv Microbial Ecol 6:17–32
Garbisu C, Carlson D, Adamkiewicz M, Yee BC, Wong JH, Resto E, Leighton T, Buchanan BB (1999) Morphological and biochemical responses of Bacillus subtilis to selenite stress. Biofactor 10:311–9
Ganther HE, OA Levander CA Sauman (1966) Dietary control of selenium volatilization in the rat. J Nutr 88:55–60
Greenberg AE, Clesceri LS, Eaton AD (eds) (1992) Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, American Water Works Association and Water Environmental Federation, Washington, DC, pp 82–93
Hunter WJ (2006) Removing selenate from groundwater with a vegetable oil-based biobarrier. Curr Microbiol 53:244–248
Hunter WJ (2007) An Azospira oryzae (syn Dechlorosoma suillum) Strain that reduces selenate and selenite to elemental red selenium. Curr Microbiol 54:376–381
Hunter WJ, Follett RF, Cary JW (1997) Use of vegetable oil to stimulate denitrification and remove nitrate from flowing water. Trans Am Soc Agric Eng 40:345–353
Hunter WJ, Kuykendall LD (2004) Determination of dimethylselenide and dimethyldiselenide by gas chromatography photoionization detection. J Chrom A 1038:295–297
Hunter WJ, Kuykendall LD (2006) Identification and characterization of an Aeromonas salmonicida (syn Haemophilus piscium) strain that reduces selenite to elemental red selenium. Curr Microbiol 52:305–309
Karlson U, Frankenberger WT Jr (1988) Determination of gaseous selenium-75 evolved from soil. Soil Sci Soc Am J 52:678–681
Kessi J, Ramuz M, Wehrli E, Spycher M, Bachofen R (1999) Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum Appl Environ Microbiol 65:4734–4740
Lawson S, Macy JM (1995) Bioremediation of selenite in oil refinery wastewater. Appl Microbiol Biotechnol 43:762–765
McCready RGL, Campbell JN, Payne JI (1966) Selenite reduction by Salmonella heidelberg. Can J Microbiol 12:703–714
Mokrasch LC, McGilvery RW (1956) Purification and properties of fructose-1,6-diphosphatase. J Biol Chem 221:909–917
Plotnikov VI (1958) Coprecipitation of small quantities of selenium with ferric hydroxide. Zh Neog Klim 3:1761–1766
Rosenberg B, Van Camp L, Kricas T (1965) Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 205:698–699
Roux M, Sarret G, Pignot-Paintrand I, Fontecave M, Coves J (2001) Mobilization of Selenite by Ralstonia metallidurans CH34 Appl Environ Microbiol 67:769–773
Schmitz RA, Albracht SPJ, Thauer RK (1992) Properties of the tungsten-substituted molybdenum formylmethanofuran dehydrogenase from Methanobacterium wolfei FEBS Lett 309:78–81
Tiedje JM (1982) Denitrification. In AL Page, RH Miller H, DR Keeney (eds) Methods of soil analysis, part 2. Chemical and microbiological properties. American Soil Association-Soil Science Society of America, Madison, WI Pages 1011–1026
Van Fleet-Stalder, Chasteen TG, Pickering IJ, George GN, Prince RC (2000) Fate of selenate and selenite metabolized by Rhodobacter sphaeroides. Appl Environ Microbiol 66:4849–4853
Whanger PD (2004) Selenium and its relation ship to cancer: an update. Br J Nutr 91:11–28
Zieve R, Ansell PJ, Young TWK, Peterson PJ (1985) Selenium volatilization by Mortierella species. Trans Br Mycol Sot 84:177–179
Acknowledgments
The author thanks Robin Montenieri and Kimberly LaCroix for their expert technical assistance. Manufacturer and product brand names are given for the reader’s convenience and do not reflect endorsement by the US government. This article was the work of US government employees engaged in official duties and is exempt from copyright.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hunter, W.J., Kuykendall, L.D. Reduction of Selenite to Elemental Red Selenium by Rhizobium sp. Strain B1. Curr Microbiol 55, 344–349 (2007). https://doi.org/10.1007/s00284-007-0202-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-007-0202-2