Abstract
Bioaerosols in cave air can serve as natural tracers and, together with physical parameters, give a detailed view of conditions in the cave atmosphere and responses to climatic changes. Airborne microbes in the Postojna Cave system indicated very dynamic atmospheric conditions, especially in the transitory seasonal periods between winter and summer. Physical parameters of cave atmosphere explained the highest variance in structure of microbial community in the winter and in the summer. The airborne microbial community is composed of different microbial groups with generally low abundances. At sites with elevated organic input, occasional high concentrations of bacteria and fungi can be expected of up to 1,000 colony-forming units/m3 per individual group. The most abundant group of airborne amoebozoans were the mycetozoans. Along with movements of air masses, airborne algae also travel deep underground. In a cave passage with elevated radon concentration (up to 60 kBq/m3) airborne biota were less abundant; however, the concentration of DNA in the air was comparable to that in other parts of the cave. Due to seasonal natural air inflow, high concentrations of biological and inanimate particles are introduced underground. Sedimentation of airborne allochthonous material might represent an important and continuous source of organic material for cave fauna.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air contains different concentrations of dust and biological particles such as insects, cells, spores, viruses, and endotoxins. For aerosols, air serves as a mode of transport from one location to another. There is variability in survival between groups of microorganisms, and generally, fungal spores, enteric viruses, and amoebic cysts are, to a certain extent, resistant to environmental stress during air transport [1]. Several environmental stressors influencing viability are: UV radiation, chemical pollutants, air ions, desiccation, oxygen (in)availability, temperature extremes, pressure fluctuations, seasonality, and cellular interactions [2, 3].
The external climate is subjected to rapid changes of atmospheric parameters due to wind, insolation, rain, changes of air pressure, etc. Some underground voids and caves are relatively well isolated from external influences, but, to a certain level, they reflect conditions of the external climate with a delay [4]. When considering large cave systems, to describe cave climate, one has to take into account the presence of different niches with special characteristics, e.g., number of entrances, proximity to the surface, sizes of underground chambers, presence of an underground stream, and ramification and fracturization of cave passages.
Study of airborne microbes in caves that enable human access offers certain advantages compared with the external environment: (1) in show caves with mass tourism, human influence and alterations of the atmosphere can be directly measured, (2) conditions reflect the external climate including seasonality and pollutants, (3) interactions between autochthonous and allochthonous airborne microbes can be unravelled and their survival rate related to different parameters. Survival rates of airborne microbes can be studied more easily because of the absence of stress factors such as UV and desiccation, but, on the other hand, elevated concentrations of radon and its progeny in some parts of caves might have a significant influence on airborne microbiota. Radon (222Rn, α radioactive transformation, half-life, t 1/2 = 3.82 days) is a ubiquitous, naturally occurring radioactive noble gas that is formed in minerals by radioactive transformation of radium (226Ra) within the uranium (238U) natural decay chain [5]. It migrates through the ground by diffusion or advection (together with carrier gases, e.g., CO2, CH4, N2), accumulates in underground rooms (e.g., karst caves and mines), and eventually enters buildings or exhales into the outdoor atmosphere [6]. Radon is always accompanied by its progeny (218Po, 214Pb, 214Bi, and 214Po) formed by its radioactive transformation and appearing in air as nano aerosols. Together, radon and radon progeny contribute more than half to the world average effective dose a member of the general public receives from all natural radioactive sources [7]. High radon concentrations are harmful for human health and are therefore a serious social concern. Similar to radon, CO2 is also dangerous in high concentrations, but its presence in air of karst caves is indispensable for their existence, because it governs speleogenetic processes [8]. In the cave air, its concentration is determined by the inflow of outside air, biogenic activity of soil, degradation of organic matter and carbonate, degassing from water, and human activity. Therefore, ventilation of the cave is crucial for its conservation [9]. Air movement in the cave, inflow of fresh air, and release of cave air into the outdoor atmosphere in caves is mainly caused by temperature differences between the cave air and outdoor air, resulting in more intensive ventilation in winter, and thus lower concentrations of both gases, than in summer.
In caves, many microorganisms already enter airborne from external air, while some become airborne underground, for example, due to water splashing. Cave air can act as a reservoir of biogenic particles. There are several important issues concerning airborne microorganisms in caves: (1) dispersion of biohazardous particles in cave air, such as Histoplasma capsulatum [10], (2) monitoring of underground health centers, which use speleotherapy to heal bronchial, allergic, and rheumatic diseases. The healing effect is attributed to special properties of cave air in the subterranean spaces, such as stability of the temperature, humidity, pressure, and content of gaseous components [11]. Additionally, (3) in caves with prehistoric art, cave air acts as a vehicle for the dispersion of microorganisms responsible for biodeterioration of paintings [12].
The objectives of this study were to test whether bioaerosols can act as indicators of changes in cave climate, to explore their distribution patterns in relation to seasonality, to compare abundances of small airborne microbes with larger ones, such as free-living amoebae, and to monitor influences of human exploitation of caves and elevated concentrations of radon and its progeny on airborne microbes.
Materials and Methods
Study Sites
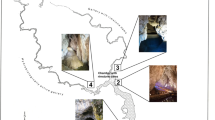
The Postojnska jama (Postojna Cave, Slovenia) cave system was selected as study site due to its large size and the existence of well-defined atmospheric niches as a result of gradually changing conditions in the atmosphere. The Postojna Cave system is composed of climatically very diverse parts, some being rather static while others are changing hourly [13]. The biggest climatic changes occur along the underground Pivka River and at the cave entrances. Decrease in humidity of the cave air is most intense in the winter; condensation is most intensive in the transitory phases between winter and summer and from summer to winter [14]. The entire cave system is 20.57 km long and comprises five historic caves with separate entrances (Postojnska jama, Otoška jama, Magdalena jama, Črna jama, and Pivka jama), which all are accessible for humans, and a few openings too small for entry, including a recently discovered passage (Rov pri Mumiji) with strong air flow in the cold season (Fig. 1). The Postojna Cave system was formed in Cretaceous limestone [15]. Because of its size, historical importance, cave formations, a regularly operating underground tourist train, and its reputation as the cradle of speleobiology research, it attracts more than 500,000 tourists annually. In the peak of tourist visits in 1985, the cave received almost one million visitors.
Postojna Cave system (Postojnska jama, Otoška jama, Magdalena jama, Črna jama, Pivka jama) with sampling sites (1 main entrance, 2 Veliki dom, 3 Pisani rov, 4 Male jame, 5 Spodnji Tartar, 6 Dvorana cevčic, 7 Rdeča dvorana, 8 Otoška jama, 9 Otoška jama/Pivka, 10 Črna jama). Ground plan from Cave Cadastre of the Karst Research Institute ZRC SAZU
Ten well-defined sampling sites were selected in the Postojna Cave system, including sites in the touristic part as well as sites in the wild part (Fig. 1). Site no. 1 is a natural opening of the cave where the external atmosphere interacts with the cave. During the cold and warm seasons, there is natural air inflow and outflow, respectively. Among the sites tested, the entrance has the largest fluctuations of atmospheric parameters. It has been used for tourist tours since 1866. Sampling site no. 2 (Veliki dom) was selected as the first site in the cave, where sampling of aerosols formed by water splash and wave action, and, consequently, mist formation from the underground Pivka River is possible. In the past, the water course in this part of the cave was regulated with a barrier constructed to maintain a permanent lake at the ponor (site where a surface stream flows underground) in order to minimize external influences on the cave environment. The next sampling site (no. 3) was selected outside the natural main air inflow and Pivka river course, in a part of the cave named Pisani rov. In the atmosphere of this passage, elevated levels of Rn concentration are constantly observed throughout the whole year by continuous monitoring. A site close to the tourist railways (5 m) along the main cave passage was selected as sampling site no. 4 in order to observe the contribution of the train to the distribution of aerosols and dust. Vermiculites give distinct leopard skin-like patterns and are sometimes found on cave walls. At one site with these formations and preserved cave sediments (in the section named Spodnji Tartar, no. 5), air was sampled to study microbe release into the air from sediments to the total airborne microbial biomass. Sampling site no. 6 was selected in the tourist walking zone Lepe jame to observe the influence of mass tourism on the air quality. Tourists visit 5.3 km of the entire cave system; the sight-seeing tour is composed of a 3.5-km round trip by the tourist train and a 1.8-km walking tour. Karst caves generally have many fissures and cracks that are connected with the external environment. Due to changes of temperature and partial air pressure linked to the cavernosity, the air circulates in caves. In Rdeča dvorana, local air circulation is present regularly, and thus sampling site no. 7 was established there. Bats sometimes use caves to roost, hibernate, and/or to reproduce. In caves, they usually form colonies on ceilings and walls not very far from the cave opening but deep enough in the cave that atmospheric conditions are stable. Sampling site no. 8 was set up in Otoška jama in the immediate vicinity of an area populated by lesser horseshoe bats Rhinolophus hipposideros, which form solitary colonies. This sampling site was selected to monitor the influence of bats (their presence and also small quantities of bat droppings) on the composition of airborne microbiota. Sampling site no. 9 was also selected in Otoška jama, at a site with the flowing underground Pivka River in order to compare the atmospheric parameters and airborne microbes 980 m downstream from the Pivka’s ponor, sampling site no. 2. Sampling site no. 10 was set up in Črna jama at the beginning of a section named Matevžev rov. This sampling site represents a site of minor human interference that rarely experiences human visits (Fig. 1).
Atmospheric Parameters
In order to get an insight into seasonal dynamics of aerosols in the cave system, sampling of cave air was performed in March, September, and December of 2009, and in June of 2010. At each sampling site in the cave, temperature, relative humidity (RH), air currents, and air pressure were measured using a portable Kestrel 4500 Pocket Weather Tracker (USA). The carbon dioxide concentration in the atmosphere was measured with a handheld carbon dioxide meter MI70 Vaisala (Finland) for 10 min, which is suitable for demanding spot measurements. For statistical analyses, the mean value of CO2 was used. For instantaneous Rn concentration measurements, grab sampling method was used. Cave air was sampled into 0.7 l alpha scintillation cells manufactured at the Jožef Stefan Institute [16]. After 3 h, when the secular equilibrium between radon and its short-lived progeny had been reached, gross alpha activity of 222Rn, 218Po, and 214Po in scintillation cells was measured in the laboratory using a PRM 145 alpha-counter (AMES, Slovenia), and activity concentration of radon was calculated. In addition, continuous all-year-round radon monitoring was performed at site no. 3 using the commercially available Barasol MC-450 probe (ALGADE, France) to record radon concentration, barometric pressure, and temperature in cave air once per hour. It consists of a cylindrical measurement chamber, which is connected with the outside air through a diffusion filter. The decay of 222Rn is detected by a solid-state silicon detector. The total amount of dust (airborne particles) was measured at each sampling site. A minimum of 60 l of air was filtered through dry and pre-weighed 0.22-μm Millipore (USA) filters with a diameter of 25 mm. After drying the filters for 72 h in a laboratory glass exsiccator, filters were weighed again, and the difference in weight was calculated as total dust concentration expressed in milligrams per cubic meter. For dust sampling in the cave, a portable pump Apex Pro-Personal Air Sampler Casella (UK) was used (Table 1).
Culture Media
Common microbiological cultivation media were used to estimate the cultivable part of the airborne microbiota; that is, 1.5 % nutrient agar (NA, Sigma, USA) for total bacterial count and 1.5 % malt extract agar (MEA, Sigma) for fungi. NA is widely used to isolate bacteria, while MEA is frequently used as a fungal isolation medium [2]. Jaworski agar (1.0 %) was adopted to estimate cyanobacterial and algal living cells [17]. No antibacterial and antifungal substances were added in the media. Each colony-forming unit was screened under a zoom stereomicroscope (Nikon SMZ800) to observe the morphology of colonies. Amoebae were cultured in Petri dishes on 1.5 % non-nutrient agar (agar no. 1, Oxoid, Vienna, Austria) coated with 100 μl of a heat-killed Escherichia coli suspension (at a concentration of ~107 cells/ml) in phosphate buffered saline (PBS).
Impacting of Bioaerosols
A Mas-100 Air Sample Device (Merck, Germany) was used to inoculate all microbiological media. The Mas-100 impactor aspirates air through a perforated plate, and the resulting air-stream, which contains aerosol particles, is directed onto the agar surface of a Petri dish. After initial experiments of impacting agar media with 50, 250, and 1,000 l and counting the colony-forming units (CFU), 500 and 1,000 l were chosen as optimal volumes due to relatively low counts in cave air. A volume of 1,000 l is the highest recommended air volume for the device with air inflow at 100 l/min (Merck). At low aspired volumes, no or very few colonies were observed. To avoid cross-contamination, the perforated plate of the impactor was sterilized with 96 % ethanol before each sampling. After impacting plates with NA and MEA, media were cultivated in Postojna Cave (11 °C) for 3 weeks. After the incubation period, bacterial and fungal colonies were counted and expressed as CFU per cubic meter. Counted CFU values were statistically corrected according to Feller [18]. This correction describes the presumption that, as the number of viable particles being impacted on a given surface increases, the probability of the next particle going onto an empty space decreases. Jaworski agar plates were cultivated under the following conditions—20 °C, in an 8:16 light/dark period with photosynthetic photon flux (PPFD) of 10 μmol photons/m2s. A Licor Li-1000 DataLogger (USA) was used to set the PPFD. After 4 weeks of incubation, Jaworski plates were screened for the presence of cyanobacterial and algal colonies, counted, and then expressed as CFU per cubic meter (Table 1). Air sampling for bacteria and fungi was performed in triplicate 1.5 m above the ground.
Gravimetric Depositional Sampling of Airborne Amoebae
We focused on the so-called amphizoic amoebae, meaning free-living amoebae that can cause disease in humans [19]. Because in a pilot survey concentrations of amoebae in cave air were found to be very low, a depositional sedimentation method using the plate culture method was employed instead of impacting. In brief, opened Petri dishes with non-nutrient agar coated with heat-killed E. coli were exposed to the cave atmosphere for 1, 3, and 5 days, with at total of three plates at each sampling site (Table 1). Then, plates were closed, sealed with parafilm, and then incubated for 3 weeks at ~11 °C to simulate cave temperature. Plates were regularly screened for amoebal growth by phase contrast microscopy (Nikon Eclipse E800). Detected amoebae were isolated from the plates with a sterile scalpel and sub-cultured as clonal strains on non-nutrient agar plates precoated with a 48-h-old culture of E. coli in brain–heart-infusion.
All amoebal isolates were identified down to the genus level according to the identification key of Page [20], mainly based on morphologic characters. All amphizoic amoebae found were additionally subjected to 18S rDNA sequencing (see below). In order to also detect non-cultivable amphizoic amoebae, sterile 0.22-μm Millipore filters (USA) with a diameter of 47 mm were exposed to the cave atmosphere for 3 or 4 days. Total DNA of sampled non-cultivable amoebae was then isolated from the filters and used for two polymerase chain reactions (PCRs), a universal amoebozoan PCR, and a PCR specific for the genus Acanthamoeba (see below).
DNA Isolation
DNA was isolated from cultures by harvesting approximately 105 amoebae from the plate cultures with a sterile swab into sterile PBS, centrifuging at 500×g for 10 min and inoculating the pellet resuspended in 200 μl PBS into the Qiamp DNA Kit (Qiagen, Hilden, Germany). For DNA isolation from the filters, the Qiamp DNA Blood Kit was used following the protocol for filter samples with prior overnight incubation at −20 °C. Total DNA concentration was measured using a Nanodrop photometer (Peqlab, Erlangen, Germany) and expressed as nanograms DNA per 100 cm2.
Molecular Identification of Amphizoic Amoebae
An Acanthamoeba-specific PCR [21] was performed with all Acanthamoeba strains isolated by the agar plate method and also with all DNA samples extracted from the filters. This PCR amplifies a 423–551 bp fragment of the 18S rRNA gene that allows genotype identification after DNA sequencing. PCR was performed with 1, 3, and 6 μl of whole cell DNA, in 50 μl reaction volumes, and the following amplification program—45 cycles; 95 °C for 1 min, 60 °C for 1 min, 72 °C for 2 min.
For all non-Acanthamoeba culture isolates and for detection of amphizoic amoebae in the filter samples, the universal eukaryotic P2 and P3r primers [22] were used, which specifically bind to strongly conserved regions within the eukaryotic 18S rRNA gene. In amoebozoans and excavates, the two taxa containing amphizoic amoebae [23], the amplicon is around 500 bp long and allows identification down to the genus level after sequencing. A standard amplification program with 30 cycles of 1 min at 95 °C, 2 min at 52 °C, and 3 min at 72 °C was used for PCR, employing 1, 3, and 6 μl of total DNA.
A genotype T4 Acanthamoeba castellanii strain (strain 1BU, ATCC PRA-105) was used as a positive control for both PCRs. The amplification of the respective PCR products was visualized by ethidium bromide in an agarose gel electrophoresis and compared with a 50-bp step marker (Peqlab, Erlangen, Germany). Amplicons were sequenced by direct sequencing from the PCR product using the ABI PRISM® BigDye sequencing kit and a 310 ABI PRISM® automated sequencer (Applied Biosystems, Langen, Germany). Sequences were obtained twice from both strands. Consensus sequences were compared with published sequences from GenBank; multiple sequence alignments were performed by stepwise pairwise alignment using the CLUSTAL X application [24]. The alignments were assessed by eye and revised manually using the GeneDoc sequence editor [25]. Acanthamoeba genotyping was achieved with the model assumption of a <5 % sequence dissimilarity within one genotype as established by Gast et al. [26].
Inflow of Aerosols Underground
The inflow of aerosols underground was calculated based on mean concentrations of microorganisms (in CFU per cubic meter) and dust (milligrams per cubic meter) in the air at the cave entrance in the cold part of the year (site no.1, measurements from 27 March 2009 and 18 December 2009). Dry weight was calculated for each microbial group separately (bacteria, fungi, and algae). To calculate biomass, we used for bacteria an estimate of dry weight for an E. coli cell [27]; for fungi, dry weight of a Penicillium spore [28], and for algae, dry weight of a Chlorella cell [29]. Furthermore, it was assumed that, in the cave opening (30.01 m2), bioaerosols were distributed homogenously. All values for individual microbial groups were summed up what represented the total microbial dry weight in the air at the cave entrance (Eq. 1). Moreover, we assumed this concentration of microbial biomass is pushed continuously in the cave with different air inflow velocities (0.1, 0.5, 0.8, 1.0, 1.2, and 1.5 m/s) for certain periods (1 day, 30, and 90 days) through the cave opening (Eq. 2).
Statistical Analyses
Canonical correspondence analysis (CCA) was used to correlate data on abundance of microbial groups, bacteria, fungi, algae, and amoebae to environmental variables for each sampling round. Parametric multivariate analyses were run by the program package CANOCO 4.5 [30]. Significance of environmental variables in CCA was tested by a Monte Carlo permutation test. In the analyses, no transformations of the environmental data were applied.
Results
Cave Atmosphere
Monitoring of atmospheric parameters (temperature, RH, CO2, radon, dust) revealed that each sampling site of the cave air had its own characteristics. As expected, throughout the 2-year monitoring period (2009–2010), temperature oscillated the most in the cave entrance (site no. 1, min. −4.6 °C, max. +15.0 °C) and at the ponor of the underground river Veliki dom (no. 2, min. +2.5 °C, max. 15.2 °C). Temperature was most stable at sites no. 5 (Spodnji Tartar, min. 10.7 °C, max. 11.9 °C) and no. 6 (Dvorana cevčic, min. 12.1 °C, max. 13.2 °C, Fig. 2).
RH had the highest oscillations in the entrance (no. 1, min. 59.2 %, max. 99.2 %) and at site no. 8 (Otoška jama, min. 66.3 %, max. 92.0 %), which is relatively close to the exterior of the cave system. Sites no. 4 (Male jame, min. 89.1 %, max. 99.2 %), no. 3 (Pisani rov, min. 78.5 %, max. 91.3 %), and no. 5 (Spodnji Tartar, min. 75.5 %, max. 88.3 %) showed the smallest RH fluctuations.
Concentrations of CO2 were the highest and had the biggest oscillations at sites no. 3 (min. 962 ppm, max. 2,483 ppm) and no. 6 (Dvorana cevčic, min. 560 ppm, max. 1,701 ppm), the latter being a site with regular tourist visits. The most stable CO2 values in the cave system were found at site no. 7 (Rdeča dvorana, min. 600 ppm, max. 966 ppm) and no. 5 (min. 872 ppm, max. 1,387 ppm, Fig. 3).
Because of the natural air flow described above, instantaneous radon concentration in the cave air exhibits a seasonal pattern, with significantly higher values in warmer than in colder periods of the year, the effect being more pronounced at measurement sites with less ventilation. The biggest fluctuations were noticed at sites no. 3 (min. 3,040 Bq/m3, max. 6,700 Bq/m3) and no. 4 (min. 152 Bq/m3, max. 2,370 Bq/m3). Radon concentration was more stable at sites no. 5 (min. 1,253 Bq/m3, max. 2,040 Bq/m3) and no. 7 (min. 620 Bq/m3, max. 1,361 Bq/m3, Fig. 3).
Total dust concentrations were highest at sites close to cave openings and gradually diminished toward the cave interior, i.e., entrance, site no. 1 (min. 1.8 mg/m3, max. 16.6 mg/m3), followed by site no. 2 (min. 0.0 mg/m3, max. 13.7 mg/m3) and no. 8 (Otoška jama, min. 0.0 mg/m3, max. 9.2 mg/m3). The lowest concentrations and fluctuations were found at sites no. 10 (Črna jama, min. 1.5 mg/m3, max. 4.9 mg/m3) and no. 9 (Otoška jama/Pivka, min. 3.3 mg/m3, max. 7.1 mg/m3, Fig. 4).
Air pressure conditions were rather constant per sampling round throughout the entire cave system.
Bioaerosols in Cave Air
Cultivating air samples on NA to enumerate bacteria and on MEA to calculate fungi revealed that average counts of fungi usually exceed bacterial counts, except at site no. 6 (Dvorana cevčic) where tourists walk by during their visit. During the 2-year sampling period concentrations of bacteria and fungi in most of the cave systems ranged between 10 and 100 CFU/m3 (Fig. 5). A notable increase of bacterial counts above 100 CFU/m3 was observed at the ponor (no. 2) where water splashes and forms aerosols and along the tourist pathway (no. 6) where tourists daily visit the cave, with a peak in the summer period. Higher counts of fungi in the cave air were detected at the entrance (no. 1), at the ponor (no. 2), and along the underground railway (no. 4). Also at this site, the biggest difference between bacterial and fungal counts was observed. Morphologically, the section named Pisani rov (site no. 3) is similar to other parts of the cave, has the highest radon concentration on one hand, and on the other the lowest concentration of airborne microorganisms (Figs. 3 and 5). Because of high radon levels at site no. 3, continuous monitoring (with frequency of data recording once an hour) was introduced. It was shown that radon concentration frequently reaches up to 60,000 Bq/m3 during summer and also in other seasons when outdoor temperature was high enough to reduce natural air draught [31]. Compared with instantaneous radon concentrations obtained during four measurements (Fig. 3), values of continuous monitoring were about ten times higher during the periods with stagnant air in the cave, i.e., when natural air draught was minimal or practically stopped. The high values recorded at site no. 3 are an exception, and at all other sites along the tourist trail, long-term average radon concentrations were much lower, in the range from 2,000 to 4,500 Bq/m3 [32]. Site no. 8, occasionally populated by individuals of the bat species R. hipposideros, did not show any elevated microbial counts in the air.
CCA analysis was performed on the basis of ten variables, if available: temperature, RH, air pressure, air flow, CO2, Rn, dust, total DNA concentration, shortest distance to the underground river from each individual sampling site, and shortest distance to a known cave entrance. Moreover, a separate analysis was performed for each sampling round because, close to sampling sites 3, 4, 5, and 7, a new passage was recently discovered, which communicates with the cave surface but is too small for human access (Fig. 1). Nevertheless, a strong air flow is reported in this passage (personal communication). The forward selection within CCA indicated that some variables explained significant and independent amounts of variation in microbial distributions (Table 2). Interestingly, physical parameters explained the highest variance in structure of microbial communities in the winter (December 2009) by 0.62 and in the summer (June 2010) by 0.49 while, in the transitory periods in March and September 2009, they explained much less, 0.25 and 0.08, respectively. In the transitory period, temperature seems to have a significant impact (p < 0.05) on bioaerosol abundance. In March 2009, besides temperature, RH and distance from the entrance also had a statistically significant impact. The “real” conditions in cave atmosphere are much more complex, as indicated by results of analysis when one additional parameter—a known cave passage that communicates with the external atmosphere—was added into the analysis. These results, for example, statistically significant impact of air pressure and dust in March 2009 on microbial abundance, indicated that there was active air flow in this passage. The conditions in the passage also had a notable impact on cave airborne microorganisms in December 2009 (air pressure, p < 0.05). On the other hand in June 2010, CCA analysis of conditional effects revealed that including this new cave opening in the analysis gave no significantly different results compared with results when only previously known historical openings were taken into consideration (Table 2 and Fig. 1).
Concentrations of DNA in the air were fluctuating; minimum and maximum concentrations varied with site and season (Fig. 6). The ratio of microbial counts vs. DNA concentration indicates the proportion of cultivable microbes. Ratios of bacterial and fungal counts vs. DNA concentration were in all seasons the lowest at site no. 3 (Pisani rov), which is characterized by Rn concentrations that are 5 to 11 times higher than the average in other parts of the cave. The highest ratio was always at sites where external airborne microbes invade underground, at the cave entrance, site no. 1, in September 2009, and in Veliki dom at the ponor, site no.2, in December 2009 and June 2010.
Airborne Algae
Presence of viable algae was screened using Jaworski solid medium. The objective of this screening was to monitor their presence at various sampling sites and the inflow of algal cells through cave openings and their eventual aerosolization from the underground Pivka River and at sites in the tourist section of the cave, where communities of phototrophs, mainly algae, grow around the lamps. These communities, named lampenflora, have significant impact on the cave’s integrity because they cause biodeterioration of cave formations, historical inscriptions, and paintings on cave walls. The highest algal concentration was detected at the ponor, site no. 2, demonstrating active aerosolization of algae from the underground Pivka River. Algae were constantly present in the air at the cave entrance, site no. 1, irrespective of whether there was air inflow or outflow. The main entrance passage continues to the underground room where the river flows (Fig. 1), explaining the presence of airborne algae at the entrance when air flows out of the cave, due to aerosolization of algae from the underground stream. However, the underground railway (no. 4) and tourists (no. 6) also contribute to spreading of algal cells. Algae in the cave air were occasionally detected also at site no. 8 (Otoška jama), located close to the cave opening, and at site no. 10 (Črna jama).
Airborne Amphizoic Amoebae
Of all microbial groups screened for, the amoebae were the largest organisms. Because the impacting sampling method of a maximum of 1,000 l of air did not reveal any amoebae, we applied gravimetric depositional sampling. Within the entire 2-year sampling period, airborne amoebae were detected at all sampling sites; however, they were constantly present only in the touristic walking zone (site no. 6, Fig. 7). Interestingly, the probability of amoebal presence did not necessarily rise with prolonged sampling times. Obviously, the density of amoebae in the air is so low that the probability of an amoebal cyst falling into a Petri dish is random. For example, in June 2010 at site no. 5, amoebae were present in the Petri dishes exposed for 1 and 3 days, respectively, but not in the one exposed for 5 days, although all three Petri dishes had been placed next to each other. Similarly, in the same period at site no. 7, amoebae were detected in the plates exposed for 1 and 5 days, respectively, but not in the one exposed for 3 days (Fig. 7).
Amoebae were most prevalent in June 2010 and least prevalent in December 2010. Of the amphizoic amoebae, only the genus Acanthamoeba was found; neither of the other potentially pathogenic amoebozoans Balamuthia mandrillaris or Sappinia diploidea nor any representative of the excavate genus Naegleria was detected in any of the samples. Acanthamoebae were detected in eight of the ten sampling sites investigated; the only constantly negative sites were site no. 1 at the entrance and site no. 5 close to the cave sediment. Altogether, four different Acanthamoeba genotypes were found, of which genotype T4 was most frequent (Table 3).
The overall most abundant group of airborne amoebozoans were the mycetozoans. In the first sampling round, a mycetozoan plasmodium of approximately 10 cm in diameter was found on the cave wall close to sampling site no. 10 and was later identified as Physarum globuliferum. Other amoebae detected were representatives of the genera Hartmannella, Echinamoeba, Vahlkampfia, and Rhizamoeba. Moreover, nematodes and insects were also found on agar plates. Insects were found on three plates altogether, but only during sampling in June 2010, and neither in September 2009 nor in December 2009.
Natural Inflow of Aerosols Underground
Natural openings to the underground are windows through which most airborne material can be introduced. As shown in Fig. 8, the total amount of biomass of viable microorganisms represents only a small portion of the total amount of particles, but one has to take into account also the presence of other microbes, which were not detected by the selected growth media. However, the calculated total amount of introduced airborne particles within a period of 90 days with a constant air inflow of 1.5 m/s is relatively high at 0.28 g/m2 biomass versus 875 kg/m2 of total dust or 0.32 ppm (Fig. 2).
Introduction of airborne material into the cave within 1 day, 30, or 90 days through the Postojna Cave main entrance, based on total dust weight per cubic meter and average values of CFU per cubic meter related to different winter air inflows of 0.1, 0.5, 0.8, 1.0, 1.2, and 1.5 m/s, respectively; a microbial biomass, b total dust particles
Discussion
Airborne Biota in the Karst Underground
The present study gives a brief temporal, spatial, and seasonal glimpse into the conditions in the air in large underground passages in the Postojna Cave system. Due to the use of a set of different methodologies, the current study provides details on a range of ecologically and/or medically important microorganisms. Impacting was shown to be a suitable method for screening most airborne microbes in cave air when the maximum of 1,000 l of air was analyzed. For larger organisms, gravimetric depositional sampling should be used. This method worked very well for sampling of amoebae, giving an overview of the presence of amoebal cysts in cave air. However, it does not allow any conclusions on amoebal density in the air, as deposition of cysts does not follow a regular pattern. This “nonlinear” dispersion can be attributed to the fact that gravitational settling of particles larger than 5.0 μm and impaction are the leading causes of “loss” of these particles. Particles between 1.0 and 5.0 μm normally follow the streamlines of the surrounding air. Larger particles, such as amoebal cysts (mostly 10–20 μm), often have the momentum to deviate from the streamlines, impact surrounding surfaces, and be deposited [3]. Nevertheless, the transport and ultimate settling of bioaerosols are affected by their physical properties and the environmental parameters that they encounter while airborne.
The airborne microbial community in the air of the Postojna Cave system was shown to be composed of different microbial groups with generally low abundance, for bacteria and fungi between 10 and 100 CFU/m3 (Fig. 5). The abundance was significantly higher at sites where the cave atmosphere directly interacts with the external climate, such as the cave entrance and river splashing zone. For example, active aerosolization from the ponor notably contributed to total airborne microbial counts at downstream sites. The highest bacterial counts in the entire cave system were detected at the ponor (min. 61 CFU/m3, max. 493 CFU/m3). Formation of mist accompanying active air flow increases transport of microbes deep into the underground. In such cases, allochthonous microbes are constantly introduced as aerosols, e.g., to a large extent as fungal spores, or they become aerosolized, such as bacteria and algae by river splashing. Viable fungal counts were highest at sites no. 1, 2, and 4 with mean values ranging from 232–281 CFU/m3, where large oscillations were observed. Large oscillations of fungal spores have also been detected in the Cave of Nerja (Spain) with a daily mean concentration of 282.195/m3 [33]. Higher concentrations of microorganisms in particular parts of the cave can be attributed to human activities. For example, along the tourist railway (no. 4), regular train transport of tourists (air flow formation ~1.7 m/s) is likely to be responsible for elevated high concentrations of fungal spores, and there are elevated bacterial counts in areas with constant tourist presence compared with non-disturbed areas of the cave. Higher numbers of bacteria in tourist walking areas indicate “fresh” and continuous microbial input from humans. A single human sheds approximately seven million particles and cells per minute, each of these carrying along an average of four microbial cells [34]. Furthermore, coughing and loud talking are known to release around 104 droplets, sneezing up to 106 droplets [1]. Altogether, human activity, particularly mass tourism, represents a significant load of microbial biomass into caves.
The limit value of 300 CFU/m3 recommended for total microbial counts for indoor commercial and residential environments [35] was occasionally exceeded at the entrance (no. 1), at the ponor (no. 2), and along the tourist railway (no. 4). This is typical for places where organic matter is directly introduced into the underground. It is not recommended to establish speleotherapy healing locations at these sites.
Amoeboid organisms are frequent dwellers in karst caves [36]. Although concentrations of amoebic trophozoites and/or cysts were lower than one organism per one cubic meter of cave air, these organisms are important for underground systems. The diversity of amoebae in the air of the Postojna Cave system was rather low; however, they were found in all parts of the system (Fig. 7). Taxa found most frequently were mycetozoans and acanthamoebae, both producing highly resistant spores and cysts, respectively. Acanthamoebae are of potential medical relevance; they can evoke a sight-threatening keratitis occurring mainly in contact lens wearers on one hand and a set of usually fatal infections, including granulomatous amoebic meningoencephalitis, in the immunocompromised host, on the other hand. Also, Hartmannella spp. and Vahlkampfia spp. have been discussed as potential pathogens, but their true medical relevance has not been clarified yet [37, 38]. Moreover, acanthamoebae, and also hartmannellae are known to act as vehicles and even as “training grounds” for several pathogenic bacteria [39].
Microorganisms and Environmental Variables
In microbial ecology, CCA analysis has been already used in many studies with microbial communities in various ecosystems [40]. CCA analysis with forward selection of airborne microorganisms and atmospheric variables showed more dynamic conditions in the cave atmosphere than believed before, although recent studies indicated that Postojna Cave system is well ventilated [41]. In the transitory seasonal period, in March and September 2009, air conditions in the cave system were rather unstable and influenced by many other factors not detected by our analysis. This was reflected by low values of variances (Table 2). This might be due to activation of many different, small cave openings and consequent air circulation. This was demonstrated for sites 3, 4, 5, and 7 in an additional CCA analysis incorporating a recently discovered passage (Rov pri Mumiji, Fig. 1). Interestingly, during September 2009, significant correlations (p = 0.006) were observed between microbial counts and temperature but with low contribution to the total variance (Table 2). The average temperature for the town of Postojna in September 2009 was 16.4 °C. This value was not much different from the measured temperature in the cave entrance (15.0 °C) when sampled in September 2009. Big differences between external and cave temperatures may not allow formation of a temperature-dependent microbial gradient. In June 2010, a correlation was established between concentration of total DNA and cultivable fraction of microorganisms (Table 2).
The ratio of microbial counts per DNA concentration showed the proportion of viable biota throughout the cave system. The lowest was at site no. 3 with the highest Rn concentrations and the lowest microbial counts, presumably because of its possible effect on airborne biota. The effect is direct and indirect. The indirect effect is due to enhanced air ionization, caused by alpha particles released during radioactive transformations of radon and its short-lived decay products. At radon concentrations in a range from about 3,000 to 60,000 Bq/m3 at site no. 3, the corresponding ionization rate (in addition to gamma and cosmic rays) to which viable organisms are exposed is in the range of 0.16–3.1 nC/kg s (2.2–44 mR/h) [42]. Bearing in mind that 1 nC/kg s means an absorbed dose rate in the air of about 40 nGy/s [43], the above ionization rate would correspond to an absorbed dose in the air in the range from 6.4 to 124 nGy/s. On the other hand, related absorbed doses in the other part of the cave would rarely exceed 2.2 pGy/s (2.5 to 50 times lower). The direct dose to the airborne biota results from absorption of the energy of α-particles released during α-transformations of 222Rn, 218Po, and 214Po, with energies of 5.5, 6.0, and 7.7 MeV, respectively [5]. The number of α-particles formed per second equals the number of transformations per second, which is expressed by the number of becquerels. A measurement has shown the following activity concentrations of 222Rn, 218Po, and 214Po (Bq/m3) at 4,175; 3,947; and 611, respectively. These 8,733 transformations produce α-energy of 4,175 × 5.5 + 3,947 × 6.0 + 611 × 7.7 = 51,350 MeV/m3 s ≈ 85 × 10–10 J/m3 s; or at the maximum radon activity concentration of 60,000 Bq/m−3, 125,500 transformations in 1 m3 per second would produce 1.2 × 10−7 J/m3 s. Although the path travelled by α-particles in biota is shorter because of its higher density (60 kg/m−3) compared with that of air (1.3 kg/m−3), its volume is too small (e. g., 6.64 × 10−15 m3 for 1.21 × 10−12 kg of 500 bacteria, or 2.04 × 10−14 m3 for 24.01 × 10−13 kg of 600 fungi in 1 m3 of air) to absorb the total energy of an impinging α-particle. Therefore, the energy is assumed to be absorbed uniformly in the medium, and the biota receives the part proportional to its volume, that is, 7.8 × 10−22 J/s for bacteria and 24 × 10−22 J/s for fungi. Taking into account 20 as the weighing factor for α-radiation, the following values are obtained for bacteria and fungi, respectively—19 and 40 nGy/s for the absorbed dose rates, 49 and 100 mGy for the monthly absorbed doses, and 0.6 and 1.2 Gy for yearly absorbed doses.
However, organisms at a low stage of evolution exhibit very high resistance to radiation. The absorbed dose of inactivation of 50 % lethal dose within 30 days for bacteria ranges from 20 to 1,000 Gy [43]. At site no. 3, the monthly doses fall in the above range only at the extreme radon concentration but are markedly below that otherwise. However, of all environmental parameters monitored at site no. 3, only radon concentrations were substantially elevated with respect to other sites. Therefore, it still remains a potential cause for the lowest rates of viable microbes and may not be simply ruled out. Additional measurements would be necessary to prove this assumption. As mentioned also by other authors, the absence of studies on the effects of radon on microbes makes it difficult to reliably estimate which radon levels are sufficient to cause cellular damage [44]. Perhaps elevated radon decay products generating high air ionization, in combination with other atmospheric parameters, for example, elevated total dust concentration (Figs. 3 and 4), might affect microbial survival rate. Dust particles in the air associated with these radioactive particles allow longer retention of radioactive products in the air. For example, if these particles are inhaled by humans in higher concentrations, they might cause pulmonary health problems, such as cancer [45]. Another important atmospheric parameter to consider would be mercury. In this cave system, changes in concentration of dissolved gaseous mercury (Hg0) in the air to a certain extent followed radon concentration (unpublished data). Mercury, even at low concentrations, represents a hazard for microorganisms [46]. It is less likely that in such a big cave system the community of airborne microorganisms is entirely different compared with other sites in the cave. However, the relationship between elevated radon and airborne biota was not confirmed by statistically significant correlation coefficients (Table 2). This raises an important question of whether certain microbes can multiply in the air despite elevated radon concentrations, or whether they become uncultivable or die. On the other hand, due to the absence of UV and desiccation stress, the viability of microbes in the cave environment is prolonged.
Air in the underground reflects external factors. Changes of bioaerosols can be directly traced and measured. Bioaerosols or aeroplankton can be considered as natural tracers that help us to understand environmental changes in the cave air, but, for complex underground systems, this is not straightforward. The present study revealed a high quantity of introduced particles in the underground (Fig. 8). The constant introduction, circulation, and finally sedimentation of allochthonous material from the air represent an important and continuous “rain” of organic carbon that serves as food for the cave fauna. Postojna Cave is known as the most biologically diverse underground cave in the world [47], and aeroplankton may be a relevant food source to sustain this biodiversity.
References
Stetzenbach LD (1997) Introduction to aerobiology. In: Hurst CJ, Knudsen GR, McInerney MJ, Stetzenbach LD, Walter MV (eds) Manual of environmental microbiology. ASM Press, Washington, D.C., pp 619–628
Buttner MP, Willeke K, Grinshpun SA (1997) Sampling and analysis of airborne microorganisms. In: Hurst CJ, Knudsen GR, McInerney MJ, Stetzenbach LD, Walter MV (eds) Manual of environmental microbiology. ASM Press, Washington, D.C., pp 629–640
Mohr AJ (1997) Fate and transport of microorganisms in air. In: Hurst CJ, Knudsen GR, McInerney MJ, Stetzenbach LD, Walter MV (eds) Manual of environmental microbiology. ASM Press, Washington, D.C., pp 641–650
Badino G (2004) Cave temperatures and global climatic change. Int J Speleol 33:103–114
Eaton RS (1988) Radon reduction: radon and its decay products in indoor air. Science 241:990
Etiope G, Martinelli G (2002) Migration of carrier and trace gases in the geosphere: an overview. Phys Earth Planet In 129:185–204
UNSCEAR (2000) United Nations Scientific Committee on the Effects of Atomic Radiation UNSCEAR 2000 report to the General Assembly, with scientific annexes. United Nations Scientific Committee on the Effects of Atomic Radiation, Vienna, Austria
Linan C, Vadillo I, Carrasco F (2008) Carbon dioxide concentration in air within the Nerja Cave (Malaga, Andalusia, Spain). Int J Speleol 37:99–106
Perrier F, Richon P (2010) Spatiotemporal variation of radon and carbon dioxide concentrations in an underground quarry: coupled processes of natural ventilation, barometric pumping and internal mixing. J Environ Radioactiv 101:279–296
Alteras I (1966) First Romanian isolation of Histoplasma capsulatum from the soil. Dermatol Int 5:69–71
Nagy K, Kávási N, Kovács T, Somlai J (2008) Radon and speleotherapy in Hungary. La Presse Thermale et Climatique 145:219–225
Saiz-Jimenez C, Gonzalez J (2007) Aerobiology and cultural heritage: some reflections and future challenges. Aerobiologia 23:89–90
Crestani G, Anelli F (1939) Ricerche di meteorologia ipogea delle grotte di Postumia. Istituto poligrafico dello stato Libreria, Roma
Gams I (1970) Zračna cirkulacija kot del jamskega okolja (na primeru Postojnske jame). In: Karaman Z, Manaković D (eds) Congrès Yugoslave de Spéléologie Cinquième session. Speleološki soujuz na Jugoslavija, Skopje
Šebela S (1998) Tectonic structure of Postojnska jama cave system. ZRC Publishing, Ljubljana
Vaupotič J, Ančik M, Škofljanec M, Kobal I (1992) Alpha scintillation cell for direct measurement of indoor radon. J Environ Sci Heal A 27:1535–1540
Warren A, Day JG, Brown S (1997) Cultivation of algae and protozoa. In: Hurst CJ, Knudsen GR, McInerney MJ, Stetzenbach LD, Walter MV (eds) Manual of environmental microbiology. ASM Press, Washington, D.C
Feller W (1950) An introduction to the probability theory and its application. John Wiley and Sons, New York
Visvesvara GS, Moura H, Schuster FL (2007) Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50:1–26
Page FC (1991) Naked rhizopods [Nackte Rhizopoda]. Gustav Fischer, Stuttgart, New York
Schroeder J, Booton G, Hay J, Niszl I, Seal D, Markus M, Fuerst P, Byers T (2001) Use of subgenic 18S ribosomal DNA PCR and sequencing for genus and genotype identification of acanthamoebae from humans with keratitis and from sewage sludge. J Clin Microbiol 39:1903–1911
Walochnik J, Michel R, Aspöck H (2004) A molecular biological approach to the phylogenetic position of the genus Hyperamoeba. J Eukaryot Microbiol 51:433–440
Adl SM, Simpson AG, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle G, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, McCourt RM, Mendoza L, Moestrup O, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MF (2005) The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J Eukaryot Microbiol 52:399–451
Thompson J, Gibson T, Plewniak F, Jeanmougin F, Higgins D (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Nicholas KB, Nicholas HBJ, Deerfield DWI (1997) Analysis and visualization of genetic variation. Embnew News 4:14
Gast R, Ledee D, Fuerst P, Byers T (1996) Subgenus systematics of Acanthamoeba: four nuclear 18S rDNA sequence types. J Eukaryot Microbiol 43:498–504
Neidhardt FC, Umbarger HE (1996) Chemical composition of Escherichia coli. In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C., pp 13–16
Schnürer J (1993) Comparison of methods for estimating the biomass of three food-borne fungi with different growth patterns. Appl Environ Microbiol 59:552–555
Chimiklis PE, Karlander EP (1973) Light and calcium interactions in Chlorella inhibited by sodium chloride. Plant Physiol 51:48–56
ter Braak CJF, Šmilauer PI, NY, USA: Microcomputer Power (2002) CANOCO reference manual and CanoDraw fo Windows user’s guide: software for Canonical Community Ordination (version 4.5). Microcomputer Power, Ithaca
Gregorič A, Zidanšek A, Vaupotič J (2011) Dependence of radon levels in Postojna Cave on outside air temperature. Nat Hazards Earth Syst Sci 11:1523–1528
Vaupotič J, Csige I, Radolić V, Hunyadi I, Planinić J, Kobal I (2001) Methodology of radon monitoring and dose estimates in Postojna Cave, Slovenia. Health Phys 80:142–147
Docampo S, Trigo MM, Recio M, Melgar M, García-Sánchez J, Cabezudo B (2011) Fungal spore content of the atmosphere of the Cave of Nerja (southern Spain): diversity and origin. Sci Total Environ 409:835–843
Binnie PWH (1991) Biological pollutants in the indoor environment. In: Kay JG, GE Keller, Miller JF (eds) Indoor air pollution, radon, bioaerosols, & VOC’s, vol 1. Lewis Publishers, Chelsea, pp 13–24
Robertson LD (1997) Monitoring viable fungal and bacterial bioaerosol concentrations to identify acceptable levels for common indoor environments. Indoor Built Environ 6:295–300
Walochnik J, Mulec J (2009) Free-living amoebae in carbonate precipitating microhabitats of karst caves and a new vahlkampfiid amoeba, Allovahlkampfia spelaea gen. nov., sp nov. Acta Protozoologica 48:25–33
Kinnear FB (2003) Cytopathogenicity of acanthamoeba, vahlkampfia and hartmannella: quantative & qualitative in vitro studies on keratocytes. J Infect 46:228–237
Lorenzo-Morales J, Martínez-Carretero E, Batista N, Alvarez-Marín J, Bahaya Y, Walochnik J, Valladares B (2007) Early diagnosis of amoebic keratitis due to a mixed infection with Acanthamoeba and Hartmannella. Parasitol Res 102:167–169
Molmeret M, Horn M, Wagner M, Santic M, Abu Kwaik Y (2005) Amoebae as training grounds for intracellular bacterial pathogens. Appl Environ Microbiol 71:20–28
Ramette A (2007) Multivariate analyses in microbial ecology. FEMS Microbiol Ecol 62:142–160
Šebela S, Turk J (2011) Local characteristic of Postojna Cave climate, air temperature, and pressure monitoring. Theor Appl Climatol 105:371–386
Pagelkopf P, Porstendorfer J (2003) Neutralisation rate and the fraction of the positive Po-218-clusters in air. Atmos Environ 37:1057–1064
Lieser KH (2001) Dosimetry and radiation protection. Wiley-VCH, Berlin, pp 417–436
Anitori R, Trott C, Saul D, Bergquist P, Walter M (2002) A culture-independent survey of the bacterial community in a radon hot spring. Astrobiology 2:255–270
Darby S, Hill D, Doll R (2001) Radon: a likely carcinogen at all exposures. Ann Oncol 12:1341–1351
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40:1335–1351
Sket B (1999) High biodiversity in hypogean waters and its endangerment—the situation in Slovenia, the Dinaric Karst, and Europe. Crustaceana 72:767–779
Acknowledgments
This study was supported by the bilateral research project “Study of amoebal viability and vehicle of their transport in the environment: case study from karst caves” (BI-AT/09-10-023) and program Karst Research P6-0119 funded by the Slovenian Research Agency. Authors are especially grateful to Andrej Mihevc, Franjo Drole, and Stanislav Glažar for assistance in locating representative sampling sites for the study; to Andreea Oarga for help during field work; to Jacek Pietrzak (Medical University of Vienna) for excellent technical assistance; and to Franko Radošič (Institute of Occupational Safety), Andrej Kozinc (Merck d.o.o., Slovenia), and Karmen Stanič (National Institute of Biology) for facility support. Useful comments on an earlier version of the manuscript were provided by Andrej Mihevc, Stanka Šebela, and Ivan Kobal. We thank Elizabeth D. Covington for terminology assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mulec, J., Vaupotič, J. & Walochnik, J. Prokaryotic and Eukaryotic Airborne Microorganisms as Tracers of Microclimatic Changes in the Underground (Postojna Cave, Slovenia). Microb Ecol 64, 654–667 (2012). https://doi.org/10.1007/s00248-012-0059-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-012-0059-1