Abstract
The South African invasive legume Dipogon lignosus (Phaseoleae) produces nodules with both determinate and indeterminate characteristics in New Zealand (NZ) soils. Ten bacterial isolates produced functional nodules on D. lignosus. The 16S ribosomal RNA (rRNA) gene sequences identified one isolate as Bradyrhizobium sp., one isolate as Rhizobium sp. and eight isolates as Burkholderia sp. The Bradyrhizobium sp. and Rhizobium sp. 16S rRNA sequences were identical to those of strains previously isolated from crop plants and may have originated from inocula used on crops. Both 16S rRNA and DNA recombinase A (recA) gene sequences placed the eight Burkholderia isolates separate from previously described Burkholderia rhizobial species. However, the isolates showed a very close relationship to Burkholderia rhizobial strains isolated from South African plants with respect to their nitrogenase iron protein (nifH), N-acyltransferase nodulation protein A (nodA) and N-acetylglucosaminyl transferase nodulation protein C (nodC) gene sequences. Gene sequences and enterobacterial repetitive intergenic consensus (ERIC) PCR and repetitive element palindromic PCR (rep-PCR) banding patterns indicated that the eight Burkholderia isolates separated into five clones of one strain and three of another. One strain was tested and shown to produce functional nodules on a range of South African plants previously reported to be nodulated by Burkholderia tuberum STM678T which was isolated from the Cape Region. Thus, evidence is strong that the Burkholderia strains isolated here originated in South Africa and were somehow transported with the plants from their native habitat to NZ. It is possible that the strains are of a new species capable of nodulating legumes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many legumes (plant family Leguminosae (Fabaceae)) have the capacity to fix atmospheric N2 via symbiotic bacteria (‘rhizobia’) in root nodules, and this can give them an advantage under low soil N conditions if other factors are favourable for growth [4, 5, 45]. Generally, legume nodules can be classified as indeterminate or determinate in growth [45]. Indeterminate nodules have a persistent apical meristem, while determinate nodules have a transient meristem. Nodule type is dependent on host plant. Indeterminate nodules are more common, but all members of the legume tribes Desmodieae, Phaseoleae and Psoraleae that have been examined, and some members of the Loteae have determinate nodules [46]. One report was found of a legume species (Sesbania rostrata) capable of forming both indeterminate and determinate nodules [26]. Evidence indicated that the switch from indeterminate to determinate nodule was mediated by the plant hormone ethylene.
Bacterial species from genera in the alphaproteobacteria (Azorhizobium, Bradyrhizobium, Devosia, Ensifer, Mesorhizobium, Methylobacterium, Microvirga, Rhizobium, Ochrobactrum, Phyllobacterium) and betaproteobacteria (Burkholderia, Cupriavidus) can form functional nodules on specific legumes [7, 29]. Recent reports of nodulation of legumes by Pseudomonas sp. in the gammaproteobacteria [33, 44] have not been confirmed. Brazil is a principal centre of diversity of Burkholderia spp. that form functional nodules on legumes [30]. In South America, Mimosa spp. have been shown to be predominantly nodulated by Burkholderia spp. with Burkholderia caribensis, Burkholderia diazotrophica, Burkholderia mimosarum, Burkholderia nodosa, Burkholderia phenoliruptrix, Burkholderia phymatum, Burkholderia sabiae, Burkholderia symbiotica and Burkholderia tuberum confirmed to produce functional nodules on species within this legume genus [12, 14, 18, 42, 43, 47] and its close relatives in the tribe Mimoseae (subfamily Mimosoideae) [13, 50]. B. phymatum STM815 has been shown to nodulate more than 40 Mimosa species, a range of species in other genera of the tribe Mimoseae as well as several Acacia spp. in the subgenus Acacia [22, 23, 30].
There is also evidence that South Africa is a centre of diversity of legume-nodulating Burkholderia spp. Specifically, strains of Burkholderia spp. have been isolated from legumes (all in the subfamily Papilionoideae) in a range of sites in the Cape Floristic Region (CFR) and confirmed to produce functional nodules on Aspalathus linearis, Cyclopia spp., Hypocalyptus spp., Lebeckia spp., Podalyria canescens, Rhynchosia ferulifolia and Virgilia oroboides from this region [10, 19, 20, 24, 28, 30–32]. The B. tuberum-type strain STM678 was isolated from Aspalathus carnosa [51], and two strains isolated from Lebeckia ambigua were formally described as the new species Burkholderia sprentiae and Burkholderia dilworthii [20, 21] and one strain from R. ferulifolia as Burkholderia rhynchosiae [19]. Recent phylogenetic analyses of housekeeping and symbiosis genes of 69 Burkholderia rhizobial strains isolated from Cyclopia spp., Hypocalyptus spp., Podalyria calyptrata or V. oroboides indicated that the majority were novel, potentially representing further new species [10]. Where tested, Burkholderia rhizobial strains isolated from South African legumes had nodulation (nod) gene sequences identical or very similar to those of B. tuberum STM678T. The nod gene sequences of Burkholderia spp. capable of nodulating South African plants are clearly separated from those of Burkholderia spp. (including B. tuberum) shown to nodulate Mimosa spp., and the South African strains did not nodulate Mimosa spp. or other members of the Mimosoideae [30]. Strains of Burkholderia spp. capable of nodulating South African plants and those nodulating species in the Mimosoideae also separated clearly on the basis of their nitrogenase iron protein (nifH) gene sequences [37].
Dipogon lignosus is an herbaceous legume (tribe Phaseoleae) native to the Fynbos Biome of the Cape of South Africa which has become invasive in the Australian-Pacific region [35, 40]. In New Zealand (NZ), it is designated as an unwanted organism and is banned from sale, propagation and distribution and is immediately eradicated when found [40]. D. lignosus is known to produce nodules in its native South Africa, but the bacteria involved have not been characterised. Here, we firstly assessed if D. lignosus nodulates in NZ soils. On a finding that it did and that in some cases the nodules appeared indeterminate in structure, we then isolated and characterised the bacteria that produce functional nodules on D. lignosus in NZ and examined nodule structure.
Materials and Methods
Bacterial Strains
Ten bacterial isolates were obtained from nodules of different D. lignosus plants sampled at Dinsdale, Hamilton, NZ (37°47′ S, 175°14′ E; field site 1), in April 2011 (three isolates) and April 2012 (two isolates); Jesmond Park, Hamilton, NZ (37°47′ S, 175°17′ E; field site 2), in December 2012 (three isolates); and Mokau, Taranaki, NZ (38°41′ S, 174°37′ E; field site 3), in December 2012 (two isolates). The Dinsdale site is unmaintained gardens with a clay loam base, the Jesmond Park site is a city council park with a sandy loam base, while the Mokau site is coastal cliff/sand dune with a nearly pure sand base and organic enrichment in the top few centimetres. Two soil cores of 5–15 cm depth were sampled at the different field sites in November 2013. Nitrate (NO3 −) and ammonium (NH4 +) in 4.0 g fresh soil samples were extracted into 40 ml of 2 M KCl [11] and were measured colorimetrically [9, 36]. An approximate measure of soil water content at water holding capacity was obtained. Soil was added to 15-cm-height × 9-cm-diameter pots with a layer of cheese cloth at their base and was kept almost immersed in a beaker of water for 36 h. The pots were then removed from the water covered with plastic wrap and left to drain for 36 h. After this, the soil was weighed, dried at 105 °C for 24 h and reweighed, and g H2O kg−1 fresh weight soil was determined. Soil pH was determined from 10 g samples of sieved (2 mm mesh), air-dried soil (25 °C for 1 week) mixed in 25 ml 0.01 M CaCl2 [11]. Phosphate (‘Olsen P’) in 1.0 g sieved, air-dry soil was extracted into 20 ml of 0.5 M NaHCO3 [11] and was measured colorimetrically [38]. Total carbon and nitrogen content of 0.5 g sieved, air-dried soil was determined using a CN elemental analyser (Elementar VarioMax CN Elemental Analyser, GmbH, Hanau, Germany).
Soil pH was 1 unit greater, but soil C, N, NO3 −–N + NH4 +–N, Olsen P and water holding capacity were substantially lower at field site 3 than at field sites 1 or 2 (Table 1).
All bacterial isolates are deposited in the International Collection of Microorganisms from Plants (ICMP), Landcare Research, Auckland, NZ. Their ICMP numbers are given in the text. B. tuberum STM678T and B. phymatum STM815T were obtained from the University of York rhizobium collection, and Burkholderia phytofirmans PsJN = LMG22487 from the Bacteriology Group, International Centre for Genetic Engineering and Biotechnology, Padriciano, Trieste, Italy.
For isolates obtained in the current study, root nodules were surface sterilised by immersion in 96 % ethanol for 5 s and 5 % sodium hypochlorite for 3 min and then were rinsed with sterile water. Surface-sterilised nodules were crushed in sterile water, and this suspension was streaked onto yeast mannitol agar (YMA) [53] and was incubated at 20 °C in the dark for 2–4 days. A purified culture was obtained by repetitive subculture. Samples of all cultures were inoculated into a suspension of yeast mannitol broth (YMB) [53] and used for preparation of DNA or inoculum.
Sequencing of the 16S Ribosomal RNA, DNA Recombinase A and Symbiosis-Related Genes
DNA was extracted from the bacterial cultures using the Gentra Puregene DNA Purification Kit (Qiagen) following the protocol for gram-negative bacteria. Depending on bacterial isolate, up to five genes were sequenced: the small subunit ribosomal RNA (16S rRNA), DNA recombinase A (recA), nitrogenase iron protein (nifH), N-acyltransferase nodulation protein A (nodA) and N-acetylglucosaminyl transferase nodulation protein C (nodC). Primers for PCR amplification with their sequences and sources are shown in Table 2. All primers were manufactured by Integrated DNA Technologies, Auckland, NZ. All PCR amplifications were performed using the FastStart™ Taq DNA Polymerase kit (Roche Applied Science, Auckland) optimised for annealing temperature and primer concentration. The PCR products were resolved via gel electrophoresis (1 % agarose gel in 1× Tris-acetate-EDTA buffer) followed by staining with ethidium bromide and viewing under UV light. PCR products were sequenced by the Bio-Protection Research Centre Sequencing Facility, Lincoln University, Lincoln, NZ, and DNA sequence data were obtained via Sequence Scanner v 1.0 software (©Applied Biosystems) and were edited and assembled using DNAMAN Version 6 (©Lynnon Biosoft Corporation, Version 4.0).
Phylogenetic Analyses
DNA sequences for all five genes examined indicated that eight isolates were Burkholderia sp. For these isolates, sequences were aligned, and maximum likelihood trees were constructed with 1,000 bootstrap replications with partial deletion and an 80 % coverage cut-off using MEGA5 software [48]. Type strains of all ‘rhizobial’ Burkholderia spp. on the GenBank sequence database (www.ncbi.nlm.nih.gov/genbank) were used in all trees where available. In addition, type strains of the most closely related non-rhizobial Burkholderia spp. were included in the 16S rRNA, recA and nifH trees. The most closely related non-type strain rhizobial Burkholderia (RAU2i) [10] was included in the 16S rRNA tree, and all closely related non-type strain rhizobial Burkholderia were included in the nifH, nodA and nodC trees. Selected Bradyrhizobium, Methylobacterium and Microvirga spp. [8, 50] were included in the nodA and nodC trees. Cupriavidus taiwanensis LMG19424T was used as an out-group in the 16S rRNA, recA and nifH trees and Azorhizobium caulinodans ORS 571T as an out-group in the nodA and nodC trees. The MEGA5 model test was performed to select a model of nucleotide substitution, and the ‘best’ model (lowest Bayesian information criterion (BIC) score) was used for each gene. Only bootstrap probability values of ≥50 % are shown on the trees. The sequences obtained in this study have been deposited in the GenBank sequence database, and their accession numbers (GenBank Acc. No.) are shown in the figures and text.
In addition, the enterobacterial repetitive intergenic consensus (ERIC) PCR and repetitive element palindromic PCR (rep-PCR) banding patterns of the eight Burkholderia isolates and the B. phytofirmans-type strain were compared [52].
Nodulation and N2 Fixation Studies
Seeds of D. lignosus, Cyclopia subternata, Hypocalyptus sophoroides, P. calyptrata and V. oroboides were purchased from Silverhill Seeds, Kenilworth, Cape Town, South Africa. Seeds of Mimosa pudica and Phaseolus vulgaris cv. Chef’s choice were purchased from Kings Seeds (NZ) Ltd, Katikati, Bay of Plenty, NZ, and Yates NZ, Auckland, NZ, respectively. Seeds were surface sterilised in 5 % sodium hypochlorite for 15 min, soaked in concentrated sulphuric acid for 10–30 min if required, rinsed with deionised water and then germinated on water agar plates at room temperature in the dark.
After germination, D. lignosus, M. pudica and P. vulgaris seedlings were transferred to polyethylene terephthalate jars (two seedlings per jar) containing vermiculite and were supplied with a complete nutrient medium (pH 6.0) as described previously [49] except that 0.1 mM NH4NO3 was replaced by 0.5 mM NH4NO3. Plants were grown in a Conviron® Adaptis A1000-controlled environment cabinet and exposed to a 16-h photoperiod (400 μmol photons m−2 s−1) at a constant 22 °C. At 5–10 days after sowing, seedlings were inoculated with 5 ml of the appropriate rhizobial strain grown to log phase (~1 × 108 cfu ml−1); uninoculated plants supplied with YMB only were used as controls. There were six replicate jars per treatment. Plants were inspected at two weekly intervals for nodulation and, at 40–50 days after inoculation, were tested for nitrogenase activity using the acetylene reduction assay (ARA) [17]. After the ARA, rhizobial strains were isolated from three to six nodules per plant, and their 16S rRNA gene was sequenced. In all cases, the 16S rRNA sequence for the strain recovered from nodules after the ARA was identical to that of the strain used as an inoculant.
C. subternata, H. sophoroides, P. calyptrata and V. oroboides were grown in glass tubes (volume = 70 ml) half filled with vermiculite/perlite (1:1) and supplied with a modified Jensens N-free nutrient solution [24]. Plants were harvested at 60 days after inoculation with ICMP 19430, and effective nodulation was assessed as the presence of pink nodules (which were thus considered to be expressing the symbiosis-essential protein leghaemoglobin; Lb), and an obviously healthy plant with green leaves. This was further confirmed by checking the structure of the nodules and their occupation by Burkholderia using light microscopy and transmission electron microscopy (TEM) combined with immunogold labelling with a Burkholderia-specific antibody (see next section).
Nodule Structure
D. lignosus plants used for examination of nodule structure were grown at 25 °C, with a 16-h photoperiod (400 μmol photons m−2 s−1) in a controlled environment room. Plants were inoculated with strain ICMP 19430 and harvested 100 days after sowing. Some nodules were removed for light microscopy and TEM studies [23, 24]. Nodules were tested for the presence of Burkholderia spp. via immunogold labelling (plus silver enhancement) with antibodies raised against B. phymatum STM815 and C. taiwanensis LMG19424 [22].
Phenotypic Characteristics of Isolates
The ability of the bacterial isolates to grow over a range of pH was tested by inoculating each isolate onto YMA adjusted to eight different pH levels (4.0, 4.5, 5.0, 6.0, 7.0, 8.0, 9.0 and 10.0) using 5 M HCl or 5 M NaOH as required. Presence or absence of bacterial growth was determined visually after 7 days. Growth of the isolates at different water potentials was determined in YMB with polyethylene glycol (PEG) 6000 added as required to give 0, 5, 10, 15, 20 or 25 % w/v PEG [1]. Here, relative growth of the isolates at different water potentials was assessed spectrophotometrically as an absorbance at 420 nm (Abs420) after 4 days for fast-growing isolates and 7 days for the single slow-growing isolate. In addition, all isolates were tested for their ability to solubilise tricalcium orthophosphate [27] and siderophore production using the chrome Azural S method [2]. All phenotypic tests were carried out in triplicate.
Results
D. lignosus was nodulated in the three sites sampled. These nodules were pink inside and assumed to be functional. Ten bacterial isolates from these nodules produced functional nodules on D. lignosus on inoculation. The 16S rRNA sequences identified one isolate (ICMP 19864) as Bradyrhizobium sp. (1,255 bp, GenBank Acc. No. KF588689), one isolate (ICMP 19865) as Rhizobium sp. (1,229 bp, GenBank Acc. No. KF588690), both isolates from field site 3, and eight of the isolates, five from field site 1 and three from field site 2, as members of the genus Burkholderia (1,262–1,469 bp, Fig. 1). All Burkholderia isolates grew at pH 4.0–10.0, while the Rhizobium sp. and Bradyrhizobium sp. grew at pH 4.5–10.0, but not at pH 4.0 (data not shown). At 0 % PEG, Abs420 ranged from 0.940 to 1.568 for all isolates. At 25 % PEG, Abs420 ranged from 0.203 to 0.387 for all Burkholderia isolates, but was 0.004 and 0.005 for the Bradyrhizobium sp. and Rhizobium sp., respectively. All Burkholderia isolates showed phosphate solubilisation ability and siderophore production, but the Bradyrhizobium sp. and Rhizobium sp. isolates did not. The 16S rRNA sequence for Bradyrhizobium strain ICMP 19864 was identical to those of several Bradyrhizobium japonicum strains isolated from the crop plants Glycine max, Arachis hypogaea and Vigna unguiculata in different countries including China, the USA and Brazil. Similarly, the 16S rRNA sequence for Rhizobium strain ICMP 19865 was identical to those of several Rhizobium sp./Rhizobium leguminosarum/Rhizobium etli strains isolated from a range of crop species including Trifolium spp., Lathyrus spp., P. vulgaris and Pisum sativum in Poland, Japan, Spain, China, the USA and Peru. It is possible that these strains originated from crop inoculum which is widely used in New Zealand [3], and they were not studied further.
Phylogenetic tree of 16S rRNA gene sequences (ca. 1,235 bp) of eight bacterial isolates from Dipogon lignosus sampled in New Zealand soils [group 1 (black filled circle), group 2 (black filled square)], selected Burkholderia spp.-type strains and the most closely related non-type strain Burkholderia (Rau2i). Cupriavidus taiwanensis LMG19424T was used as an out-group. This tree was constructed using the MEGA5 software with the Tamura and Nei (TN93) gamma distribution (+G) with invariant sites (+I) model. GenBank accession numbers are in parentheses. Numbers on branches are bootstrap per cent from 1,000 replicates (shown only when ≥50 %). Scale bar = 5 % sequence divergence (five substitutions per 100 nucleotides). Superscript T indicates type strain
The eight Burkholderia isolates were separated into two groups on the basis of their 16S rRNA sequences: one of five isolates from field site 1 sampled over 2 years (group 1) and the other three isolates from field site 2 (group 2) (Fig. 1). Isolates within each group were identical, and the groups showed 99.83 % similarity (1,154 bp) to each other. Both groups were most closely related to but clearly separated from the B. phytofirmans-type strain (99.68 % similarity, 1,235 bp, group 1; 99.7 % similarity, 1,331 bp, group 2) (Fig. 1). Both groups were also closely related to Burkholderia sp. RAU2i isolated from Hypocalyptus coluteoides sampled at Storms River Bridge, CFR [10] (99.66 % similarity, 1,189 bp, group 1; 99.55 % similarity, 1,331 bp, group 2).
The eight Burkholderia isolates separated into the same two groups for their recA (406 bp), nifH (267–293 bp), nodA (363–426 bp) and nodC (507–519 bp) sequences as for their 16S rRNA sequences (Figs. 2, 3 and 4). Isolates within each group were identical for the recA, nifH and nodC sequences. For the recA sequences, the groups showed 99.17 % similarity (406 bp) to each other, and as for 16S rRNA sequences, both groups were most closely related to but clearly separate from the B. phytofirmans-type strain (98.28 % similarity, 406 bp, group 1; 99.01 % similarity, 406 bp, group 2).
Phylogenetic tree of recA gene sequences (ca. 406 bp) of eight bacterial isolates from Dipogon lignosus sampled in New Zealand soils [group 1 (black filled circle), group 2 (black filled square)] and selected Burkholderia spp.-type strains. Cupriavidus taiwanensis LMG19424T was used as an out-group. This tree was constructed using the MEGA5 software with the Tamura three-parameter (T92) gamma distribution (+G) model. GenBank accession numbers are in parentheses. Numbers on branches are bootstrap per cent from 1,000 replicates (shown only when ≥50 %). Scale bar = 5 % sequence divergence (five substitutions per 100 nucleotides). Superscript T indicates type strain
Phylogenetic tree of nifH gene sequences (ca. 267 bp) of eight bacterial isolates from Dipogon lignosus sampled in New Zealand soils [group 1 (black filled circle), group 2 (black filled square)], all closely related strains and selected type strains of Burkholderia. Cupriavidus taiwanensis LMG19424T was used as an out-group. This tree was constructed using the MEGA5 software with the Tamura three-parameter (T92) gamma distribution (+G) with invariant sites (+I) model. GenBank accession numbers are in parentheses. Numbers on branches are bootstrap per cent from 1,000 replicates (shown only when ≥50 %). Scale bar = 5 % sequence divergence (five substitutions per 100 nucleotides). Superscript T indicates type strain
Phylogenetic tree of a nodA gene sequences (ca. 363 bp) and b nodC gene sequences (ca. 507 bp) of eight bacterial isolates from Dipogon lignosus sampled in New Zealand soils [group 1 (black filled circle), group 2 (black filled square)], all closely related strains and selected type strains of Bradyrhizobium, Burkholderia, Methylobacterium and Microvirga. Azorhizobium caulinodans ORS 571T was used as an out-group. Both trees were constructed using the MEGA5 software, with the Tamura three-parameter (T92) gamma distribution (+G) model for nodA and the T92 + G invariant sites (+I) model for nodC. GenBank accession numbers are in parentheses. Numbers on branches are bootstrap per cent from 1,000 replicates (shown only when ≥50 %). Scale bar = 10 % sequence divergence (one substitution per ten nucleotides). Superscript T indicates type strain
The groups showed 98.22 % similarity (225 bp) to each other for nifH sequences. Here, in contrast with the 16S rRNA and recA sequences, the isolates were most closely related to B. tuberum STM678T isolated from A. carnosa in South Africa [51], B. rhynchosiae WSM3937T isolated from R. ferulifolia growing in relic rangeland near Darling, South Africa [19], and nine other strains isolated from different plants and sites in the CFR (Fig. 3). Indeed, nifH sequences (267–270 bp) for the five isolates of group 1 were identical to those of Burkholderia sp. UCT56 isolated from Cyclopia meyeriana sampled at Hottentots Holland mountains, CFR, and Burkholderia sp. RAU2c and Burkholderia sp. RAU2d2 isolated from H. coluteoides sampled at Storms River Bridge, CFR [10]. Also, nifH sequences (283–285 bp) for the three isolates of group 2 were identical to those of B. rhynchosiae WSM3937T and WSM3930 isolated from R. ferulifolia near Darling, South Africa [19].
The nodA sequences were identical for isolates within group 2 (427 bp) but showed 99.45–100 % similarity (363 bp) for group 1, and the groups showed 95.04–95.32 % similarity (363 bp) to each other. The nodC sequences for the two groups showed 96.06 % similarity (507 bp) to each other. As for the nifH sequences, both the nodA and nodC sequences clustered with B. tuberum STM678T, B. rhynchosiae WSM3937T and several other strains isolated from different plants and sites in the CFR (Fig. 4a, b). For nodA and nodC sequences, this group included the recently described B. sprentiae WSM5005T and B. dilworthii WSM3556T. Overall, sequences of the five genes examined were identical for the three Burkholderia isolates of group 2 and, with the exception of small differences in nodA sequences, identical for the five isolates of group 1. This indicates that each group of isolates may consist of clones of one strain. ERIC PCR and rep-PCR banding patterns indicated that this could be the case (Fig. S1).
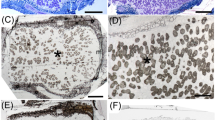
The structure of nodules formed on D. lignosus after inoculation with the Burkholderia strains isolated from plants growing in NZ soils suggested that they were effective, N2-fixing symbioses (Fig. 5a–d). Immunogold labelling with an antibody specific to the genus Burkholderia [22] confirmed that the bacteroids within the nodules were, indeed, Burkholderia (Fig. 5b, d–f). The D. lignosus nodules had two morphologies, spherical and determinate (Fig. 5a, b), and elongated with an apical meristem (Fig. 5c, d). The latter possessed an invasion zone behind the meristem that consisted of newly divided cells being invaded by infection threads, conveying and then releasing rhizobia into the host cytoplasm (Fig. 5g, h). Taken together, Fig. 5c, d, g, h indicates that the elongated nodules are of the indeterminate type. D. lignosus was nodulated by both B. tuberum STM678 and B. phymatum STM815 (Table 2). B. phytofirmans did not nodulate D. lignosus, and nod genes were not detected in this strain.
Light (a–d) and transmission electron microscopy (TEM) (e–h) of sections from the nodules of Dipogon lignosus at 100 days after inoculation with Burkholderia sp. strain ICMP 19430. a Spherical, determinate type nodule. The N2-fixing, infected cells are indicated with asterisk. b Serial section to a which has been immunogold labelled with an antibody against B. phymatum STM815, followed by silver enhancement to reveal that the antibody reacts strongly with the N2-fixing, infected cells (asterisk). c Elongated, indeterminate type nodule with a distinct branched apical meristem (arrows). The N2-fixing, infected cells are indicated with asterisk and the invasion zone with an arrowhead. d Serial section to c which has been immunogold labelled with an antibody against B. phymatum STM815, followed by silver enhancement to reveal that the antibody reacts strongly with the N2-fixing, infected cells (asterisk). e Bacteroids (b) in the infected cells that have been immunogold labelled with an antibody against B. phymatum STM815. The antibody has labelled the cell walls of the bacteroids (arrows). f Serial section to e that has been treated with non-immune serum substituted for the primary antibody. There is no gold labelling of the bacteroids (b). g Cells in the invasion zone of an elongated nodule similar to that shown in c and d that are in the process of being invaded by a transcellular infection thread (arrow). h An invasion zone cell containing infection threads (arrows). Note that a rhizobial cell has been released into the host cell cytoplasm (arrowheads). v vacuole, n nucleus, w cell wall. Bars, 100 μm (a, b), 200 μm (c, d), 1 μm (e–h)
Five Burkholderia strains isolated from D. lignosus nodulated P. vulgaris, but not M. pudica (Table 3). One strain (ICMP 19430) was tested and shown to produce N2-fixing nodules on C. subternata, H. sophoroides, P. calyptrata and V. oroboides.
Discussion
D. lignosus is known to produce nodules in its native South Africa, but the bacteria involved have not been characterised. Here, ten bacterial isolates were shown to produce functional nodules on D. lignosus. The 16S rRNA, recA, nifH, nodA and nodC gene sequences clearly identified eight of the isolates as members of the genus Burkholderia, while the two other isolates were in the alphaproteobacteria. The 16S rRNA sequences identified one as Bradyrhizobium sp. and the other as Rhizobium sp. D. lignosus was also nodulated by B. tuberum STM678 and B. phymatum STM815 (Table 2). These findings indicate that D. lignosus is promiscuous in relation to its rhizobial partners. This is the first description of rhizobia that nodulate D. lignosus. Also, this study has confirmed using microscopic techniques that papilionoid legumes endemic to the Western Cape region from the tribe Phaseoleae can contain Burkholderia as their symbionts [28]. The D. lignosus nodules were unusual in that they had two morphologies: spherical and determinate as generally observed in all other tribe Phaseoleae nodules [45] and elongated with an apical meristem, indicating that they are of the indeterminate nodule type. The ability to form dimorphic nodules is rare, but it does occur in S. rostrata [26], and there are unconfirmed reports of its occurrence in Kennedia and Erythrina spp., both in the tribe Phaseoleae [45].
A wide range of Bradyrhizobium, Mesorhizobium, Rhizobium and Ensifer strains have been isolated previously from legumes in NZ (www.landcareresearch.co.nz/resources/collections/icmp) [49, 55], but this is the first report of a Burkholderia sp. in NZ soils capable of nodulating a legume. The available data indicate that legume-nodulating Burkholderia are commonly, but not exclusively, associated with legumes growing in acidic, low nutrient/N and often periodically dry soils [22, 25, 37, 47]. Here, the Burkholderia isolates were obtained from D. lignosus sampled at field sites 1 and 2 which had a soil pH of 4.4 and 4.8, respectively, while the Bradyrhizobium sp. and Rhizobium sp. were obtained from plants at field site 3 which had a soil pH of 5.9. The Burkholderia isolates were able to grow at pH 4.0–10.0, while the Bradyrhizobium and Rhizobium isolates grew at pH 4.5–10.0, but not at pH 4.0. Thus, the Burkholderia isolates may have an advantage over the Bradyrhizobium and Rhizobium isolates in low pH soils. Soil N and P availability and water holding capacity were lower at field site 3 than at field sites 1 and 2, and these may be factors why Bradyrhizobium sp. and Rhizobium sp. were the D. lignosus symbionts here. However, this may not be the case as growth of the bacteria under different PEG concentrations indicated that the Burkholderia isolates had greater tolerance of water stress than the Bradyrhizobium sp. or Rhizobium sp. Also, all Burkholderia isolates showed phosphate solubilisation ability and siderophore production, but the Bradyrhizobium sp. and Rhizobium sp. did not. These abilities could give the Burkholderia isolates and their legume hosts an advantage in low P and Fe soils.
Evidence to date indicates that Brazil and South Africa are principal centres of diversity of Burkholderia that form functional nodules on legumes [30]. The South American and South African strains separated clearly on the basis of their nifH, nodA and nodC sequences and strains isolated from legumes in South Africa did not nodulate Mimosa spp. or other members of the Mimosoideae [30, 37]. The eight Burkholderia isolates from D. lignosus showed a very close relationship to Burkholderia rhizobia strains isolated from South African plants with respect to their nifH, nodA and nodC gene sequences. Also, the five isolates tested nodulated the promiscuous legume species P. vulgaris [34] (Table 2) which, like D. lignosus, is in the legume tribe Phaseoleae, but did not nodulate M. pudica which is nodulated by a wide range of Burkholderia spp. isolated from Mimosa and Piptadenia group spp. including B. phymatum STM815, but not B. tuberum STM678 and other South African isolates [12–14, 23, 37, 50]. One of the isolates (ICMP 19430) did, however, nodulate four South African species, including C. subternata and P. calyptrata, both of which have previously been shown to be nodulated by B. tuberum STM678 [24, 30]. These findings provide evidence that the strains originated in South Africa and were somehow transported with the plants from their native habitat to NZ. There is strong evidence that such long-distance transfer of Burkholderia spp. symbionts has occurred previously with South American Mimosa pigra naturalised in Taiwan [15]. Similarly, Cupriavidus strains sociated with Mimosa diplotricha and M. pudica in the Philippines are likely to have originated in Central America [6].
Against this, the eight strains separated clearly from all Burkholderia rhizobia species with respect to their 16S rRNA and recA gene sequences and showed greatest similarity to B. phytofirmans which has not been shown to be capable of nodulating a legume [47]. Also, in the current study, the B. phytofirmans-type strain did not nodulate D. lignosus, and neither nodA nor nodC genes were detected in this strain, indicating that it does not have the ability to nodulate legumes. However, recent work has shown that Burkholderia rhizobia associated with legumes of the CFR are highly diverse, and some such as Burkholderia sp. RAU2i have 16S rRNA sequences similar to B. phytofirmans [10]. The Burkholderia strains isolated here may be a novel Burkholderia sp. capable of nodulating legumes.
It is concluded that D. lignosus is promiscuous in relation to its rhizobial symbionts. Strains of alphaproteobacteria and Burkholderia sp. exist in NZ soils that can form functional nodules on D. lignosus; these nodules show both determinate and indeterminate characteristics. Burkholderia strains isolated from D. lignosus in NZ showed a much closer relationship to Burkholderia spp. isolated from South African plants than to those isolated from Mimosa spp., and it is likely that they originated in South Africa in association with D. lignosus. Further work is required to test if the strains are a new Burkholderia sp. capable of nodulating legumes.
References
Abdel-Salem MS, Ibrahim SA, Abd-El-Halim MM, Badawy FM, Abo-Aba SEM (2010) Phenotypic characterization of indigenous Egyptian rhizobial strains for abiotic stresses performance. J Am Sci 60:498–503
Alexander DB, Zuberer DA (1991) Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fertil Soils 12:39–45
Andrews M, Edwards GR, Ridgway HJ, Cameron KC, Di HJ, Raven JA (2011) Positive plant microbial interactions in perennial ryegrass dairy pasture systems. Ann Appl Biol 159:79–92
Andrews M, James EK, Sprent JI, Boddey RM, Gross E, dos Reis Jr FB (2011) Nitrogen fixation in legumes and actinorhizal plants in natural ecosystems: values obtained using 15N natural abundance. Plant Ecol Divers 4:131–140
Andrews M, Raven JA, Lea PJ (2013) Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann Appl Biol 163:174–199
Andrus AD, Andam C, Parker MA (2012) American origin of Cupriavidus bacteria associated with invasive Mimosa legumes in the Philippines. FEMS Microbiol Ecol 80:747–750
Ardley JK, Parker MA, De Meyer SE, Trengove RD, O’Hara GW, Reeve WG, Yates RJ, Dilworth MJ, Willems A, Howieson JG (2012) Microvirga lupini sp. nov., Microvirga lotononidis sp. nov., and Microvirga zambiensis sp. nov. are alphaproteobacterial root-nodule bacteria that specifically nodulate and fix nitrogen with geographically and taxonomically separate legume hosts. Int J Syst Evol Microbiol 62:2579–2588
Ardley JK, Reeve WG, O’Hara GW, Yates RJ, Dilworth MJ, Howieson JG (2013) Nodule morphology, symbiotic specificity and association with unusual rhizobia are distinguishing features of the genus Listia within the southern African crotalarioid clade Lotononis s.l. Ann Bot 112:1–15
Baethgen WE, Alley MM (1989) A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjeldahl digests. Commun Soil Sci Plant Anal 20:961–969
Beukes CW, Venter SN, Law IJ, Phalane FL, Steenkamp ET (2013) South African papilionoid legumes are nodulated by diverse Burkholderia with unique nodulation and nitrogen-fixation loci. PLoS One 8(7):e68406
Blakemore LC, Searle PL, Daly BK (1987) Methods for chemical analysis of soils. New Zealand Soil Bureau Scientific Report 80. Department of Scientific and Industrial Research, Lower Hutt, 103 pp
Bontemps C, Elliott GN, Simon MF, dos Reis Jr FB, Gross E, Lawton RC, Neto NE, Loureiro Mde F, de Faria SM, Sprent JI, James EK, Young JPW (2010) Burkholderia species are ancient symbionts of legumes. Mol Ecol 19:44–52
Bournaud C, de Faria SM, dos Santos JMF, Tisseyre P, Silva M, Chaintreuil C, Gross E, James EK, Prin Y, Moulin L (2013) Burkholderia species are the most common and preferred nodulating symbionts of the Piptadenia Group (tribe Mimoseae). PLoS One 8(5):e63476
Chen W-M, de Faria SM, Straliotto R, Pitard RM, Simões-Araùjo JL, Chou Y-J, Chou J-H, Barrios E, Prescott AR, Elliott GN, Sprent JI, Young JPW, James EK (2005) Proof that Burkholderia forms effective symbioses with legumes: a study of novel Mimosa-nodulating strains from South America. Appl Environ Microbiol 71:7461–7471
Chen W-M, James EK, Chou J-H, Sheu S-Y, Yang S-Z, Sprent JI (2005) β-Rhizobia from Mimosa pigra, a newly discovered invasive plant in Taiwan. New Phytol 168:661–675
Chen WM, Moulin L, Bontemps C, Vandamme P, Bena G, Boivin-Masson C (2003) Legume symbiotic nitrogen fixation by β-proteobacteria is widespread in nature. J Bacteriol 185:7266–7272
Cummings SP, Gyaneshwar P, Vinuesa P, Farruggia FT, Andrews M, Humphry D, Elliott GN, Nelson A, Orr C, Pettitt D, Shah GR, Santos SR, Krishnan HB, Odee D, Moreira FMS, Sprent JI, Young JPW, James EK (2009) Nodulation of Sesbania species by Rhizobium (Agrobacterium) strain IRBG74 and other rhizobia. Environ Microbiol 11:2510–2525
de Cunha C O, Zuleta LFG, de Almeida LGP, Ciapina LP, Borges WL, Pitard RM, Baldani JI, Straliotto R, de Faria SM, Hungria M, Cavada BS, Mercante FM, de Vasconcelos ATR (2012) Complete genome sequence of Burkholderia phenoliruptrix BR3459a (CLA1), a heat-tolerant, nitrogen-fixing symbiont of Mimosa flocculosa. J Bacteriol 194:6675–6676
De Meyer SE, Cnockaert M, Ardley JK, Trengove RD, Garau G, Howieson JG, Vandamme P (2013) Burkholderia rhynchosiae sp. nov. isolated from Rhynchosia ferulifolia root nodules from South Africa. Int J Syst Evol Microbiol 63:3944–3949
De Meyer SE, Cnockaert M, Ardley JK, Maker G, Yates R, Howieson JG, Vandamme P (2013) Burkholderia sprentiae sp. nov. isolated from Lebeckia ambigua root nodules from South Africa. Int J Syst Evol Microbiol 63:3950–3957
De Meyer SE, Cnockaert M, Ardley JK, Van Wyk B-E, Van Damme PA, Howieson JG (2014) Burkholderia dilworthii sp. nov isolated from Lebeckia ambigua root nodules from South Africa. Int J Syst Evol Microbiol. doi:10.1099/ijs.0.058602-0
dos Reis Jr FB, Simon MF, Gross E, Boddey RM, Elliott GN, Neto NE, Loureiro Mde F, de Queiroz LP, Scotti MR, Chen W-M, Norén A, Rubio MC, de Faria SM, Bontemps C, Goi SR, Young JPW, Sprent JI, James EK (2010) Nodulation and nitrogen fixation by Mimosa spp. in the Cerrado and Caatinga biomes of Brazil. New Phytol 186:934–946
Elliott GN, Chen W-M, Chou J-H, Wang H-C, Sheu S-Y, Perin L, Reis VM, Moulin L, Simon MF, Bontemps C, Sutherland JM, Bessi R, de Faria SM, Trinick MJ, Prescott AR, Sprent JI, James EK (2007) Burkholderia phymatum is a highly effective nitrogen-fixing symbiont of Mimosa spp. and fixes nitrogen ex planta. New Phytol 173:168–180
Elliott GN, Chen W-M, Bontemps C, Chou J-H, Young JPW, Sprent JI, James EK (2007) Nodulation of Cyclopia spp. (Leguminosae, Papilionoideae) by Burkholderia tuberum. Ann Bot 100:1403–1411
Elliott GN, Chou J-H, Chen W-M, Bloemberg GV, Bontemps C, Martinez-Romero E, Velázquez E, Young JPW, Sprent JI, James EK (2009) Burkholderia spp. are the most competitive symbionts of Mimosa, particularly under N-limited conditions. Environ Microbiol 11:762–778
Fernández-López M, Goormachtig S, Gao M, D’Haeze W, van Montagu M, Holsters M (1998) Ethylene-mediated phenotypic plasticity in root nodule development on Sesbania rostrata. Proc Natl Acad Sci U S A 95:12724–12728
Frey-Klett P, Chavatte M, Clausse M-L, Courrier S, Le Roux C, Raaijmakers J, Martinotti MG, Pierrat J-C, Garbaye J (2005) Ectomycorrhizal symbiosis affects functional diversity of rhizosphere fluorescent pseudomonads. New Phytol 165:317–328
Garau G, Yates RJ, Deiana P, Howieson JG (2009) Novel strains of nodulating Burkholderia have a role in nitrogen fixation with papilionoid herbaceous legumes adapted to acid, infertile soils. Soil Biol Biochem 41:125–134
Graham PH (2008) Ecology of the root nodule bacteria of legumes. In: Dilworth MJ, James EK, Sprent JI, Newton WE (eds) Nitrogen fixing leguminous symbioses. Springer, Dordrecht, pp 23–58
Gyaneshwar P, Hirsch AM, Moulin L, Chen W-M, Elliott GN, Bontemps C, Estrada-de los Santos P, Gross E, dos Reis Jr FB, Sprent JI, Young JPW, James EK (2011) Legume-nodulating betaproteobacteria: diversity, host range, and future prospects. Mol Plant Microbe Interact 24:1276–1288
Hassen AI, Bopape FL, Habig J, Lamprecht SC (2012) Nodulation of rooibos (Aspalathus linearis Burm. f.), an indigenous South African legume, by members of both the α-proteobacteria and β-proteobacteria. Biol Fertil Soils 48:295–303
Howieson JG, De Meyer SE, Vivas-Marfisi A, Ratnayake S, Ardley JK, Yates RJ (2013) Novel Burkholderia bacteria isolated from Lebeckia ambigua—a perennial suffrutescent legume of the fynbos. Soil Biol Biochem 60:55–64
Huang B, Lv C, Zhao Y, Huang R (2012) A novel strain D5 isolated from Acacia confusa. PLoS One 7(11):e49236
Laguerre G, Nour SM, Macharet V, Sanjuan J, Drouin P, Amarger N (2001) Classification of rhizobia based on nodC and nifH analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 147:981–993
Lewis G, Schrire B, Mackinder B, Lock M (2005) Legumes of the World. Royal Botanic Gardens, Kew
Mackereth FJH, Heron J, Talling JR (1978) Water analysis: some revised methods for limnologists. Sci Publ Freshw Biol Assoc 27:1–139
Mishra RPN, Tisseyre P, Melkonian R, Chaintreuil C, Miché L, Klonowska A, Gonzalez S, Bena G, Laguerre G, Moulin L (2012) Genetic diversity of Mimosa pudica rhizobial symbionts in soils of French Guiana: investigating the origin and diversity of Burkholderia phymatum and other beta-rhizobia. FEMS Microbiol Ecol 79:487–503
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural water. Anal Chim Acta 27:31–36
Poly F, Ranjard L, Nazaret S, Gourbière F, Monrozier LJ (2001) Comparison of nifH gene pools in soils and soil microenvironments with contrasting properties. Appl Environ Microbiol 67:2255–2262
Popay I, Champion P, James T (2010) Common weeds of New Zealand, 3rd edn. New Zealand Plant Protection Society, Inc., Christchurch
Sarita S, Sharma PK, Priefer UB, Prell J (2005) Direct amplification of rhizobial nodC sequences from soil total DNA and comparison to nodC diversity of root nodule isolates. FEMS Microbiol Ecol 54:1–11
Sheu S-Y, Chou J-H, Bontemps C, Elliott GN, Gross E, James EK, Sprent JI, Young JPW, Chen W-M (2012) Burkholderia symbiotica sp. nov., isolated from root nodules of Mimosa spp. native to North East Brazil. Int J Syst Evol Microbiol 62:2272–2278
Sheu S-Y, Chou J-H, Bontemps C, Elliott GN, Gross E, dos Reis Jr FB, Melkonian R, Moulin L, James EK, Sprent JI, Young JPW, Chen W-M (2013) Burkholderia diazotrophica sp. nov., isolated from root nodules of Mimosa spp. Int J Syst Evol Microbiol 63:435–441
Shiraishi A, Matsushita N, Hougetsu T (2010) Nodulation in black locust by the gammaproteobacteria Pseudomonas sp. and the betaproteobacteria Burkholderia sp. Syst Appl Microbiol 33:269–274
Sprent JI (2009) Legume nodulation a global perspective. Wiley-Blackwell, Chichester
Sprent JI, James EK (2007) Legume evolution: where do nodules and mycorrhizas fit in? Plant Physiol 144:575–581
Suárez-Moreno ZR, Caballero-Mellado J, Coutinho BG, Mendonça-Previato L, James EK, Venturi V (2012) Common features of environmental and potentially beneficial plant-associated Burkholderia. Microb Ecol 63:249–266
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tan HW, Weir BS, Carter N, Heenan PB, Ridgway HJ, James EK, Sprent JI, Young JPW, Andrews M (2012) Rhizobia with 16S rRNA and nifH similar to Mesorhizobium huakuii but novel recA, glnll, nodA and nodC genes are symbionts of New Zealand Carmichaelinae. PLoS One 7(10):e47677
Taulé C, Zabaleta M, Mareque C, Platero R, Sanjurjo L, Sicardi M, Frioni L, Battistoni F, Fabiano E (2012) New betaproteobacterial rhizobium strains able to efficiently nodulate Parapiptadenia rigida (Benth.) Brenan. Appl Environ Microbiol 78:1692–1700
Vandamme P, Goris J, Chen WM, de Vos P, Willems A (2002) Burkholderia tuberum sp. nov. and Burkholderia phymatum sp. nov., nodulate the roots of tropical legumes. Syst Appl Microbiol 25:507–512
Versalovic J, Koeuth T, Lupski JR (1991) Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19:6823–6831
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. IBP Handbook No 15. Blackwell Scientific Publications, Oxford
Vinuesa P, Silva C, Lorite MJ, Izaguirre-Mayoral ML, Bedmar EJ, Martinez-Romero E (2005) Molecular systematics of rhizobia based on maximum likelihood and Bayesian phylogenies inferred from rrs, atpD, recA and nifH sequences, and their use in the classification of Sesbania microsymbionts from Venezuelan wetlands. Syst Appl Microbiol 28:702–716
Weir BS, Turner SJ, Silvester WB, Park D-C, Young JM (2004) Unexpectedly diverse Mesorhizobium strains and Rhizobium leguminosarum nodulate native legume genera of New Zealand, while introduced legume weeds are nodulated by Bradyrhizobium species. Appl Environ Microbiol 70:5980–5987
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Young JM, Park D-C, Weir BS (2004) Diversity of 16S rDNA sequences of Rhizobium spp. implications for species determinations. FEMS Microbiol Lett 238:125–131
Acknowledgments
WYYL was financially supported by a Lincoln University doctoral scholarship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Agarose gel electrophoresis of ERIC-PCR and rep-PCR fingerprinting patterns from genomic DNA of Burkholderia sp. isolates recovered from the nodules of Dipogon lignosus and the Burkholderia phytofirmans-type strain. Lanes 1 and 20, 1-kb plus DNA ladder (Invitrogen, Australia); lanes 2-10, ERIC-PCR fingerprinting patterns; lanes 11-19, rep-PCR fingerprinting patterns. Isolates are indicated at the top of each lane (GIF 97 kb)
Rights and permissions
About this article
Cite this article
Liu, W.Y.Y., Ridgway, H.J., James, T.K. et al. Burkholderia sp. Induces Functional Nodules on the South African Invasive Legume Dipogon lignosus (Phaseoleae) in New Zealand Soils. Microb Ecol 68, 542–555 (2014). https://doi.org/10.1007/s00248-014-0427-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-014-0427-0