Abstract
The article deals with the publications of recent years on the formation of galls on the leaves of flowering plants infected with four-legged mites (Acariformes, Eriophyoidea). The literature data on several parasite–host pairs were used, since there is no universal model system for the experimental analysis of this problem. The gallogenesis is a complex growth reaction that occurs in the leaf tissues under the influence of mite saliva. Data on the possibility of transmission of phytohormones and symbiotic microorganisms from parasites to gall-forming plants are considered, but an important question about the nature of agents inducing mite galls remains open. In recent years, progress has been made in the study of gene expression during the development of galls on strawberry leaves. The mite galls are characterized by the presence of nutritive tissue, and comparative cytological and molecular genetic studies of its development and differentiation are of interest. It is also necessary to analyze the role and dynamics of changes in cell proliferation during gallogenesis, since the widespread ideas about the activation of cell divisions in the early stages of gall formation are based only on a qualitative evaluation, without quantitatively taking into account dividing cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Plant galls are specialized structures that appear on plants in response to the effects of various parasites, such as herbivorous insects, mites and nematodes, viruses, bacteria, fungi (Sinnott, 1960; Raman, 2011; Chetverikov et al., 2015; de Lillo et al., 2018; Ferreira et al., 2019; Harris and Pitzschke, 2020; etc.). Many researchers are engaged in the analysis of galls, but most often botanists, zoologists, entomologists, parasitologists, and ecologists. Despite the extensive literature on the process of gallogenesis, it rarely attracts the attention of developmental biologists. Meanwhile, animal-induced galls (synonym: zoocecidia) “have a constant and specific form, size, and structure and a very considerable amount of histological differentiation… In most cases they undergo a definite period of development, or life cycle, correlated with that of the parasite” (Sinnott, 1960, p. 283).
This review attempts to consider the induction of mite gallogenesis as a developmental biology problem. For this purpose, in particular, we attract some classical publications on the study of intercellular interactions in animal ontogeny (Toivonen et al., 1976; De Robertis and Kuroda, 2004), since the greatest success in the research of induction processes was achieved precisely in the field of experimental embryology of animals. This concerns such aspects as genetic control of embryonic induction, identification of inducing molecules, and modes of transmission of inducing stimuli. Naturally, the processes of cell differentiation and morphogenesis in higher plants have their own specifics. Nevertheless, when plants and parasitic arthropods interact, the stimuli that modify the development program of the host plant and induce gallogenesis are emitted from the animal’s body.
Galls can form on a wide variety of plant parts, however, in this article we limit ourselves to reviewing publications on mite galls induced only on leaves. Traditionally, leaf galls developing on plants infected with insects have been studied more often. However, episodically in review articles on this topic (for example, Raman, 2011, 2021; Fernandes et al., 2012; Gätjens-Boniche, 2019; Miller and Raman, 2019), the authors consider in the same context data on galls induced not only by insects, but also four-legged mites (harmful species with high economic significance). Therefore, in contrast to the above-mentioned reviews, in which minimal attention was paid to the mite galls, we mainly discuss the literature on gallogenesis on leaves infected with four-legged mites, but at the same time we also use some important data of recent years on the development of insect-induced galls. On the other hand, in reviews on plant interactions with the four-legged mites (Petanović and Kielkiewicz, 2010a, 2010b; Chetverikov et al., 2015; de Lillo et al., 2018), the authors tried to cover all aspects of mite gallogenesis and, perhaps, for this reason, they actually did not use the literature on gall-forming insects.
BRIEF CHARACTERIZATION OF THE FOUR-LEGGED MITES
Gall mites or eriophyoids (Acariformes: Eriophyoidea) are an aberrant group of microscopic acariform mites (Fig. 1). They have an elongated worm-like body covered with annular folds, a piercing-sucking mouth apparatus and only two pairs of walking limbs, therefore they are often called “four-legged mites.” These structural features of eriophyoids are associated with adaptation to phytoparasitism and miniaturization (Nuzzaci and Alberti, 1996). The size of eriophyoids on average ranges from 200 to 300 μm, and the smallest representatives do not exceed 90 μm in length (Polilov, 2015). Despite their microscopic size, four-legged mites play an important role in ecosystems, making a significant contribution to the regulation of the quantitative and qualitative (structural) composition of phytocoenoses due to the ability of eriophyoids to inhibit plant growth and induce gallogenesis (Sukhareva, 1992; de Lillo et al., 2018). Recent works on the phylogeny of Acariformes (Bolton et al., 2017, 2018; Klimov et al., 2018) showed that eriophyoids have common ancestors with the ancient group of soil worm-like nematalycide mites (Nematalycidae) and, probably, passed to phytophagy through an intermediate association with the mycorrhiza of higher plants.
Lateral view of a gall mite exemplified by Phyllocoptes bilobospinosus Chetverikov et al., 2019 (female, confocal laser scanning microscopy). Notations: AL—anal lobe, G—gnathosoma (stylet-like chelicerae are everted), GC—genital coverflap, PSh—prodorsal shield, OP—opisthosoma with opisthosomal cuticular annuli.
Currently, about 5 thousand species of gall mites are known, but it is assumed that most of the world diversity of eriophyoids has not yet been described, and the total number of species is estimated at about 50 thousand (Amrine et al., 2003). However, only about 500 species (about 10% of the total number) induce the formation of galls, while other species of eriophyoids do not possess this ability and often live on plants openly (Michalska et al., 2010; Chetverikov et al., 2015; de Lillo et al., 2018). Mites are able to form galls on all above ground unbarked parts of plants, but most often infect leaves. Although attempts to classify various types of leaf galls have been made many times (Nalepa, 1929; Keifer, 1975; Westphal, 1992; Westphal and Manson, 1996; Chetverikov et al., 2015), at the moment there is no single classification accepted by all experts. As the main classification criteria, such traits of galls as their appearance and shape, color, and localization on the plant are usually considered. Since many eriophyoid galls mites are characterized by the presence of a variety of trichomes formed under the influence of mite saliva in the gall, the form of trichomes (hairy, capitate, clavate, etc.) is often used to distinguish the types of galls. On the leaves of woody plants, mites cause the following main types of galls (Fig. 2): pouch and nail galls, erinea (open galls, characterized by the formation of a leaf area densely covered with trichomes, a kind of “trichome rug”), marginal leaf rolling, vein galls, and parenchymatous galls (in this case, mites penetrate under the epidermis and cause necrotic changes in the parenchyma).
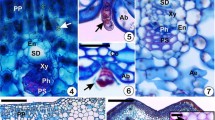
Some common types of leaf galls caused by eriophyoid mites. (a) Marginal leaf rolling on the beech Fagus sylvatica caused by the mite Acalitus stenaspis Nalepa; (b) pouch galls on the alder Alnus incana caused by the mite Eriophyes laevis Nalepa; (c) vein (“serpentine”) galls on the leaf of the hornbeam Carpinus orientalis caused by the mite Aculops macrotricus Nalepa; (d) parenchymatous (blister) galls on the rowan Sorbus torminalis caused by the mite Eriophyes sorbi Canestrini; (e) nail galls on the linden Tilia cordata caused by the mite Eriophyes tiliae Pagenstecher; (f) schematic structure of the pouch gall (modified after Fereira et al., 2019). Notations: GC—gall chamber, LL—leaf lamina, NT—nutritive tissue, CB—conductive bundle, E—entrance to the gall chamber.
It is pertinent to note that in the class of insects (Insecta), which includes over a million species, there are, according to various authors, from 13 thousand to 211 thousand gall-forming species (Stone and Schönrogge, 2003; Hardy and Cook, 2010; Takeda et al., 2021). Such insects are found in six orders, most often in the order Diptera (in the family Cecidomyiidae) and in the order Hymenoptera (in the family Cynipidae). The ability to induce gallogenesis appeared in the class of insects repeatedly and independently (Hardy and Cook, 2010; Miller and Raman, 2019; de Araújo et al., 2019; Takeda et al., 2021), as well as in different phylogenetic lineages of gall mites (Chetverikov et al., 2021).
BRIEF DESCRIPTION OF GALLOGENESIS INDUCED BY FOUR-LEGED MITES ON THE LEAVES OF FLOWERING PLANTS
There is no universal model system for the analysis of mite gallogenesis on leaves. Different authors studied different parasite–host pairs from different climatic zones of the globe: temperate climate (Kendall, 1930; Kane et al., 1997), subtropics (de Lillo et al., 2020), tropics (Moura et al., 2009).
Gallogenesis induced by the mite Eriophyes laevis Nalepa on two species of European alder Alnus glutinosa (L.) Gaertn. and Alnus incana (L.) Moench. (Betulaceae) (Kane et al., 1997), as well as induced by the mite Aceria lantanae Cook on the neotropical evergreen shrub Lantana camara L. (Verbenaceae) (Moura et al., 2009) is described in relative detail. In both cases, the initial stages of gallogenesis are associated with active cell division in the epidermis and parenchyma of the leaf. Invagination occurs on the abaxial side of the leaf lamina, resulting in the formation of a pouch gall (Kane et al., 1997). The gall chamber, in which mites reproduce and develop, is lined with nutritive tissue that serves as a direct food source for parasites (Moura et al., 2009). However, it should be noted that in the aforementioned articles (as, incidentally, in the works on the analysis of insect-induced gallogenesis), no quantitative accounting of dividing cells was made during the formation of leaf galls. Therefore, unfortunately, at present nothing can be said about the duration of cell cycles (and possibly also about the dynamics of their changes) during the development of leaf galls induced by the eriophyoid mites.
About 50 years ago, polyploidization of the nuclei of nutritive tissue cells in the leaf galls of host plants (plants of the genera Alnus, Campanula, Prunus, Ulmus, etc.; mites of the genera Aceria, Eriophyes, Phytoptus) was shown on several systems for the study of mite gallogenesis (Hesse, 1968, 1971a; Westphal, 1974). At the same time, polyploid nuclei were also found in the cells of the nutritive tissue of leaf galls induced by insects (Hesse, 1968, 1971b, 1972). However, out of 60 pairs “flowering plant–parasitic arthropod” studied in the works of M. Hesse, polyploid nuclei in the cells of galls were clearly identified only in the case of 26 pairs. In 25 pairs, the result was unequivocally negative, and in 9 pairs it was presumably negative (Hesse, 1968). Unfortunately, in subsequent years, the perspective of further study of polyploidy in gall cells induced by mites or insects did not attract the attention of researchers.
The most detailed analysis of mite gallogenesis is still a long-term series of studies conducted in France by E. Westphal and her colleagues (Westphal et al., 1981, 1990; Westphal, 1982, 1983, 1992; Bronner et al., 1989; Westphal and Manson, 1996; etc.). These authors cultivated the perennial bittersweet shrub Solanum dulcamara L. (Solanaceae) in a laboratory or greenhouse and infected the plants with the eriophyoid mite Aceria cladophthirus Nalepa. An experimental study using 240 young plants at the age of 3–4 weeks, each infested with 20–50 gall mites, revealed two completely different responses of the host organism to the action of parasites (Westphal et al., 1981). Only 36% of plants were susceptible to gallogenic effects of mite bites, in which leaf galls were formed as a result. Already within the first hour after the mite pierced the wall of the epidermal cell of the bittersweet leaf with chelicerae, the plant polysaccharide callose began to accumulate near the puncture site. The nuclei and nucleoli of epidermal cells around the puncture site increased in size and chromatin dispersion occurred. In the following hours, the division of these cells was noted and the gradual formation of the gall began. The epidermal cell damaged by the mite died. However, the surrounding epidermal cells, apparently, somehow received a signal from this cell to differentiate into the nutritive tissue of the gall, whose cells were characterized by slightly increased size and larger nuclei and nucleoli, although ploidy analysis was not performed in this case (Westphal et al., 1981; Westphal, 1982; Westphal and Manson, 1996). Thus, in this series of works, it was shown that the mite acts on a single plant cell (injecting saliva), but the gall-forming effect captures an entire section of the leaf.
On the other hand, almost 2/3 of the mite-infected bittersweet plants (64%) did not form galls (Westphal et al., 1981; Westphal, 1982). Nevertheless, in these plants, mites within a few hours induced a hypersensitivity reaction in the leaf epidermis. Around the puncture sites, cell death began within the first hour, no callose deposition occurred, and after 4 hours, polyphenolic compounds were found in necrotic areas, which were clearly delimited from normal (intact) tissue. After about 3 weeks, the spots of necrotic lesions on slightly deformed leaves of resistant plants were 300–400 µm in diameter. The survival time of mites on these resistant plants did not exceed 2–3 weeks from the moment of infection of the bittersweet (Westphal et al., 1990). According to the works of recent years (Golan et al., 2017; Wallis and Galarneau, 2020; Singh et al., 2021), polyphenols, widespread metabolites of flowering plants, provide their chemical protection against a variety of pests, including parasitic arthropods.
Of particular interest are the data of Westphal (1992) obtained in the study of another parasite–host system: the eriophyoid mite Eriophyes eupadi Newkirk and the common bird cherry Prunus padus L. (Rosaceae). The analysis of the dependence of the start of gallogenesis on the duration of mite feeding was carried out. For this purpose, mites were removed from the leaves at various intervals after the start of the experiment, which lasted 10 days. By the end of the ten-day period, full-fledged young galls with differentiated nutritive tissue were formed on the control leaves. The minimum time of contact of the parasite with the plant, necessary for the appearance of only small primary protrusions of the leaf lamina area (“abortive galls”), ranged from 8 to 24 hours, and further gallogenesis did not continue after the removal of mites. In order to form a small pouch gall, which does not yet have nutritive tissue (“defective gall”), the presence of a feeding mite on the bird cherry leaf was required for 48 hours.
Thus, the experiments carried out in the Westphal laboratory show that (1) the degree of development of a gall depends on the duration of exposure to the mite; perhaps the determining factor is the minimum critical amount of saliva that the mite injects into the plant cell in order to start gallogenesis; (2) plants differ in the degree of resistance to mites and are capable of one of two types of reactions: either the formation of galls, or the hypersensitivity reaction, accompanied by tissue necrosis.
BRIEF OVERVIEW OF RECENT RESEARCH ON MITE GALLOGENESIS
There are several lines of research related to the induction of gallogenesis. First, in special experiments (de Lillo and Monfreda, 2004), it was found that the saliva of four-legged mites acts on wheat coleoptiles in a similar way to how auxins and cytokinins act on coleoptiles (enhancing their growth). These phytohormones play an important role in the process of normal plant development, participating in the control of cell division, differentiation, and morphogenesis (see, for example: Fambrini and Pugliesi, 2013; van Berkel et al., 2013). Subsequently, data on the presence of auxins and cytokinins (mainly in the salivary glands) were obtained in the immunochemical analysis of a large number of insect species (Yamaguchi et al., 2012; Andreas et al., 2020; Ponce et al., 2021). Thus, a hypothesis arose that these phytohormones (or their analogs) are synthesized in the salivary glands of gall-forming arthropods and, entering the tissues of the host plant, modify the program of its normal development.
Second, it is well known that many species of arthropods (both mites and insects) are often in symbiotic relationships with various bacteria (see, for example: Zhang et al., 2016; Gätjens-Boniche, 2019; Hammer and Moran, 2019), and such bacterial symbionts are both intracellular and intracavitary. On this basis, one could assume that, together with the saliva of a parasitic animal, the host plant also receives bacteria that stimulate the formation of galls. Nonetheless, a recent study of 12 insect species (which included both gall-inducing species and closely related non-gall-inducing species) did not support this hypothesis. As the authors pointed out (Hammer et al., 2021, p. 1), “there were no specific bacterial taxa that were consistently associated with gall induction.” However, the number of species studied in this work was small. Besides, a number of characteristic gall-forming insects from the families Cynipidae and Cecidomyiidae were not analyzed. Hammer et al. (2021) studied mainly representatives of the families Aphididae (Hemiptera), Gelechiidae (Lepidoptera), and Tephritidae (Diptera). No mites were included in their analysis. Finally, some species of eriophyoid mites can be vectors of phytopathogenic viruses (de Lillo et al., 2018; Mansouri et al., 2021; Trzmiel et al., 2021; etc.). However, even in this case, nothing is known about ability of the symbiotic viruses of mites to cause the formation of galls. Thus, it seems premature to speak about the emergence of a clearly formulated “symbiotic” (or “infectious”) hypothesis of the induction of gallogenesis.
In recent years, a system consisting of the eriophyoid mite Fragariocoptes setiger Nalepa and the green strawberry Fragaria viridis Weston (Rosaceae) has become a promising model for the analysis of leaf gallogenesis (Pautov et al., 2016; Paponova et al., 2018). It is assumed that “morphologically, gall formation is a bending of cell sheets that compose the leaf lamina, accompanied by a change in the direction of differentiation of their cells” (Pautov et al., 2016, p. 1406). Discussing a hypothetical scheme for the formation of leaf galls, these authors attempt to attract ideas from the experimental embryology of multicellular animals (Beloussov, 2005), according to which mechanical stress plays an important role in the morphogenesis of cell sheets. However, it seems that such an attempt is not entirely correct due to the well-known differences in morphogenetic mechanisms between multicellular animals and higher plants (Ivanov, 2011). In particular, due to the presence of cell walls in the plant organism during its development, there is neither migration of individual cells, nor movements of cell layers. Anyway, a detailed analysis of the possible role of mechanical stress in the morphogenesis of mite galls on the leaves of strawberries (or any other plants) has not yet been published.
Subsequently, the same group of authors (Paponova et al., 2018) performed a complex morphological (histological) and molecular genetic analysis of galls on strawberries. Four stages of gall growth have been identified: the first stage roughly corresponds to the “abortive gall” of Westphal (1992), the second stage roughly corresponds to the “defective gall” of Westphal, while the third and fourth stages correspond to full-fledged young and mature galls of Westphal. Gall ontogenesis begins with the activation of anticlinal cell divisions in the mesophyll and epidermis (at the stage 1). Later, both anticlinal and periclinal divisions take place; gall cell proliferation continues at the developmental stages 2 and 3. The discovery of the inversion of the adaxial-abaxial polarity of the epidermis during the growth of galls can be considered a very interesting finding of these authors. Finally, the same publication (Paponova et al., 2018) provides detailed data on changes of the expression of the CYCD3 and CYCB1 cell cycle genes, as well as genes encoding homeodomain transcription factors from the KNOX and WOX families, during the process of gallogenesis. The expression intensity of all mentioned genes increased during the stage 2, remained at a high level at the stage 3, and dropped sharply by the stage 4. It is known from the literature that homeobox genes KNOX and WOX are universal regulators of normal plant development and diversity (Hake et al., 2004; Hay and Tsiantis, 2010; Gao et al., 2015; Radoeva et al., 2019; Conklin et al., 2020).
Thus, in the example of mite gallogenesis considered above (Paponova et al., 2018), there is a change (modification) of the normal genetic program of a young leaf development in the host plant. Similar data on changes in the genetic program of development of the host plant were recently obtained in the study of insect-induced leaf gallogenesis (Hirano et al., 2020). The parasite–host system included the aphid Schlechtendalia chinensis Bell (Aphididae, Hemiptera, Insecta) and sumac Rhus javanica L. (Anacardiaceae). In the early stages of gall development, an increase in the expression of KNOX genes and a suppression of the expression of genes associated with the regulation of photosynthesis were shown. The suppression of photosynthesis during gallogenesis under the influence of insects and mites has been shown in many works (Patankar et al., 2011; Carneiro et al., 2014; Kmieć et al., 2018; Takeda et al., 2019; Pestov and Ogorodnikova, 2020; Shih et al., 2020).
In the latest literature, data have appeared on the participation of elements of reproductive development programs in the implementation of gallogenesis: the activation of genes that ensure the formation of reproductive organs has been found (Schultz et al., 2019; Takeda et al., 2019). An illustrative example is the grape phylloxera Daktulosphaira vitifoliae Fitch (Phylloxeridae, Hemiptera, Insecta) and the coastal grape Vitis riparia Michx (Vitaceae) (Schultz et al., 2019). Some leaf galls of grape resemble flowers and fruits in appearance. Similar data are not yet available for gall-forming eriophyoid mites.
Westphal (1983) claimed that the nutritive tissue lining the gall chambers (with large cells and polyploid nuclei) is present only in the mite galls, while it is absent in the galls induced by insects. Such a point of view turned out to be erroneous, and the presence of typical nutritive tissue was recently shown also in galls induced by representatives of insects from the orders Diptera, Hymenoptera, and Lepidoptera (Ferreira et al., 2017, 2019). However, these authors suggest that nutritive tissue is absent in galls induced by insects from the order Hemiptera (for example, aphids). The latter seem to feed directly on phloem sap, sucking nutrients from the conductive bundles.
In publications of the Westphal laboratory (Westphal et al., 1981; Westphal, 1982, 1992), it was emphasized that the cells of the nutritive tissue in mite-induced leaf galls differentiate from epidermal cells. Modern researchers (Ferreira et al., 2017, 2019, 2020) argue that the nutritive tissue in mite galls can differentiate not only from the leaf epidermis, but also from parenchymal cells. Speaking about these transformations of cell types during gallogenesis, the authors (Ferreira et al., 2019) use the term “redifferentiation.” However, in developmental biology and cell biology, the term “transdifferentiation” is usually preferred to denote changes in differentiation at the cellular level (e.g., Eguchi and Kodama, 1993). Neither a comparative study of the nutritive tissue of mite galls, nor a molecular-genetic analysis of its development has yet been carried out.
CONCLUSIONS
Leaf gall formation is a complex growth reaction that occurs in plant leaf tissues in response to an injection of saliva from four-legged mites. Analyzing gallogenesis, researchers are faced with a number of phenomena and processes that are traditionally in the field of developmental biology: changes in gene expression and cell proliferation activity, the search for factors that induce morphogenesis, and transdifferentiation of cells and tissues.
To date, the greatest progress has been achieved in the study of changes in gene expression during gallogenesis induced by mites on leaves (genes from the KNOX and WOX families, as well as the CYCD3 and CYCB1 cell cycle genes). However, the analysis of proliferation in works on gallogenesis is based only on a qualitative assessment, without a quantitative account of dividing cells.
The nature of agents inducing mite gallogenesis and the specific mechanisms of their transmission to the plant have not been fully elucidated despite intensive research in this direction. It cannot be excluded that in different interacting parasite–host systems, the details of the “cellular and molecular dialogue” of these two participants differ. This idea resonates with the concepts of classical experimental animal embryology, according to which various interacting systems have a number of common features, but the method of transmission of the inducing stimulus is not universal (Toivonen et al., 1976). In addition, the molecules that induce the differentiation of the same structure in the early development of an animal can be very diverse (see, for example: De Robertis and Kuroda, 2004).
Special attention should be paid to the comparative cytological and molecular genetic analysis of the development of the nutritive tissue of mite galls, since it is not yet unequivocally clear from which source (cells of the parenchyma or the leaf epidermis) it originates.
Thus, despite a significant number of publications on the induction of leaf gallogenesis by the four-legged mites, progress in the development of this problem from the point of view of a developmental biologist has been relatively small to date. In our opinion, in the future it is necessary to use several (at least 2 or 3) parasite–host model systems, and it is desirable that several groups of researchers work in parallel on each of these systems. Main discoveries in the study of developmental biology of the mite galls have not yet been made.
REFERENCES
Amrine, J.W., Jr., Stasny, T.A., and Flechtmann, C.H.W., Revised Keys to the World Genera of the Eriophyoidea (Acari: Prostigmata), West Bloomfield (Michigan), USA: Indira Publishing House, 2003.
Andreas, P., Kisiala, A., Emery, R.J.N., et al., Cytokinins are abundant and widespread among insect species, Plants (Basel), 2020, vol. 9, no. 2, p. 208. https://doi.org/10.3390/plants9020208
de Araújo, W.S., de Freitas, É.V.D., Kollár, J., et al., Host specialization in plant-galling interactions: contrasting mites and insects, Diversity, 2019, vol. 11, no. 10, p. 180. https://doi.org/10.3390/d11100180
Beloussov, L.V., Osnovy obshchei embriologii (Fundamentals of General Embryology), Moscow: Mosk. Gos. Univ., 2005.
van Berkel, K., de Boer, R.J., Scheres, B., et al., Polar auxin transport: models and mechanisms, Development, 2013, vol. 140, no. 11, pp. 2253–2268.
Bolton, S.J., Chetverikov, P.E., and Klompen, H., Morphological support for a clade comprising two vermiform mite lineages: Eriophyoidea (Acariformes) and Nematalycidae (Acariformes), Syst. Appl. Acarol., 2017, vol. 22, no. 8, pp. 1096–1131.
Bolton, S.J., Bauchan, G.R., Chetverikov, P.E., et al., A rudimentary sheath for the smallest of “biting” chelicerae: the mouthparts of Cunliffea (Nematalycidae) and a new hypothesis on the origin of the stylet sheath of Eriophyoidea (Acariformes), Int. J. Acarol., 2018, vol. 44, no. 8, pp. 374–381.
Bronner, R., Westphal, E., and Dreger, F., Chitosan, a component of the compatible interaction between Solanum dulcamara L. and the gall mite Eriophyes cladophthirus Nal., Physiol. Mol. Plant Pathol., 1989, vol. 34, no. 2, pp. 117–130.
Carneiro, R.G.S., Castro, A.C., and Isaias, R.M.S., Unique histochemical gradients in a photosynthesis-deficient plant gall, South Afr. J. Bot., 2014, vol. 92, pp. 97–104.
Chetverikov, P.E., Vishnyakov, A.E., Dodueva, I.E., et al., Gallogenesis induced by eryophyoid mites (Acariformes: Eriophyoidea), Entomol. Rev., 2015, vol. 95, no. 8, pp. 1137–1143.
Chetverikov, P.E., Bolton, S.J., Gubin, A.I., et al., The anal secretory apparatus of Eriophyoidea and description of Phyllocoptes bilobospinosus n. sp. (Acariformes: Eriophyidae) from Tamarix (Tamaricaceae) from Ukraine, Crimea and USA, Syst. Appl. Acarol., 2019, vol. 24, no. 1, pp. 139–157.
Chetverikov, P.E., Craemer, C., Cyrković, T., et al., Molecular phylogeny of the phytoparasitic mite family Phytoptidae (Acariformes: Eriophyoidea) identified the female genitalic anatomy as a major macroevolutionary factor and revealed multiple origins of gall induction, Exp. Appl. Acarol., 2021, vol. 83, no. 1, pp. 31–68.
Conklin, P.A., Johnston, R., Conlon, B.R., et al., Plant homeodomain proteins provide a mechanism for how leaves grow wide, Development, 2020, vol. 147, no. 20, art. dev193623. https://doi.org/10.1242/dev.193623
Eguchi, G. and Kodama, R., Transdifferentiation, Curr. Opin. Cell Biol., 1993, vol. 5, no. 6, pp. 1023–1028.
Fambrini, M. and Pugliesi, C., Usual and unusual development of the dicot leaf: involvement of transcription factors and hormones, Plant Cell Rep., 2013, vol. 32, no. 6, pp. 899–922.
Fernandes, G.W., Carneiro, M.A.A., and Isaias, R.M.S., Gall-inducing insects: from anatomy to biodiversity, in Insect Bioecology and Nutrition for Integrated Pest Management, Panizzi, A.R. and Parra, J.R.P., Eds., Boca Raton, FL: CRC, 2012, pp. 369–395.
Ferreira, B.G., Álvarez, R., Avritzer, S.C., et al., Revisiting the histological patterns of storage tissues: beyond the limits of gall-inducing taxa, Botany, 2017, vol. 95, no. 2, pp. 173–184.
Ferreira, B.G., Álvarez, R., Bragança, G.P., et al., Feeding and other gall facets: patterns and determinants in gall structure, Bot. Rev., 2019, vol. 85, no. 1, pp. 78–106.
Ferreira, B.G., Bragança, G.P., and Isaias, R.M.S., Cytological attributes of storage tissues in nematode and eriophyid galls: pectin and hemicellulose functional insights, Protoplasma, 2020, vol. 257, no. 1, pp. 229–244.
Gao, J., Yang, X., Zhao, W., et al., Evolution, diversification, and expression of KNOX proteins in plants, Front. Plant Sci., 2015, vol. 6, p. 882. https://doi.org/10.3389/fpls.2015.00882
Gätjens-Boniche, O., The mechanism of plant gall induction by insects: revealing clues, facts, and consequences in a cross-kingdom complex interaction, Rev. Biol. Trop., 2019, vol. 67, no. 6, pp. 1359–1382.
Golan, K., Sempruch, C., Górska-Drabik, E., et al., Accumulation of amino acids and phenolic compounds in biochemical plant responses to feeding of two different herbivorous arthropod pests, Arthropod–Plant Interact., 2017, vol. 11, no. 5, pp. 675–682.
Hake, S., Smith, H.M.S., Holtan, H., et al., The role of knox genes in plant development, Ann. Rev. Cell Dev. Biol., 2004, vol. 20, pp. 125–151.
Hammer, T.J. and Moran, N.A., Link between metamorphosis and symbiosis in holometabolous insects, Philos. Trans. R. Soc., B, 2019, vol. 374, art. 20190068.
Hammer, T.J., De Clerck-Floate, R., Tooker, J.F., et al., Are bacterial symbionts associated with gall induction in insects?, Arthropod–Plant Interact., 2021, vol. 15, no. 1, pp. 1–12.
Hardy, N.B. and Cook, L.G., Gall-induction in insects: evolutionary dead-end or speciation driver?, BMC Evol. Biol., 2010, vol. 10, p. 257. http://www.biomedcentral.com/1471-2148/10/257.
Harris, M.O. and Pitzschke, A., Plants make galls to accommodate foreigners: some are friends, most are foes, New Phytol., 2020, vol. 225, no. 5, pp. 1852–1872.
Hay, A. and Tsiantis, M., KNOX genes: versatile regulators of plant development and diversity, Development, 2010, vol. 137, no. 19, pp. 3153–3165.
Hesse, M., Karyologische Anatomie von Zoocecidien und ihre Kernstrukturen, Österr. Bot. Z., 1968, vol. 115, no. 1, pp. 34–83.
Hesse, M., Über Mehrkernigkeit und Polyploidisierung der Nährgewebe einiger Milbengallen, Österr. Bot. Z., 1971a, vol. 119, nos. 1–3, pp. 74–93.
Hesse, M., Häufigkeit und Mechanismen der durch gallbildene Organismen ausgelösten somatischen Polyploidisierung, Österr. Bot. Z., 1971b, vol. 119, nos. 4–5, pp. 454–463.
Hesse, M., Über die Galle von Dechtiria nigrifasciata WLSM. (Lepidoptera) an Blättern von Periploca laevigata AIT (Asclepiadaceae), Österr. Bot. Z., 1972, vol. 120, no. 3, pp. 213–222.
Hirano, T., Kimura, S., Sakamoto, T., et al., Reprogramming of the developmental program of Rhus javanica during initial stage of gall induction by Schlechtendalia chinensis, Front. Plant Sci., 2020, vol. 11, p. 471. https://doi.org/10.3389/fpls.2020.00471
Ivanov, V.B., Kletochnye mekhanizmy rosta rarstenii (Cellular Mechanisms of Plant Growth), Moscow: Nauka, 2011.
Kane, N.A., Jones, C.S., and Vuorisalo, T., Development of galls on leaves of Alnus glutinosa and Alnus incana (Betulaceae) caused by the eriophyid mite Eriophyes laevis (Nalepa), Int. J. Plant Sci., 1997, vol. 158, no. 1, pp. 13–23.
Keifer, H.H., Eriophyoidea Nalepa, in Mites Injurious to Economic Plants, Jeppson, L.R., Keifer, H.H., and Baker, E.W., Eds., Berkeley, USA: Univ. California Press, 1975, pp. 327–533.
Kendall, J., The structure and development of certain eriophyid galls, Z. Parasitenkd., 1930, vol. 2, no. 4, pp. 477–501.
Klimov, P.B., OConnor, B.M., Chetverikov, P.E., et al., Comprehensive phylogeny of acariform mites (Acariformes) provides insights on the origin of the four-legged mites (Eriophyoidea), a long branch with presumably massive basal extinction, Mol. Phylogenet. Evol., 2018, vol. 119, pp. 105–117.
Kmieć, K., Rubinowska, K., Michalek, W., et al., The effect of galling aphids feeding on photosynthesis photochemistry of elm trees (Ulmus sp.), Photosynthetica, 2018, vol. 56, no. 4, pp. 989–997.
de Lillo, E. and Monfreda, R., ‘Salivary secretions’ of eriophyoids (Acari: Eriophyoidea): first results of an experimental model, Exp. Appl. Acarol., 2004, vol. 34, nos. 3–4, pp. 291–306.
de Lillo, E., Pozzebon, A., Valenzano, D., et al., An intimate relationship between eriophyoid mites and their host plants—a review, Front. Plant Sci., 2018, vol. 9, art. 1786. https://doi.org/10.3389/fpls.2018.01786
de Lillo, E., Fanelli, E., Valenzano, D., et al., Characterisation of Aceria massalongoi and a histopathological study of the leaf galls induced on chaste trees, Exp. Appl. Acarol., 2020, vol. 82, no. 1, pp. 33–57.
Mansouri, F., Richert-Pöggeler, K., Lewandowski, M., et al., Transmission characteristics of allexiviruses by the eriophyid mite, Aceria tulipae (Keifer) (Acari: Eriophyidae) from naturally mixed infected garlic (Allium sativum L.), Eur. J. Plant Pathol., 2021, vol. 160, no. 4, pp. 789–796.
Michalska, K., Skoracka, A., Navia, D., et al., Behavioural studies on eriophyoid mites: an overview, Exp. Appl. Acarol., 2010, vol. 51, nos. 1–3, pp. 31–59.
Miller, D.G. and Raman, A., Host–plant relations of gall-inducing insects, Ann. Entomol. Soc. Am., 2019, vol. 112, no. 1, pp. 1–19.
Moura, M.Z.D., Soares, G.L.G., and Isaias, R.M.S., Ontogênese da folha e das galhas induzidas por Aceria lantanae Cook (Acarina: Eriophyidae) em Lantana camara L. (Verbenaceae), Rev. Brasil. Bot., 2009, vol. 32, no. 2, pp. 271–282.
Nalepa, A., Neuer Katalog der bisher Beschriebenen Gallmilben, ihrer Gallen und Wirtspflanzen, Marcellia, 1929, vol. 25, nos. 1–4, pp. 67–183.
Nuzzaci, G. and Alberti, G., Internal anatomy and physiology, in Eriophyoid Mites—Their Biology, Natural Enemies and Control, Lindquist, E.E., Sabelis, M.W., and Bruin, J., Eds., Amsterdam: Elsevier, 1996, pp. 101–150.
Paponova, S.S., Chetverikov, P.E., Pautov, A.A., et al., Gall mite Fragariocoptes setiger (Eriophyoidea) changes leaf developmental program and regulates gene expression in the leaf tissues of Fragaria viridis (Rosaceae), Ann. Appl. Biol., 2018, vol. 172, no. 1, pp. 33–46.
Patankar, R., Thomas, S.C., and Smith, S.M., A gall-inducing arthropod drives declines in canopy tree photosynthesis, Oecologia, 2011, vol. 167, no. 3, pp. 701–709.
Pautov, A.A., Krylova, E.G., Vishnyakov, A.E., et al., Influence of growth parameters of the leaves of Fragaria viridis (Rosaceae) on gallogenesis, Bot. Zh., 2016, vol. 101, no. 12, pp. 1401–1410.
Pestov, S.V. and Ogorodnikova, S.Yu., Status of the photosynthetic apparatus of woody plants damaged by gall mites, Biol. Bull., 2020, vol. 47, no. 10, pp. 1392–1397.
Petanović, R. and Kielkiewicz, M., Plant–eriophyoid mite interactions: cellular biochemistry and metabolic responses induced in mite-injured plants. Part I, Exp. Appl. Acarol., 2010a, vol. 51, nos. 1–3, pp. 61–80.
Petanović, R. and Kielkiewicz, M., Plant–eriophyoid mite interactions: specific and unspecific morphological alterations. Part II, Exp. Appl. Acarol., 2010b, vol. 51, nos. 1–3, pp. 81–91.
Polilov, A.A., Small is beautiful: features of the smallest insects and limits to miniaturization, Ann. Rev. Entomol., 2015, vol. 60, pp. 103–121.
Ponce, G.E., Fuse, M., Chan, A., et al., The localization of phytohormones within the gall-inducing insect Eurosta solidaginis (Diptera: Tephritidae), Arthropod–Plant Interact., 2021, vol. 15, no. 3, pp. 375–385.
Radoeva, T., Vaddepalli, P., Zhang, Z., et al., Evolution, initiation and diversity in early plant embryogenesis, Dev. Cell, 2019, vol. 50, no. 5, pp. 533–543.
Raman, A., Morphogenesis of insect-induced plant galls: facts and questions, Flora, 2011, vol. 206, no. 6, pp. 517–533.
Raman, A., Gall-inducing insects and plants: the induction conundrum, Curr. Sci., 2021, vol. 120, no. 1, pp. 66–78.
De Robertis, E.M. and Kuroda, H., Dorsal-ventral patterning and neural induction in Xenopus embryos, Ann. Rev. Cell Dev. Biol., 2004, vol. 20, pp. 285–308.
Schultz, J.C., Edger, P.P., Body, M.J.A., et al., A galling insect activates plant reproductive programs during gall development, Sci. Rep., 2019, vol. 9, no. 1, p. 1833. https://doi.org/10.1038/s41598-018-38475-6
Shih, T.-H., Lin, K.-H., Chen, Y.-J., et al., Photosynthesis-related proteins of cup-shaped galls in Litsea acuminata leaves, Taiwania, 2020, vol. 65, no. 3, pp. 407–412.
Singh, S., Kaur, I., and Kariyat, R., The multifunctional roles of polyphenols in plant–herbivore interactions, Int. J. Mol. Sci., 2021, vol. 22, no. 3, p. 1442. https://doi.org/10.3390/ijms22031442
Sinnott, E.W., Plant Morphogenesis, New York: McGrow-Hill Book Co., 1960.
Stone, G.N. and Schönrogge, K., The adaptive significance of insect gall morphology, Trends Ecol. Evol., 2003, vol. 18, no. 10, pp. 512–522.
Sukhareva, S.I., Chetyrekhnogie kleshchi na zlakakh (Four-Legged Mites on Cereals), St. Petersburg: St.-Petersb. Gos. Univ., 1992.
Takeda, S., Yoza, M., Amano, T., et al., Comparative transcriptome analysis of galls from four different host plants suggests the molecular mechanism of gall development, PLoS One, 2019, vol. 14, no. 10, article e0223686. https://doi.org/10.1371/journal.pone.0223686
Takeda, S., Hirano, T., Ohshima, I., et al., Recent progress regarding the molecular aspects of insect gall formation, Int. J. Mol. Sci., 2021, vol. 22, no. 17, p. 9424. https://doi.org/10.3390/ijms22179424
Toivonen, S., Tarin, D., and Saxen, L., The transmission of morphogenetic signals from amphibian mesoderm to ectoderm in primary induction, Differentiation, 1976, vol. 5, no. 1, pp. 49–55.
Trzmiel, K., Szydło, W., and Hasiów-Jaroszewska, B., Biological and molecular characterisation of the two polish wheat streak mosaic virus isolates and their transmission by wheat curl mites, Plant Protect. Sci., 2021, vol. 57, no. 3, pp. 171–178.
Wallis, C.M. and Galarneau, E.R.-A., Phenolic compound induction in plant–microbe and plant–insect interactions: a meta-analysis, Front. Plant Sci., 2020, vol. 11, art. 580753. https://doi.org/10.3389/fpls.2020.580753
Westphal, E., Cécidogenèse et aspects structuraux de la galle en bourse de l’Eriophyes padi Nal. sur la feuille de Prunus padus L., Marcellia, 1974, vol. 38, pp. 77–93.
Westphal, E., Modification du pH vacuolaire des cellules épidermiques foliaires de Solanum dulcamara soumises á l’action d’un acarien cécidogène, Can. J. Bot., 1982, vol. 60, no. 12, pp. 2882–2888.
Westphal, E., Adaptation of gall mites (Acari, Eriophyoidea) to live in galls, in Adaptations to Terrestrial Environments, Margaris, N.S., Ed., New York: Plenum, 1983, pp. 69–75.
Westphal, E., Cecidogenesis and resistance phenomena in mite-induced galls, in Biology of Insect-Induced Galls, Shorthouse, J. and Rohfritsch, O., Eds., New York: Oxford Univ. Press, 1992, pp. 141–156.
Westphal, E., Bronner, R., and Le Ret, M., Changes, in leaves of susceptible and resistant Solanum dulcamara infested by the gall mite Eriophyes cladophthirus (Acarina, Eriophyoidea), Can. J. Bot., 1981, vol. 59, no. 5, pp. 875–882.
Westphal, E. and Manson, D.C.M., Feeding effects on host plants: gall formation and other distortions, in Eriophyoid Mites—Their Biology, Natural Enemies and Control, Lindquist, E.E., Sabelis, M.W., and Bruin, J., Eds., Amsterdam: Elsevier, 1996, pp. 231–242.
Westphal, E., Dreger, F., and Bronner, R., The gall mite Aceria cladophthirus. 1. Life cycle, survival outside the gall and symptoms' expression on susceptible or resistant Solanum dulcamara plants, Exp. Appl. Acarol., 1990, vol. 9, nos. 3–4, pp. 183–200.
Yamaguchi, H., Tanaka, H., Hasegawa, M., et al., Phytohormones and willow gall induction by a gall-inducing sawfly, New Phytol., 2012, vol. 196, no. 2, pp. 586–595.
Zhang, Y.-K., Chen, Y.-T., Yang, K., et al., A review of prevalence and phylogeny of the bacterial symbiont Cardinium in mites (subclass: Acari), Syst. Appl. Acarol., 2016, vol. 21, no. 7, pp. 978–990.
ACKNOWLEDGMENTS
The authors are grateful to S.I. Sukhareva for the comments made while reading the manuscript of this review. The authors are also grateful to two reviewers for critical comments on the original version of the article.
Funding
This study was supported by the Russian Foundation for Basic Research (grant 21-54-46003 СТ_а) and research project of ZIN RAS АААА-А19-119020790133-6.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest. In preparing this review, humans and animals were not used as objects of experimental research.
Rights and permissions
About this article
Cite this article
Desnitskiy, A.G., Chetverikov, P.E. Induction of Leaf Galls by Four-Legged Mites (Eriophyoidea) as a Problem of Developmental Biology. Russ J Dev Biol 53, 6–14 (2022). https://doi.org/10.1134/S1062360422010039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360422010039