Abstract
We investigated the association between a gall midge, Illiciomyia yukawai, and its symbiotic fungi on Japanese star anise, Illicium anisatum. The number of fungal species isolated from the galls increased with development of the galls, whereas those from the leaves showed a different trend. Botryosphaeria dothidea was dominant in the galls from June to October, and after that Phomopsis sp. 1, Colletotrichum sp., and Pestalotiopsis sp. became dominant. Although B. dothidea was not isolated from the leaves, it was detected from mycangia (abdominal sternite VII) of egg-laying adults at a high isolation frequency (>90%). However, B. dothidea was not isolated from mycangia of adults emerging from galls that were enclosed by plastic bags. This indicates that I. yukawai is closely associated with B. dothidea and that its newly emerged adults do not take the fungus into mycangia directly from the galls where they had developed. Also, the fungus from the fungal layers of ambrosia galls has less ability to propagate on artificial media despite the presence of its mycelial mass in mature galls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insect–fungal symbioses exist widely in nature, and their mechanisms and interactions have been of considerable interest in ecology. For example, ambrosia beetles have specific fungi in their mycangia, which are structures specialized to carry fungal spores, and their larvae mainly feed on the fungi which are introduced into wood from the mycangia [3, 18]. Wood wasps inoculate Amylostereum fungi into wood with their eggs. Their larvae can make use of cellulose decomposed by digestive enzymes which are derived from their fungal symbionts [20]. Fungus-growing ants cultivate basidiomycetous fungi as a main food source in their nest (fungus garden) into which they carry various substrates [8, 21, 24].
“Ambrosia galls” are induced by gall midges (especially species in the tribes Asphondyliini, Lasiopterini, and Alycaulini, [28]) and are characterized by endo-mycetophagous larval feeding [4, 22]. Ambrosia galls have a fungal layer instead of nutrient plant tissue which is usually present in galls [40]. The gall midge larvae are thought to be dependent on the fungal mycelium growing on the inner surface of the galls as a food source [4, 16]. Female adults have mycangia, as in ambrosia beetles and wood wasps, which are located in the abdominal sternite VII in Asphondyliini and the abdominal segment VIII or IX in Lasiopterini and Alycaulini [5, 26, 27]. In addition, mycangial conidia are found with eggs in or on host plants [4, 26]. Therefore, relationships between gall midges inducing ambrosia galls and associated fungi are considered as mutualisms [1, 4, 13, 25].
Previously, fungal symbionts have been determined only by the isolation of fungi from galls [2, 14] or by morphological identification based on mycangial conidia [5]. Adair et al. [1] and Janson et al. [17] investigated fungal symbionts by molecular basis and identified fungi associated with distinct tribe gall midges as a single species Botryosphaeria dothidea, but the primary fungal symbionts are not yet understood in many gall midge species responsible for ambrosia galls. Therefore, we focused on the gall midge Illiciomyia yukawai, which is not yet examined to isolate symbiotic fungi, although fungal layers can be obviously seen inside its mature galls. Because the genus Illiciomyia is situated at one of the most basal groups of the subtribe Asphondyliina [35, 36], this species is critically important in understanding the evolutionary process of gall midges in relation to acquisition of their associated fungi.
I. yukawai induces hemiglobular and single-chambered galls on leaves of Illicium anisatum (Illiciaceae), a broad-leaved evergreen tree, and the galls remain on the tree throughout the year [34]. In order to understand the nature of gall midge–fungus relationships, fungal floras in galls and mycangia as well as their seasonal changes should be compared intensively.
In this study, we investigated associations between the gall midge I. yukawai and its fungal symbionts from both spatial and temporal points of view. We isolated fungi from the galls and mycangia and revealed successions of fungal floras in them. We also focused on micro-space within the gall by separating it into inner (fungal) and outer (plant tissue) layers and then compared fungal floras between them to reveal interaction between gall midge larvae and fungal symbionts within the galls.
Materials and Methods
Study Insect
I. yukawai is a monotypic species of the tribe Asphondyliini [34]. In May, adults emerge from galls on the tree in the morning and females lay eggs into new leaves. Larvae induce galls, overwinter at the third (final) instar, and pupate in the following spring [34, 39]. Although I. yukawai is fundamentally univoltine, some individuals exhibit prolonged diapause at the first instar stadium and require 2 years to complete a generation [34, 39].
Study Sites
The study was conducted in an I. anisatum plantation at Kameyama City (34º50′ N, 136º18′ E, 200 m asl), Mie Prefecture, central Japan, and in secondary forests consisting of I. anisatum, Cryptomeria japonica, and Pieris japonica, Eurya japonica in Taiki Town (34º18′ N, 136º18′ E, 800 m asl) and Komono Town (35º01′ N, 136º26′ E, 300 m asl), Mie Prefecture, central Japan, and Shimanto Town (33º12′ N, 133º02′ E, 450 m asl), Kochi Prefecture, western Japan. Five galled trees (approximately 3 m in height) of I. anisatum were randomly selected as sampling trees at each site.
Fungal Isolation from Galls and Leaves
From May 2006 to April 2007, five galled shoots (including about 40 galls) were randomly collected at 2–4-week intervals in Kameyama, Taiki, and Shimanto. Collected galls were surface-sterilized in 70% ethanol for 1 min and then dipped for 1 min in sterile distilled water. Afterwards, the galls were aseptically dried on sterile filter paper, and they were then dissected and placed on four kinds of agar media (ten plates each of MA, YM, MPY, MM; “Electronic supplementary material”). The leaves, from which the galls were removed, were also used as the control for fungal isolation. After surface sterilization, leaf discs (approximately 2 × 2 mm) were aseptically cut and placed on the agar plates.
Fungal Isolation from Inner and Outer Layers of Galls
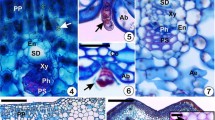
In November 2007, when the larvae were fully grown, the galls were collected from Kameyama (n = 36), Komono (n = 23), and Shimanto (n = 26). They were surface-sterilized in 70% ethanol for 1 min and 1% NaClO for 2 mins and then dipped for 1 min in sterile distilled water. After the same process of drying, the galls were separated into inner layer (fungal tissue) and outer layer (lignified plant tissue) (Fig. 1a). Each layer was placed on MPY and MM agar media (each about half).
a Cross-section of a leaf gall induced by I. yukawai, showing inner (fungal) and outer (plant tissue) layers. b Mycangia on the abdominal sternite VII and retracted ovipositor of I. yukawai. c A picture of B. dothidea cultured on PDA plate after 30 days. d Conidiophores (left) and conidia (right) of B. dothidea induced on WA with pine needles
Fungal Isolation from Female Adults
In Kameyama and Komono, some galled shoots were enclosed in plastic bags (340 × 240 mm) before the emergence of I. yukawai. In May 2007, the shoots were observed almost daily in order to collect female adults that emerged within plastic bags. In total, 16 female adults (enclosed adults) have emerged from the enclosed galls. At the same time, 15 female adults that were laying eggs into new host leaves (egg-laying adults) were collected from the census sites.
In the laboratory, abdomens of the collected adults were aseptically pressed on agar plates to isolate fungi adherent to the body surface. Then, without surface sterilization, conidia in the mycangia on the abdominal sternite VII (Fig. 1b) were inoculated into agar plates with a sterilized insect pin. MPY was used for both surface and mycangial fungal isolations of enclosed and egg-laying adults.
Identification
All cultures were incubated at 15°C. After fungal colonies were purified, isolated fungi were classified based on morphology and rDNA informations (LSU D1/D2 and/or ITS sequence data). Cultural morphologies and fungal structures on the media were observed under an optical microscope. Spore production on the representative isolates of each fungus was induced on water agar with pine needles under BLB light. If fungal structures were found on the media, they were picked up and mounted on glass slides and observed with a stereomicroscope.
The DNA of each isolate was extracted by the high-throughput method of Kikuchi et al. [19]. It was amplified using polymerase chain reaction (PCR) on a GeneAmp 9700 thermal cycler (Perkin-Elmer Applied Biosystems, Foster City, CA, USA). The oligonucleotide primer pairs, NL1 and NL4, ITS5 and ITS4 [38], were used for PCR. Then, 50 μl of reaction mixture containing 25 μl Qiagen GoTaq premix and 10 pmol each of primer and distilled water were added to the template. The reactions were initiated with 3 min of denaturation at 95°C, followed by 40 cycles of two-step PCR, consisting of 20 s at 95°C and 60 s at either 56°C for the primer pairs NL1 and NL4 or 54°C for ITS5 and ITS4, with a final extension for 10 min at 72°C. Amplification products were purified by QIAquick PCR Purification Kit (QIAGEN) and used for sequencing with a Big Dye Terminator Cycle Sequencing FS Ready Reaction kit ver. 3.1 and ABI PRISM 3100 genetic analyzer (Perkin-Elmer Applied Biosystems). Both strands of a fragment were sequenced. Blast homology search against the sequence data obtained was conducted (http://blast.ddbj.nig.ac.jp/top-j.html). We identified using the results of homology search together with morphological observations. The representative isolates (FFPRI411066-FFPRI411073) were deposited on FFPRI culture collection (Forestry & Forest Products Research Institute, Japan). Selected sequence data (accession no. AB645744-AB645759) obtained in this study were deposited on DDBJ (DNA Data Bank of Japan).
Data Analysis
For the number of species and isolation frequency, data were pooled in each experiment because there were only small differences among the media kinds. Isolation frequencies of mycangial fungi were also combined irrespective of study sites.
The isolation frequency of each fungal species was calculated according to the following formula:
where N i and N t are the numbers of samples from which the fungus was isolated and the total number of samples examined, respectively.
For the number of fungal species isolated from the inner and outer layers of galls, statistical significance was examined using Mann–Whitney U-test. Fisher’s exact test was used to compare the isolation frequency of each fungus between inner and outer layers. All data were analyzed by SPSS ver. 11.5.1 j.
Results
Over 30 species of fungi were isolated in this study. They included plant-parasitic fungi such as B. dothidea, Colletotrichum sp., Phomopsis spp., and Pestalotiopsis sp. and saprophytic fungi such as Cladosporium spp. and Penicillium spp. Among the obtained fungal species, B. dothidea (Fig. 1c) was most frequently isolated from mycangia and ambrosia galls and was identified based on sequence data and its anamorphic characteristics. Its conidiomata were often produced on the medium, showing multilocular and eustromatic opening through several non-papillate pores. Conidiogenous cells were initially holoblastic, becoming enteroblastic, integrated, hyaline, smooth, 8–18 × 2–5 μm, cylindrical producing one or more conidia apically, often proliferating percurrently to produce conidia at successively higher levels on annellate conidiogenous cells or proliferating at the same level, resulting in periclinal thickenings. Conidia were fusiform to elliptical with a subobtuse apex and a truncate base bearing a minute basal frill, 20.4–25.6 × 4.9–6.5 μm in size (average of 84 conidia, 23.0 × 5.7 μm, L/W ratio = 4.0), hyaline, aseptate, thin-walled, rarely becoming darker, 1-septate, and well fitted with the description of its anamorph by Slippers et al. [30] (Fig. 1d).
Seasonal changes in the number of fungal species isolated from the galls and leaves are shown in Figs. 2 and 3, respectively. From June to October, several species of fungi were detected from the galls in all census sites (Fig. 2). The numbers tended to increase gradually from June to December and sharply during winter. The maximum numbers of fungal species detected were 14, 13, and 15 in Kameyama, Taiki, and Shimanto, respectively. In contrast, the fungi isolated from the leaves fluctuated from three to eight species after November, although the numbers reached peaks from September to October, in Taiki and Shimanto. In Kameyama, the number of fungal species fluctuated from five to ten through the study period (Fig. 3). Thus, there were clear differences in the isolation trend between the galls and the leaves rather than between the three study sites.
B. dothidea predominated among all of the isolates from May to September when the galls were still immature, and the isolation frequency was 10–62.5%. However, the frequency decreased to 0–35% following the development of galls (Fig. 4). Other common fungi at the three sites were Phomopsis sp. 1, Colletotrichum sp., and Pestalotiopsis sp. In contrast to B. dothidea, the isolation frequencies of these fungi were low from May to September, but they increased gradually during winter. Thus, they were not isolated frequently from the immature galls and then tended to increase gradually. The frequency of Phomopsis sp. 1 eventually reached about 90% and 40% in Kameyama and Shimanto, respectively, and that of Colletotrichum sp. about 60% in Taiki. Pestalotiopsis sp. was less dominant and its isolation frequency did not exceed 15% in all study sites throughout the survey.
In the leaves, B. dothidea, which is the dominant fungus in the galls, was seldom detected (< 10%) in all study sites throughout the survey. Although Phomopsis sp. 1, Colletotrichum sp., and Pestalotiopsis sp. were also isolated from the leaves, their frequencies were less than 50% (Fig. 5).
Table 1 summarizes the number of fungal species isolated from the inner and outer layers of the galls at each site (mean ± SE, per gall). In all study sites, the number of species isolated was significantly larger in the outer layer than in the inner layer (Mann–Whitney U-test, p < 0.01).
Figure 6 illustrates the isolation frequency of three fungal species isolated from the inner and outer layers of the galls dominant at each site, including B. dothidea. Though the isolation frequency of B. dothidea showed no significant differences between the layers at all study sites (Fisher’s exact test, p > 0.05), that of Phomopsis sp. 1 was significantly more frequent in the outer layer than in the inner layer at all sites. Similar results and trends were obtained for Colletotrichum sp. in Komono (Fisher’s exact test, p < 0.01) and in Shimanto (Fisher’s exact test, p > 0.05), respectively. An unidentified fungal species (unidentified sp. 1) was isolated in Kameyama, but the frequency was very rare and no significant differences were detected between the layers (Fisher’s exact test, p > 0.05).
From mycangia of egg-laying adults, B. dothidea was the dominant species in immature galls with a frequency of 93.3%, but other fungi were seldom detected (Table 2). However, on the surface of the abdomen, the isolation frequency of B. dothidea was much lower (6.7%) than in mycangia, and that of the other fungi was higher (33.3%). In contrast to egg-laying adults, B. dothidea was not isolated at all from both parts of enclosed adults (both 0%), although other fungi were isolated in some cases (12.5% in mycangia, 75.0% on body surface).
Discussion
Fungal flora in the galls and the leaves changed spatio-temporally. The number of fungal species isolated from the leaves increased from May to September or October and then decreased or fluctuated (Fig. 3). Generally, it is well known that fungal flora in leaves fluctuate with time [7, 12]. On the contrary, the number of fungal species isolated from the galls increased consistently with the development of galls (Fig. 2). Because the isolation frequency of each fungus was also different between leaves and galls (Figs. 4 and 5), galls are considered to have unique chemical and physical properties as fungal habitats distinct from the leaves, even though the gall tissue is a part of the leaf.
B. dothidea, Phomopsis sp. 1, Colletotrichum sp., and Pestalotiopsis sp. were dominant fungi commonly isolated from the galls at all study sites (Fig. 4). These fungi are known as endophytes of various plant species [6, 11, 23, 29, 31]. Among them, B. dothidea and Phomopsis sp. 1 showed different trends in temporary occurrence. B. dothidea was the most abundant during the immature-gall period but the isolation frequency became low after the galls were fully developed. In contrast, the isolation frequency of Phomopsis sp. 1 increased steadily with gall development and was often higher than that of B. dothidea. Although Phomopsis sp. 1 was also isolated from the leaves in some degree, B. dothidea was rarely detected from them throughout the year. These results suggest that B. dothidea is a symbiotic fungus particularly associated with the gall.
We can propose a hypothesis why B. dothidea had low isolation frequency at mature-gall period (Fig. 4). Although the fungal inner layer existed inside the gall used for fungal isolation, the number of species and the isolation frequency of the isolated fungi including B. dothidea were extremely low in the inner layer (Table 1; Fig. 6). Generally, fungi should be isolated at high frequency from the mature gall with huge mycelia, but not in this study. Therefore, B. dothidea may have less ability to propagate on artificial media, showing physiological (trophic mode) change. This change is possibly caused by biological effects such as larval secretions of the gall midge. Manipulation of fungal symbionts by gall midges was also suggested in the case of Asteromyia carbonifera, goldenrod-galling midge, which belongs to the tribe Alycaulini [13, 17].
The dominant fungus in the galls, B. dothidea, was found frequently in mycangia of egg-laying adults, but not on their body (Table 2). Thus, we would strongly conclude that B. dothidea is the primary symbiotic fungus for I. yukawai. The enclosed adults, just emerging from the galls, had no B. dothidea in their mycangia or body surface (Table 2) as in previous reports [1, 4, 5, 13, 14]. This fact demonstrates that gall midges cannot take the symbiotic fungus into mycangia from the galls where they had developed. In other words, B. dothidea is not inherited via vertical (mother-to-infant) transmission as indicated by Janson et al. [17]. Females of gall midge could collect conidia from the environment such as old non-natal galls in the leaf litter [4], incomplete galls where midge or parasitoids have died [1], and other parts of host plant [26]. Future studies are needed to clarify how and when the females search for conidia of symbiotic fungus and where they collect them. These are important study subjects to understand biological interactions between the gall midge and fungus.
The genus Botryosphaeria is a common endophyte in many plants [31, 32] and is also recognized as an economically important pathogen affecting various crops [15, 33]. Some species, including B. dothidea, are known to have a wide host range [31]. In this study, B. dothidea showed low isolation frequency from I. anisatum leaves (Fig. 5); however, it may possibly attack other parts, such as branches and stems of I. anisatum trees and/or other plants.
Bissett and Borkent [4] have predicted that fungal symbionts of gall midge species belonging to the tribe Asphondyliini and the supertribe Lasiopteridi may be Botryosphaeria and its related anamorphs (Fusicoccum, Diplodia, Dothiorella, and Macrophoma). For example, Adair et al. [1] suggested that fungal associates of some Asphondylia species distributed in Australia and South Africa are B. dothidea. Our study clarified that the symbiotic fungus associated with Illiciomyia was also Botryosphaeria. Asphondylia is the largest and radiating genus in tribe Asphondyliini [41] and contains nearly 300 described species [9, 10]. In contrast, Illiciomyia comprises only one species, as mentioned earlier, and is situated at one of the most basal clades of the subtribe Asphondyliina [35, 36]. To verify their prediction, we have to examine the symbionts of many other genera and species of gall midges further. However, the currently available evidence indicates that Botryosphaeria fungi appear to be common among Asphondyliini midges, all of which can provide a great opportunity for the fungi to disperse its conidia. Janson et al. [17] suggested that fungal symbionts of A. carbonifera are B. dothidea and identical to free-living populations with lack of genetical specialization. In our isolates of the symbionts of I. yukawai, further detailed genetic analysis is required to discuss this possibility.
We propose two hypotheses about the benefits of symbiotic fungus for I. yukawai. First, the fungus is directly utilized as a food source as predicted in ambrosia gall midges by Bissett and Borkent [4]. Furthermore, Heath and Stireman [13] showed that fungal proliferation and gall development are dependent on larvae of the gall midge A. carbonifera, indicating direct gall midge–fungal interaction. In the case of I. yukawai, full-grown larvae were obviously covered with fungal layers in galls. So, the larvae probably rely upon the fungus as the food resource. This observation supports the first hypothesis, although we will need to detect fungal DNA from larval gut contents. Secondly, gall midge-associated fungus may function as a biological defense against antagonistic organisms [37], especially fungi. For example, mortality of the gall midge, Contarinia sp., which does not associate with symbiotic fungi, was higher on Douglas fir leaves infected by endophytes than on those uninfected [6]. Actually, many fungi other than B. dothidea existed in the leaves of I. anisatum and the outer layer of galls induced by I. yukawai (Figs. 5 and 6). Thus, B. dothidea might protect the larva from attack by antagonistic fungi.
Throughout the survey, we revealed the succession of fungal flora on galls, host leaves, and female adults of I. yukawai and identified the primary symbiotic fungus as B. dothidea. This is the first report to investigate the symbiosis between a gall midge and associated fungi from both spatial and temporal points of view.
References
Adair RJ, Burgess T, Serdani M, Barber P (2009) Fungal associations in Asphondylia (Diptera: Cecidomyiidae) galls from Australia and South Africa: implications for biological control of invasive acacias. Fung Ecol 2:121–134
Batra LR, Lichtwardt RW (1963) Association of fungi with some insect galls. J Kans Entomol Soc 36:262–278
Beaver RA (1989) Insect–fungus relationships in the bark and ambrosia beetles. In: Insect–fungus interactions. Academic, London, pp 121–143
Bissett J, Borkent A (1988) Ambrosia galls: the significance of fungal nutrition in the evolution of the Cecidomyiidae (Diptera). In: Coevolution of fungi with plants and animals. Academic, London, pp 203–225
Borkent A, Bissett J (1985) Gall midge (Diptera: Cecidomyiidae) are vectors for their symbionts. Symbiosis 1:185–194
Carroll GC (1986) The biology of endophytism in plants with particular reference to woody perennials. In: Microbiology of the phylloplane. Cambridge University Press, Cambridge, pp 205–222
Carroll GC (1995) Forest endophytes: pattern and process. Can J Bot 73:1316–1324
Cherrett JM, Powell RJ, Stradling DJ (1989) Mutualism between ants and their fungus. In: Insect–fungus interactions. Academic, London, pp 93–116
Gagné RJ (2004) A catalog of the Cecidomyiidae (Diptera) of the world. Mem Entomol Soc Wash 25:1–408
Gagné RJ (2010) Update for a catalog of the Cecidomyiidae (Diptera) of the world. Digital version 1. (http://www.ars.usda.gov/SP2UserFiles/Place/12754100/Gagne_2010_World_Catalog_Cecidomyiidae.pdf)
Hata K, Arari R, Sone K (2002) Isolation of endophytic fungi from leaves of Pasania edulis and their within-leaf distributions. Mycoscience 43:369–373
Hata K, Tsuda M, Futai K (1998) Seasonal and needle age-dependent changes of the endophytic mycobiota in Pinus thunbergii and Pinus densiflora needles. Can J Bot 76:245–250
Heath JJ, Stireman JO (2010) Dissecting the association between a gall midge, Asteromyia carbonifera, and its symbiotic fungus, Botryosphaeria dothidea. Entomol Exp Appl 137:36–49
Herman RP, Bynum HG, Alexander AB (1993) Interaction between the black yeast Aureobasidium pullulans and the galls midge Lasioptera ephedricola in gall formation on the desert shrub Ephedra trifurca. Ecography 16:261–268
Inderbitzin P, Bostock RM, Trouillas FP, Michailides TJ (2010) A six locus phylogeny reveals high species diversity in Botryosphaeriaceae from California almond. Mycologia 102:1350–1368
Janson EM, Grebenok RJ, Behmer ST, Abbot P (2009) Same host-plant, different sterols: variation in sterol metabolism in an insect herbivore community. J Chem Ecol 35:1309–1319
Janson EM, Peeden ER, Stireman JO III, Abbot P (2010) Symbiont-mediated phenotypic variation without co-evolution in an insect–fungus association. J Evol Biol 23:2212–2228
Kajimura H, Hijii N (1992) Dynamics of the fungal symbionts in the gallery system and the mycangia of the ambrosia beetle, Xylosandrus mutilatus (Blandford) (Coleoptera: Scolytidae) in relation to its life history. Ecol Res 7:107–117
Kikuchi T, Karim N, Masuya H, Ota Y, Kubono T (2009) An inexpensive high-throughput method to extract high yields of good quality DNA from fungi. Mol Ecol Res 9:41–45
Kukor JJ, Martin MM (1983) Acquisition of digestive enzymes by siricid woodwasps from their symbiont. Science 220:1161–1163
Mueller UG, Schultz TR, Currie CR, Adams RMM, Malloch D (2001) The origin of attine ant-fungus mutualism. Q Rev Biol 76:169–197
Neger FW (1910) Ambrosiapilze III. Weitere beobachtungen an Ambrosiagallen. Ber Deutsch Bot Ges 28:455–480
Okane I, Nakagiri A, Ito T (1998) Endophytic fungi in leaves of ericaceous plants. Can J Bot 76:657–663
Quinlan RJ, Cherrett JM (1978) Studies on the role the infrabuccal poket of the leaf-cutting ant Acromymex octospinosus (Reich) and its food fungus. Ecol Entomol 2:161–170
Rohfritsch O (1992) A fungus associated gall midge, Lasioptera arundinis (Shiner), on Phragmites austrakis (Cav.). Trin Bull Soc Bot Fr Lett Bot 139:45–59
Rohfritsch O (1997) Morphological and behavioral adaptations of the gall midge Lasioptera arundinis (Shiner) (Diptera: Cecidomyiidae) to collect and transport conidia of its fungal symbiont. Tijdschr Entomol 140:59–66
Rohfritsch O (2008) Plants, gall midges, and fungi: a three component system. Entomol Exp Appl 128:208–216
Roskam HC (2005) Phylogeny of gall midges (Cecidomyiidae). In: Biology, ecology, and evolution of gall-inducing arthropods. Science, pp 305–319
Sahashi N, Kubono T, Miyasawa Y, Ito S (1999) Temporal variations in isolation frequency of endophytic fungi of Japanese beech. Can J Bot 77:197–202
Slippers B, Crous PW, Denman S, Coutinho TA, Wingfield BD, Wingfield MJ (2004) Combined multiple gene genealogies and phenotypic characters differentiate several species previously identified as Botryosphaeria dothidea. Mycologia 96:83–101
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fung Biol Rev 21:90–106
Smith H, Wingfield MJ, Crous PW, Coutinho TA (1996) Sphaeropsis sapinea and Botryosphaeria dothidea endophytic in Pinus spp. and Eucalyptus spp. in South Africa. S Afr J Bot 62:86–88
Swart WJ, Wingfield MJ (1991) Biology and control of Sphaeropsis sapinea on Pinus species in South Africa. Plant Dis 75:761–766
Tokuda M (2004) Illiciomyia Tokuda, a new genus for Illiciomyia yukawai sp.n. (Diptera: Cecidomyiidae: Asphondyliini) inducing leaf galls on Illicium anisatum (Illiciaceae) in Japan. Esakia 44:1–11
Tokuda M, Yukawa J (2006) First records of genus Bruggmanniella (Diptera: Cecidomyiidae: Asphondyliini) from palaearctic and oriental regions, with descriptions of two new species that induce stem galls on Lauraceae in Japan. Ann Entomol Soc Am 99:629–637
Tokuda M, Yukawa J (2007) Biogeography and evolution of gall midges (Diptera: Cecidomyiidae) inhabiting broad-leaved evergreen forests in oriental and eastern palearctic regions. Orient Insects 41:121–139
Weis AE (1982) Use of a symbiotic fungus by the gall maker Asteromyia carbonifera to inhibit attack by the parasitoid Torymus capite. Ecology 63:1602–1605
White T J, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Yukawa J, Masuda H (1996) In: Insect and mite galls of Japan in colors. Zenkoku Noson Kyoiku Kyokai, Tokyo, pp 826 (in Japanese)
Yukawa J, Rohfritsch O (2005) Biology and ecology of gall-inducing Cecidomyiidae. In: Biology, ecology, and evolution of gall-inducing arthropods. Science, pp 273–304
Yukawa J, Uechi N, Tokuda M, Sato S (2005) Radiation of gall midges (Diptera: Cecidomyiidae) in Japan. Bas Appl Ecol 6:453–461
Acknowledgements
The authors would like to thank S. Sato for sample collection. Thanks are also due to A. Sano and S. Saka for utilization of study sites. We are grateful to K.M. Harris and M. Tokuda for providing valuable comments on the manuscript. We thank all the members of Forest Protection Laboratory of Nagoya University for their helpful suggestion and assistance. The research was partly funded by the Sasakawa Scientific Research Grant from the Japan Science Society and Grant-in-Aid for Scientific research (B) from Japan Society for the Promotion of Science (20405025).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLSX 11 kb)
Rights and permissions
About this article
Cite this article
Kobune, S., Kajimura, H., Masuya, H. et al. Symbiotic Fungal Flora in Leaf Galls Induced by Illiciomyia yukawai (Diptera: Cecidomyiidae) and in Its Mycangia. Microb Ecol 63, 619–627 (2012). https://doi.org/10.1007/s00248-011-9962-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9962-0