Abstract
A few dominant species of plants often disproportionately contribute to primary production; however, dominance has an underappreciated influence on ecosystem processes and functioning. Cascading impacts of dominant species have been documented in ecosystems undergoing eutrophication, but competitive exclusion may also influence dominance structures when limiting nutrients become scarce (i.e., in lakes experiencing oligotrophication) or with exposure to stressors to which few species are adapted (i.e., desiccation stress in wetlands). To predict impacts of widespread changes in nutrients and hydrology on dominance structures in aquatic ecosystems, we need quantitative assessments of dominance of important primary producers, including algae and cyanobacteria, which can regulate other structural and functional properties of ecosystems. We used a highly spatiotemporally resolved (7 years, 165 sites) dataset from the abundant microbial mats of the Florida Everglades to assess how and why the degree of dominance and the identity of dominant taxa vary across nutrient and desiccation gradients. Using algal counts and the dimensions of algal units (cells, coenobia, colonies, and filaments), we measured dominance as relative biovolume. As hypothesized, the relative biovolume of dominant taxa increased and the number of taxa comprising 95% of the biovolume decreased with lower concentrations of limiting nutrient in the mats (phosphorus; P) and higher desiccation stress. Algal taxa that regulate the structural integrity of mats, such as the filamentous, calcium carbonate precipitating cyanobacterium Scytonema sp., strongly influenced these patterns through their tolerance of P scarcity and desiccation. Our indicators and approach can be used to test whether dominance of microscopic primary producers, and other organisms, increases with nutrient scarcity and desiccation stress in other aquatic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diverse communities tend to be more resilient to environmental changes than less diverse ones via different ecological mechanisms operating at multiple scales, such as redundancy among different species performing the same functions (Oliver et al. 2015). In low diversity ecosystems, rare species may not be sufficiently numerous to compensate for losses of dominant ones (Flöder et al. 2010). Dominant taxa (in biomass or abundance terms) often play an important and overlooked resilience function in many ecosystems undergoing considerable biodiversity loss (Hillebrand et al. 2008). Species that best compete for scarce limiting nutrients may dominate, or monopolize, community biomass, and thus primary production, therefore influencing species interactions and ecosystem functions (Grman et al. 2010). Dominant taxa that not only dominate biomass, but create, modify, and maintain habitats for other species, and contribute to nutrient cycling are called ecosystem engineers (Jones et al. 1994); these are crucial for ecological status assessment in terrestrial (e.g., McCullough Hennessy et al. 2016) and aquatic ecosystems (e.g., Vu et al. 2017). The most commonly studied taxa in this context include plants (e.g., shrubs; Gilad et al. 2007) and animals (e.g., beavers: Wright et al. 2002; and crabs: Vu et al. 2017); here we investigate microscopic primary producers acting as ecosystem engineers, a less covered topic. Dominance is usually measured as relative abundance or biomass of single taxa (population-level) or groups of species (community-level). Biomass better reflects resource use and partitioning among competing species than abundance (Guo and Rundel 1997); for example, a pool of species can be considered dominant when their cumulative (community-level) relative biomass is higher than a certain threshold (e.g., >50%; Downing et al. 2001). Thus, here we focus on dominance as expressed by relative biomass estimates of algae to reveal underlying drivers of patterns that are meaningful for broader ecosystem functions.
Benthic microbial mats (also called periphyton) are three-dimensional ecosystems in which algae exchange nutrients with both water and substrate (Passy 2008). In these systems, algae substantially contribute to primary production and carbon cycling, provide food, habitat, and oxygen to consumers (Hagerthey et al. 2011), and are sensitive to water diversion and eutrophication (Gaiser 2009; Gaiser et al. 2006). In oligotrophic and desiccation-prone systems, a number of algae and cyanobacteria species have adaptive traits that allow them to dominate primary production. Some filamentous cyanobacteria cope with low P concentrations and/or desiccation by enzymatically hydrolyzing phosphate from organic solutes (Coleman 1992) and by secreting extracellular polysaccharides (EPS) to store nutrients and water (Hagerthey et al. 2011), respectively. Moreover, in proximity of cyanobacteria heterotrophic bacteria can cope with low P by producing phosphatase (Sharma et al. 2005). Although nutrient fertilization tends to increase dominance by homogenizing available resources to various organisms in many ecosystems (Hillebrand et al. 2008), nutrient reduction/oligotrophication can also increase dominance by taxa with adaptive traits. One or a few algae may dominate biomass; for example, the diatom Didymosphenia geminata forms blooms in benthic systems with low concentrations of limiting P (Bothwell et al. 2014). In some cyanobacteria, pigments protect cells from dehydration and high UV radiation that may, for example, cause the aggregation of macromolecules that can disintegrate organelles (Karsten and Holzinger 2014). Here we investigate dominance by algal taxa adapted to low nutrient levels and dry conditions in an oligotrophic ecosystem subject to P enrichment and desiccation stress (Gaiser et al. 2011).
In shallow wetlands, nutrient and water availability are key factors determining phytoplankton and phytobenthic community structure changes (Huszar and Reynolds 1997; Gaiser et al. 2011; Brasil et al. 2016; Marazzi et al. 2017). Hydrology and P drive algal richness in subtropical wetlands, such as the Okavango Delta and the Everglades (Marazzi et al. 2017). Wetlands are facing multiple environmental stresses including habitat loss, eutrophication (Junk 2002), and droughts causing carbon loss (Fenner and Freeman 2011). On the other hand, nutrient removal via drainage (in particular of the less replenishable P) has caused oligotrophication in wetlands and downstream areas (Stockner et al. 2000). Both eutrophication and oligotrophication cause community shifts, respectively, toward algae better adapted to high P (Smith 2003) and to scarce P (Horn 2003) in various aquatic ecosystems. Therefore, testing how nutrient and hydrology influence algal dominance can help predict how communities of primary producers may change under different oligotrophication/eutrophication (Duarte 2009) and drying/wetting scenarios (Obeysekera et al. 2015; Brasil et al. 2016). We use a globally important and threatened wetland, the Florida Everglades, as a model of the interplay between key environmental stressors and algal dominance in benthic mats, fundamental systems for primary production, nutrient cycling, and other ecosystem functions (e.g., dissolved oxygen production and food provision to consumers; Hagerthey et al. 2011; Trexler and Loftus 2016). Our indicators and approach can be applied to other organisms and ecosystems facing nutrient scarcity and drying trends to support predictions of the occurrence of dominant species (including key ecosystem engineers), with changing resource and water availability.
To predict whether and how primary producer communities will cope with future nutrient and water scarcity, we need to understand how and why their dominance varies and identify which adaptive traits allow certain taxa to become dominant (Hillebrand et al. 2008). To this end we need spatiotemporally resolved and extensive data series that include environmental covariates across multiple locations and sampling periods (Horn 2003). Long-term research studies in the Everglades provide such data on algal community composition, biomass, and environmental conditions (Gaiser et al. 2011). Using recent data, we assess whether and how algal dominance changes with limiting nutrient concentrations and hydrology in this wetland. To describe and explain patterns of algal dominance in the Everglades, we assessed how much of the total biovolume is represented by the top 5 taxa, i.e., top 5 biovolume (%) and the number of taxa comprising 95% of the biovolume, i.e., N taxa = 95% biovolume. We also used evenness as a complementary measure of dominance, as communities with lower evenness are characterized by taxa with less equal abundance/biomass (Magurran and McGill 2011). Our hypotheses are that in Everglades habitats where P limitation and desiccation stress are more severe, (1) the top five taxa with the highest biovolume comprise a higher share of the total biovolume; (2) fewer taxa dominate the total biovolume, resulting in lower evenness; and (3) adaptive traits allow these taxa to dominate biovolume.
Methods
Sampling protocol
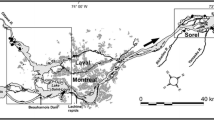
To describe and explain how the degree and structure of dominance in benthic algal mats vary across resource and stress gradients in the Everglades, we analyzed an extensive dataset originated by the Comprehensive Everglades Restoration Plan Monitoring and Assessment Plan (CERP-MAP). To investigate how water chemistry (in particular nutrients) and hydrology affect benthic algae during the wet season, we collected 947 algal samples from 165 sites (Fig. 1). We conducted this sampling each year from 2005 to 2011 between September and December in these representative regions (approximately north to south): Lake Okeechobee; the Arthur R. Marshall Loxahatchee National Wildlife Refuge; Pal Mar; Holey Land Wildlife Management Area; Water Conservation Area 2 and 3; Pennsuco; Lostman’s Creek; the ‘Oligohaline’ area; Southern Marl Prairie; Shark River Slough; and Taylor Slough (Marazzi et al. 2017). Using generalized random-tessellation stratification (Stevens and Olsen 2004), we sampled sites drawn from a pool of GPS coordinates (Philippi 2005) in representative locations (800 m × 800 m principal sampling units, PSUs). Within a 1-m3 enclosure open at top and bottom (Gunderson 1994), we removed all benthic microbial mats from the surface of the water and any substrate and placed them into a 2000-mL perforated graduated cylinder. To comprehensively assess all benthic algae, if no mats were present (in ~8% of the samples), we took flocculent detritus from the benthos (Pisani et al. 2011). A 120-mL homogenized subsample was taken from the total harvested material, placed into weighed plastic bags, and transferred to a laboratory freezer (Jordan et al. 1997). We measured water depth (cm) with a metal ruler and mat TP concentrations (μg g−1 dry weight) by means of colorimetry after dry combustion (Solorzano and Sharp 1980; EPA 1983). We used water-level approximations from the Everglades Depth Estimation Network (EDEN; http://sofia.usgs.gov/eden) to evaluate desiccation stress by estimating the days since last dry (depth ≤5 cm; DSLD) and the annual number of dry days (ANDD) for each sampling site. Mat TP concentrations are a better proxy of potential primary productivity than concentrations of P in the water; the latter are often below detection limits, while algae rapidly uptake P from oligotrophic waters (Gaiser et al. 2004). Lower TP indicates limiting nutrient scarcity, while shorter hydroperiod, lower depth and DLSD, and higher ANDD correspond to higher desiccation stress for algae and cyanobacteria (Gaiser 2009).
Algal analyses
To analyze algal community structure, we used modified methods to prepare wet mounts for algal counts (APHA et al. 2005) from frozen benthic mat samples (Stevenson and Bahls 1999). We thawed and homogenized 0.1–1 mL samples with a hand blender; when CaCO3 crystals were present on the slide, we added a solution of 0.01 mL 10% HCl and then dried the cover slip; otherwise, we dried the samples directly. Finally, we inverted the samples onto microscope slides in 0.020 mL of water and sealed them by ringing the glass with fingernail polish (Stevenson et al. 2002). We counted at least 500 algal units (i.e., cells, coenobia, colonies, and filaments of at least 100 µm length) in each sample at 1000× magnification using a compound light microscope. We identified the algae observed to the lowest possible taxonomic unit (genus, species, or variety) using Prescott (1962), Komárek and Hindak (1975), and Komárek and Anagnostidis (1986, 1989, 1999). We calculated algal biovolume by measuring ~20 cells covering the size range of each taxon and applying geometric formulas representative of the different shapes of the taxa observed (Hillebrand et al. 1999); we then estimated relative biovolume (%) for each taxon.
Data analyses
To investigate changes in cumulative (community-level) dominance of algae in the Everglades, we used three indicators, calculated from count and biovolume data: (1) the relative biovolume (%) of the top 5 taxa—hereafter top 5 biovolume (%); (2) the number of taxa comprising 95% of the biovolume—hereafter N taxa = 95% biovolume; and (3) the identity and frequency of the single most dominant taxa (i.e., those with the highest relative biovolume). In addition, we calculated Shannon Evenness, a complement of dominance widely used in diversity studies (Magurran and McGill 2011), to identify its key drivers and verify that, as expected, evenness decreased when dominance increased. To identify statistically significant differences of mean algal dominance between sampling sites, we divided P and hydrology gradients into quartiles (Table 1). Our TP classes are rather similar to those identified by Gaiser et al. (2006) (high TP: >172.8 µg g−1, see Table 1, as compared to 250 µg g−1 in Gaiser et al. 2006). To assess the influence of limiting nutrient levels and desiccation on the life-history strategies of dominant taxa, we classified the 26 taxa most dominant in at least one site according to six morphological and functional traits (Table 2): coccoid, epiphytic, unicellular motile, filamentous producing scytonemin, filamentous not producing scytonemin, and unicellular non-motile (Wehr et al. 2015).
To illustrate the relationship between environmental stressors, we graphed TP concentrations in the benthic mats in relation to desiccation stress (i.e., DSLD and ANDD) by quartiles in scatterplots in SPSS® (version 20). To identify statistically significant differences in algal dominance across P and hydrology gradients, we used parametric tests (one-way ANOVA and Tukey’s HSD) and nonparametric ones (Kruskal–Wallis and Mann–Whitney U) when the assumptions of normality and homogeneity of variance were / were not met (assessed via the Kolmogorov–Smirnov and Levene’s tests), respectively. We graphed dominance changes across P and desiccation gradients via box plots using the package ggplot2 in R (RStudio Team 2015). To quantify and compare the influence of P scarcity and hydrology/desiccation stress on algal dominance, we ran linear, quadratic, and cubic univariate models. By means of multiple linear regressions, we assessed the influence of alternative hydrological variables—water depth, hydroperiod, days since last dry (DSLD), and annual number of dry days (ANDD), and of the water chemistry variables mat TP, water pH, and conductivity on the response variables top 5 biovolume (%), N taxa = 95% biovolume, and Shannon Evenness. To avoid multicollinearity, we evaluated the correlations between hydrological and water chemistry predictors and the variance inflation factors (VIF) prior to fitting the models with the candidate explanatory variables (Zuur et al. 2007); we used the recommended maximum VIF value of 10 to retain the models (Marquardt 1970; Hair et al. 1995). We chose the best univariate and multiple regression models (Tables 4, 5) on the basis of the highest \(R_{\text{adj}}^{2}\), while also checking the Akaike information criterion for model selection (AIC; Johnson and Omland 2004). For the multiple linear regressions, we visually assessed whether the distributions of the residuals of our twelve models were stationary (i.e., the residuals oscillated above and below 0), in relation to the hydrological predictor defining each model (see Table 5). We conducted all the regression analyses in R (RStudio Team 2015). To assess the contribution of adaptive traits to the dominance of specific taxa, we analyzed their trait occurrence frequency in relation to P and desiccation gradients.
Results
Mat TP was significantly lower at Everglades sites that, prior to sampling, had been wet for a shorter time (i.e., <154 days since last dry; DSLD) and/or had been dry for a longer period on an annual basis (i.e., ANDD >36 days) than in sites that had been wet for a longer time and/or had been dry for a shorter period (Fig. 2). Our indicators were about 5–50% higher/lower where mat TP was lower and desiccation stress higher (Figs. 3, 4). Top 5 biovolume (%) was higher where mat TP was lower (Fig. 3A), DSLD lower (Fig. 3B), and ANDD higher (Fig. 3C). N taxa = 95% biovolume and Shannon Evenness were lower (i.e., higher dominance) with lower mat TP (Figs. 4A, 5A) and with lower DSLD (Figs. 4B, 5B), and higher ANDD (i.e., higher desiccation stress; Figs. 4C, 5C). All the hydrological variables considered (depth, hydroperiod, DSLD, ANDD) were highly correlated with one another (Table 3). The response variables were also highly correlated (Table 3); where top 5 biovolume (%) was higher, N taxa = 95% biovolume and Shannon Evenness were lower, and vice versa.
Relationship between mat TP concentrations and: A days since last dry (DSLD): Kruskal–Wallis (p < 0.05): χ 2: 19.088; Mann–Whitney U (p < 0.05); B annual number of dry days (ANDD): one-way ANOVA (p < 0.05): F = 5.096; Tukey’s HSD (p < 0.05). Statistically significant differences are indicated by different letters
Top 5 biovolume (%) in relation to quartiles of A mat TP (one-way ANOVA, O.W.A.: F = 90.63; p = 2 × 10−16***; Tukey’s HSD: p < 0.05); B days since last dry (DSLD; O.W.A.: F = 23.27; p = 3.53 × 10−6***); and C annual number of dry days (ANDD; O.W.A.: F = 8.640; p < 0.001; Tukey’s HSD: p < 0.05). Statistically significant differences are indicated by different letters; the y axis does not start at 0
N taxa = 95% biovolume in relation to quartiles of A mat TP (O.W.A.: F = 122.8; p = 2 × 10−16***; Tukey’s HSD test: p < 0.05); B days since last dry (DSLD; one-way ANOVA, O.W.A.: F = 19.06; p = 2.4 × 10−05***); C annual number of dry days (ANDD; O.W.A.: F = 3.178; p < 0.026; Tukey’s HSD: p < 0.05). Statistically significant differences are indicated by different letters
Shannon Evenness in relation to quartiles of A mat TP (O.W.A.: F = 14.07; p = 3.25 × 10−8***; Tukey’s HSD test: p < 0.05); B days since last dry (DSLD; O.W.A.: F = 7.135; p = 1.69 × 10−4***); C annual number of dry days (ANDD; O.W.A.: F = 7.079; p = 1.88 × 10−4***). Statistically significant differences are indicated by different letters; the y axis does not start at 0
On a univariate basis, according to highest \(R_{\text{adj}}^{2}\) values, often corresponding to the lowest AIC values, mat TP explained, on average, 32% of the variance of top 5 biovolume (%), N taxa = 95% biovolume, and Shannon Evenness, as compared to 20% for pH, 17% for hydrological variables, and 13% for conductivity (Table 4). Apart from depth and pH, which best predicted our response variable via quadratic models, the other predictors mostly attained the best fit via cubic models (Table 4). On a multivariate basis, due to the high correlation coefficients between hydrological predictors (mostly >0.8, as compared to <0.4 between these and the other variables; see Table 3), we created competing models to explain the three response variables, each including TP, conductivity, and pH, and each hydrological predictor in separate models (Table 5). We did not observe any multicollinearity, as all the VIF values of our models were <10 (Table S1). According to the models \(R_{\text{adj}}^{2}\) values, often corresponding to the lowest Akaike information criterion (AIC) values, among the hydrological variables used, ANDD best predicted top 5 biovolume (%), N taxa = 95% biovolume, and Shannon Evenness. Models with ANDD had higher explained variance (53%) than those with hydroperiod (47%), DSLD (42%), and depth (29%) (Table 5). The residuals of our twelve models were stationary (Fig. S1).
When mean site-specific data are considered, the filamentous cyanobacterium Scytonema sp. showed a stable degree of dominance across the mat TP gradient (Fig. 6a); by contrast, it was more frequently dominant where a shorter time had passed since the last dry conditions (i.e., lower DSLD; see ‘producing Scytonemin’ group in Fig. 6b), and where annual dry periods were longer (i.e., higher ANDD; Fig. 6c). Overall, coccoid, epiphytic, motile, and unicellular non-motile taxa were less dominant than filamentous ones (producing scytonemin or not; Fig. 6). Coccoid taxa were slightly more dominant in low P and desiccation-prone sites, and motile taxa were slightly more dominant with intermediate mat TP concentrations and DSLD (Fig. 6a, b). When all the dominance data by sample are considered, a total of 26 taxa (9 cyanobacteria, 8 Bacillariophyta, 8 Chlorophyta, and 1 Xanthophyta) were the single most dominant ones in at least one site (4 coccoid, 1 epiphytic, 7 filamentous not producing scytonemin, 1 filamentous producing scytonemin, 8 motile, and 5 unicellular taxa; Tables 2, 6). Over 84% of these dominant taxa were filamentous and coccoid cyanobacteria and filamentous taxa not producing scytonemin (e.g., Schizothrix sp. and a few Chlorophyta; Table 6). Scytonema sp. was more frequently dominant with lower mat TP (63% in quartiles 1 and 2), whereas filamentous taxa not producing scytonemin, such as Mougeotia and Schizothrix, were more dominant with higher TP and lower ANDD (Table 6). Aphanothece sp., the most frequently dominant coccoid cyanobacterium, was more frequently dominant with higher mat TP (Table 6). Unicellular Chlorophyta and Bacillariophyta were more dominant with higher TP; unicellular Chlorophyta were also more dominant with lower ANDD (Table 6).
Discussion
In the Everglades, mean algal dominance increased with oligotrophy and desiccation stress, as evidenced by the increase of top 5 biovolume (%) and the decrease of N taxa = 95% biovolume and Shannon Evenness with lower TP and drier conditions (Figs. 3, 4, 5; Tables 4, 5). This means that changing nutrient loads and water availability can substantially reshape algal communities over space and time and that environmental conditions favoring dominant native taxa need to be preserved to ultimately enhance ecosystem functions, such as the provision of habitat for consumers.
Algal dominance increases with oligotrophy
The proportion of biovolume accounted for by dominant taxa, as measured by top 5 biovolume (%), increased with P scarcity and was >60% in all the sites (Fig. 3). Changes of mean algal dominance across space significantly increased with oligotrophy, i.e., lower mat TP concentrations (Tables 4, 5). In other benthic systems, reduced P has been also shown to increase dominance by diatoms and/or cyanobacteria that tolerate oligotrophy (Horn 2003; Bothwell et al. 2014). In the case of Didymosphenia geminata, P additions have even been suggested to limit its blooms (James et al. 2015). In the Everglades, freshwater drainage, canalization, and impoundment for agricultural and urban development purposes historically caused P to increase downstream of the Everglades Agricultural Area (Fig. 1). This has caused substantial impacts on periphyton mats (Gaiser et al. 2006), but other areas have preserved their ecological character, such as the marl prairie further south. Scenarios of increasing freshwater flow and thus potentially higher P load to the Everglades marshes are likely to favor heterotrophic bacteria with abundant fatty acids and more edible green algae and diatoms (which contain more lipids than cyanobacteria); thus, consumers may benefit from a higher nutritional quality of periphyton (Bornhoeft 2016; Naja et al. 2017). However, enforcing stringent P load and concentration criteria while restoring freshwater flow to the Everglades (Naja et al. 2017) remains crucial for the preservation of cohesive periphyton mats with native algal species adapted to low P (Gaiser 2009; Gaiser et al. 2011).
Looking at the occurrence of the dominant taxa, we found that filamentous cyanobacteria were more dominant than eukaryotic algae in oligotrophic sites (see Table 6; Fig. 6) likely due to their ability to store P intracellularly by using phosphatases, enzymes that hydrolyze phosphate from organic solutes (Carey et al. 2012). These cyanobacteria are known to be abundant in oligotrophic sites with higher pH in the Everglades (Gaiser et al. 2011). With increasing P, periphyton mats break down due to heterotrophic bacteria rapidly taking up this nutrient from the mats and thus lowering the pH via CO2 release; this in turn causes the dissolution of calcium carbonate (Gottlieb et al. 2005; Gaiser et al. 2011, 2015). Scytonema sp. was more frequently the taxon with the highest relative biovolume in oligotrophic sites (quartiles 1 and 2) than in mesotrophic and eutrophic sites (quartiles 3 and 4; Table 6). This and other cyanobacteria, such as Schizothrix calcicola, thrive in low P areas and are considered ecosystem engineers; they form compact layers of filaments and secrete extracellular polysaccharide (EPS) where P and water are stored (Swift and Nicholas 1987; Hagerthey et al. 2011). Epiphytic green algae, such as Mougeotia, Oedogonium, and a few unicellular desmids, were more frequently dominant with higher TP (Table 6), consistent with established knowledge of their ability to successfully compete for P in oligotrophic–mesotrophic conditions (Reynolds 2006). In these deeper sites with higher TP, for example in Water Conservation Areas (Fig. 1), pH tends to be lower, which favors desmids (Table 6), as previously observed in the Everglades (see, e.g., Bellinger et al. 2010). A less clear trend is shown by the coccoid cyanobacterium Aphanothece, which was the taxon with the highest relative biovolume more frequently where TP was higher (Table 6), whereas it is usually widespread in this wetland (Gleason and Spackman 1974).
Algal dominance increases with desiccation stress
We demonstrated that higher dominance, especially of cyanobacteria, was caused by desiccation stress, alongside mat TP, in a globally important wetland (Tables 4, 5). Drier conditions (i.e., higher ANDD, shorter hydroperiod, and lower depth and DSLD), alongside higher pH, significantly increased top 5 biovolume (%) and reduced N taxa = 95% biovolume, and Shannon Evenness (Tables 4, 5). This means that, in the Everglades, the combined stress of nutrient and water scarcity is best tolerated by a few dominant benthic taxa, especially cyanobacteria (Table 6). Cyanobacteria were also found to dominate in other wetlands with receding water levels, for example in various shallow lakes in South America (Huszar and Reynolds 1997; Kosten et al. 2012; Brasil et al. 2016). Chase (2007) observed similar trends in experimental ponds where green algae, tolerant to desiccation, dominated following drought, while more diverse algal (and plant) assemblages were dominant in the absence of drought. In this study, algal dominance was also predicted by increasing water pH (Tables 4, 5) that causes calcium carbonate to dissolve from limestone and to be re-precipitated by algae that use bicarbonates, rather than carbon dioxide (Hagerthey et al. 2011). Although high pH might favor cyanobacteria dominance per se (Caraco and Miller 1998), in our dataset pH was positively correlated with ANDD (Table 3) so that higher desiccation stress and pH jointly increase algal dominance (Tables 4, 5). The dominance of filamentous cyanobacteria with higher pH is not surprising; contrary to eukaryotic algae, these organisms favor low concentrations of metals, such as manganese, which become less soluble as pH increases (Patrick et al. 1969).
Cyanobacteria, such as Scytonema sp., dominated this wetland’s prairies and marshes (Fig. 6; see the ‘filamentous producing scytonemin’ group), consistent with previous observations (Gleason and Spackman 1974; Swift and Nicholas 1987; Gaiser et al. 2011). Although we did not measure scytonemin, the capability to produce this pigment that protects photosystem II (Potts 1994) likely contributes to the dominance of Scytonema sp. in 63% of our samples (Table 6). The mean frequency of the most dominant traits by site was more variable across the desiccation gradient (Fig. 6b, c) than the P gradient (Fig. 6a) indicating that the production of scytonemin may be the most important adaptation in this wetland. Further research aimed to measure this pigment is however needed to confirm this interpretation of our findings. Previous work concluded that desiccation contributes to algal community structure changes in the Everglades (Gleason and Spackman 1974; Swift and Nicholas 1987; Gaiser et al. 2011) and other periodically dry ecosystems, such as savannahs (Williams et al. 2014). The ability to store water and nutrients in EPS (Hagerthey et al. 2011) allows several taxa to dominate at numerous sites: filamentous (e.g., Schizothrix sp.; 188 sites) and coccoid cyanobacteria (e.g., Aphanothece sp.; 18 sites), as well as filamentous green algae (e.g., Mougeotia; 55 sites; Table 6). Future environmental scenarios indicate that warmer temperatures, caused by global warming, may compound with the possibility of more frequent and/or longer droughts (Obeysekera et al. 2015) in changing species distribution and dominance patterns across ecosystems. As these adaptive traits have evolved over very long periods of time to counter nutrient scarcity and/or drying, these likely confer resilience to benthic primary producer communities. Experimental studies testing which adaptive traits best support the viability and productivity of dominant algae are therefore needed to better understand how benthic mats will react to ongoing environmental changes.
Using the Everglades as a model ecosystem to predict dominance patterns
In the interior of the Everglades, P tends to be scarce and desiccation stress high, especially in shallow sites that are frequently dry (Gaiser et al. 2011); in this wetland we found that algal dominance is high (Figs. 3, 4) and taxon richness is low, compared to other subtropical wetlands, such as the more pristine Okavango Delta (Marazzi et al. 2017). Diverse phytoplankton communities in a shallow lake in New Zealand were observed to have enough species to compensate for the loss of dominant taxa impacted by salt intrusion (Flöder et al. 2010). By contrast, rare species in the Everglades are likely not sufficiently numerous to compensate for the performance loss of dominant ones with possibly even drier conditions in the future (Obeysekera et al. 2015). However, dominant/ecosystem engineer cyanobacteria tolerate desiccation, high temperatures, and UV radiation in aquatic ecosystems (Gaiser et al. 2011; Karsten and Holzinger 2014; Brasil et al. 2016). In lakes and estuaries that are becoming warmer, more polluted, and/or more saline, the dominance of microscopic primary producers is also likely to increase (Carey et al. 2012; Kosten et al. 2012; Paerl and Otten 2013). In biological soil crusts limited by water scarcity, early succession cyanobacteria are becoming more dominant than late succession primary producers, such as mosses and lichens (Ferrenberg et al. 2015). Dominance was also observed to increase in tundra shrub under experimental warming (Walker et al. 2006). Further research using our indicators could evaluate whether nutrient scarcity (or excess) and desiccation stress may be increasing (decreasing) the occurrence of dominant taxa with adaptive traits in prairie wetlands (Johnson et al. 2010), lakes (Paerl and Otten 2013), floodplains (Finlayson 2013; Brasil et al. 2016), and peatlands (Fenner and Freeman 2011).
Dominance patterns of other organisms in the Everglades and elsewhere can be interpreted with the approach we eveloped. For example, primary production in this wetland’s oligotrophic marshes is dominated by sawgrass (Cladium jamaicense) and spike rush (Eleocharis cellulosa), while muhly grass (Muhlenbergia filipes) prevails in its marl prairies (Gunderson 1994). These species have been subject to the encroachment of other plants tolerating high soil P, notably cattail (Typha sp.; Osborne et al. 2014) as well as the advancement of mangroves due to higher salinity caused by sea-level rise (Teh et al. 2008). In other ecosystems facing environmental threats, algae and/or plants also dominate benthic mats (Fenner and Freeman 2011; Johnson et al. 2010; Muster et al. 2015). The impacts of nutrient load changes and drought in these landscapes may change the dominance structure of various primary producers, a topic that warrants further research.
Conclusions
We demonstrated that phosphorus scarcity and desiccation stress increase the occurrence of dominant taxa and their prevalence in biovolume terms in benthic communities in the Everglades, a globally important wetland. More oligotrophic and drier habitats host communities with fewer taxa monopolizing higher shares of algal biovolume than habitats with higher P and deeper waters. Our indicators and approach for analyzing dominance can support predictions of community structure changes in other ecosystems subject to oligotrophic and dry conditions. This can in turn help devise conservation decisions; depending on the level of anthropogenic impacts, ecosystem management requires a focus on biodiversity and/or ecosystem services (see, e.g., Schneiders et al. 2012). On the one hand, maintaining/restoring the natural flood pulse in subtropical wetlands may enhance diversity-mediated resilience to environmental changes (Marazzi et al. 2017). However, dominant taxa with adaptive traits are often key for ecosystem functioning and provide resilience to environmental changes such as eutrophication (Grman et al. 2010), oligotrophication (Horn 2003), and droughts (Brasil et al. 2016). Therefore, both species diversity and dominance should be assessed to better understand how and why communities change. In aquatic ecosystems subject to nutrient limitation and droughts, conservation should not only protect threatened and charismatic species, but also preserve shallow habitats hosting crucial microscopic primary producers. As some of the most dominant taxa perform an ecosystem engineer role (e.g., Scytonema sp.), this will likely benefit organisms with evident cultural, recreational, and economic value (e.g., fish and birds) that rely on healthy microbial mats for food, nutrients, habitat, and/or oxygen.
References
American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF) (2005) Standard methods for the examination of water and wastewater, 21st edn. APHA-AWWA-WEF, Washington DC, USA
Bellinger BJ, Gretz MR, Domozych DS, Kiemle SN, Hagerthey SE (2010) Composition of extracellular polymeric substances from periphyton assemblages in the Florida Everglades. J Phycol 46:484–496
Bornhoeft SC (2016) Influence of experimental sheet flow on aquatic food webs of the Central Everglades. Master’s thesis, Florida International University
Bothwell ML, Taylor BW, Kilroy C (2014) The Didymo story: the role of low dissolved phosphorus in the formation of Didymosphenia geminata blooms. Diatom Res 29:229–236
Brasil J, Attayde JL, Vasconcelos FR, Dantas DD, Huszar VL (2016) Drought-induced water-level reduction favors cyanobacteria blooms in tropical shallow lakes. Hydrobiologia 770:1–20
Caraco NF, Miller R (1998) Effects of CO2 on competition between a cyanobacterium and eukaryotic phytoplankton. Can J Fish Aquat Sci 55:54–62
Carey CC, Ibelings BW, Hoffmann EP, Hamilton DP, Brookes JD (2012) Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res 46:1394–1407
Chase JM (2007) Drought mediates the importance of stochastic community assembly. Proc Natl Acad Sci USA 104:17430–17434
Coleman JE (1992) Structure and mechanism of alkaline phosphatase. Annu Rev Biophys Biomol Struct 21:441–483
Downing J, Watson S, McCauley E (2001) Predicting cyanobacteria dominance in lakes. Can J Fish Aquat Sci 58:1905–1908
Duarte C (2009) Coastal eutrophication research: a new awareness. Hydrobiologia 629:263–269
EPA (1983) Methods for chemical analysis of water and wastes. Chapter 365.1 revision March 83. United States Environmental Protection Agency, Cincinnati, OH
Fenner N, Freeman C (2011) Drought-induced carbon loss in peatlands. Nat Geosci 4:895–900
Ferrenberg S, Reed SC, Belnap J (2015) Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proc Natl Acad Sci USA 112:12116–12121
Finlayson CM (2013) Climate change and the wise use of wetlands—information from Australian wetlands. Hydrobiologia 708:145–152
Flöder S, Jaschinski S, Wells G, Burns CW (2010) Dominance and compensatory growth in phytoplankton communities under salinity stress. J Exp Mar Biol Ecol 395:223–231
Gaiser EE (2009) Periphyton as an indicator of restoration in the Florida Everglades. Ecol Indic 9:537–545
Gaiser EE, Scinto LJ, Richards JH, Jayachandran K, Childers DL, Trexler JC, Jones RD (2004) Phosphorus in periphyton mats provides the best metric for detecting low-level P enrichment in an oligotrophic wetland. Water Res 38:507–516
Gaiser EE, Richards JH, Trexler JC, Jones RD, Childers DL (2006) Periphyton responses to eutrophication in the Florida Everglades: cross-system patterns of structural and compositional change. Limnol Oceanogr 51:617–630
Gaiser EE, McCormick PV, Hagerthey SE, Gottlieb AD (2011) Landscape patterns of periphyton in the Florida Everglades. Crit Rev Environ Sci Technol 41(S1):92–120
Gaiser EE, Gottlieb AD, Lee SS, Trexler JC (2015) The importance of species-based microbial assessment of water quality in freshwater Everglades wetlands. In: Entry J, Jayachandran K, Gottlieb AD, Ogram A (eds) Microbiology of the Everglades ecosystem. CRC Press, Boca Raton, pp 115–130
Gilad E, von Hardenberg J, Provenzale A, Shachak M, Meron E (2007) A mathematical model of plants as ecosystem engineers. J Theor Biol 244:680–691
Gleason PJ, Spackman WS Jr (1974) Calcareous periphyton and water chemistry in the Everglades. In: Gleason PJ (ed) Environments of South Florida: present and past. Miami Geological Society, Miami, pp 146–181
Gottlieb A, Richards J, Gaiser E (2005) Effects of desiccation duration on the community structure and nutrient retention of short and long-hydroperiod Everglades periphyton mats. Aquat Bot 82:99–112
Grman E, Lau JA, Schoolmaster DR Jr, Gross KL (2010) Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol Lett 13:1400–1410
Gunderson LH (1994) Vegetation: determinants of composition. In: Davis SM, Ogden J (eds) Everglades: the ecosystem and its restoration. St. Lucie, Delray Beach, pp 323–340
Guo Q, Rundel PW (1997) Measuring dominance and diversity in ecological communities: choosing the right variables. J Veg Sci 8:405–408
Hagerthey SE, Bellinger BJ, Wheeler K, Gantar M, Gaiser EE (2011) Everglades periphyton: a biogeochemical perspective. Crit Rev Environ Sci Technol 41(S1):309–343
Hair JF Jr, Anderson RE, Tatham RL, Black WC (1995) Multivariate data analysis, 3rd edn. Macmillan, New York
Hillebrand H, Durselen CD, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Hillebrand H, Bennett DM, Cadotte MW (2008) Consequences of dominance: a review of evenness effects on local and regional ecosystem processes. Ecology 89:1510–1520
Horn H (2003) The relative importance of climate and nutrients in controlling phytoplankton growth in Saidenbach Reservoir. Hydrobiologia 504:159–166
Huszar VLM, Reynolds CS (1997) Phytoplankton periodicity and sequences of dominance in an Amazonian flood-plain lake (Lago Batata, Pará, Brasil): responses to gradual environmental change. Hydrobiologia 346:169–181
James DA, Bothwell ML, Chipps SR, Carreiro J (2015) Use of phosphorus to reduce blooms of the benthic diatom Didymosphenia geminata in an oligotrophic stream. Freshw Sci 34:1272–1281
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108
Johnson WC, Werner B, Guntenspergen GR, Voldseth RA, Millet B, Naugle DE, Tulbure M, Carroll RWH, Olawsky C (2010) Prairie wetland complexes as landscape functional units in a changing climate. Bioscience 60:128–140
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69:373–386
Jordan F, Coyne S, Trexler JC (1997) Sampling fishes in vegetated habitats: effects of habitat structure on sampling characteristics of the 1-m2 throw trap. Trans Am Fish Soc 126:1012–1020
Junk WJ (2002) Long-term environmental trends and the future of tropical wetlands. Environ Conserv 29:414–435
Karsten U, Holzinger A (2014) Green algae in alpine biological soil crust communities: acclimation strategies against ultraviolet radiation and dehydration. Biodivers Conserv 23:1845–1858
Komárek J, Hindak F (1975) Taxonomy of new isolated strains of Chroococcidiopsis (Cyanophyceae). Arch Hydrobiol 46(Suppl.):311–329
Komárek J, Anagnostidis K (1986) Modern approach to the classification system of cyanobacteria. 2 Chroococcales. Algol Stud 56(43):157–226
Komárek J, Anagnostidis K (1989) Modern approach to the classification system of cyanobacteria. 4 Nostocales. Algol Stud 56:247–345
Komárek J, Anagnostidis K (1999) Cyanoprokaryota. I. Teil Chlorococcales. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa. Gustav Fischer, Stuttgart, pp 13–145
Kosten S, Huszar VLM, Bécares E, Costa LS, van Donk E, Hansson LA, Jeppesen E, Kruk C, Lacerot G, Mazzeo N, Meester LD, Moss B, Lürling M, Nõges T, Romo S, Scheffer M (2012) Warmer climates boost cyanobacterial dominance in shallow lakes. Glob Change Biol 18:118–126
Magurran AE, McGill BJ (2011) Biological diversity: frontiers in measurement and assessment. Oxford University Press, Oxford
Marazzi L, Gaiser EE, Jones VJ, Tobias FAC, Mackay AW (2017) Algal richness and life-history strategies are influenced by hydrology and phosphorus in two major subtropical wetlands. Freshw Biol 62:274–290
Marquardt DW (1970) Generalized inverses, ridge regression, biased linear estimation, and nonlinear estimation. Technometrics 12:591–612
McCullough Hennessy S, Deutschman DH, Shier DM, Nordstrom LA, Lenihan C, Montagne JP, Wisinski CL, Swaisgood RR (2016) Experimental habitat restoration for conserved species using ecosystem engineers and vegetation management. Anim Conserv 6:506–514
Muster C, Gaudig G, Krebs M, Joosten H (2015) Sphagnum farming: the promised land for peat bog species? Biodivers Conserv 24:1989–2009
Naja GM, Childers DL, Gaiser EE (2017) Water quality implications of hydrologic restoration alternatives in the Florida Everglades USA. Restor Ecol. doi:10.1111/rec.12513
Obeysekera J, Barnes J, Nungesser M (2015) Climate sensitivity runs and regional hydrologic modeling for predicting the response of the greater Florida Everglades ecosystem to climate change. Environ Manag 55:749–762
Oliver TH, Heard MS, Isaac NJ, Roy DB, Procter D, Eigenbrod F, Freckleton R, Hector A et al (2015) Biodiversity and resilience of ecosystem functions. Trends Ecol Evol 30:673–684
Osborne TZ, Reddy KR, Ellis LR, Aumen NG, Surratt DD, Zimmerman MS, Sadle J (2014) Evidence of recent phosphorus enrichment in surface soils of Taylor Slough and northeast Everglades National Park. Wetlands 34(Suppl 1):S37–S45
Paerl HW, Otten TG (2013) Harmful cyanobacterial blooms: causes, consequences and controls. Microb Ecol 65:995–1010
Passy SI (2008) Continental diatom biodiversity in stream benthos declines as more nutrients become limiting. Proc Natl Acad Sci USA 105:9663–9667
Patrick R, Crum B, Coles J (1969) Temperature and manganese as determining factors in the presence of diatom or blue–green algal floras in streams. Proc Natl Acad Sci USA 64:472–478
Philippi T (2005) CERP MAP implementation: transect and sentinel site sampling design. Final Report SFWMD Agreement CP 040131:42
Pisani O, Yamashita Y, Jaffé R (2011) Photo-dissolution of flocculent, detrital material in aquatic environments: contributions to the dissolved organic matter pool. Water Res 45:3836–3844
Potts M (1994) Desiccation tolerance of prokaryotes. Microbiol Rev 58:755–805
Prescott GW (1962) Algae of the western Great Lakes Area, revised edn. Wm.C. Brown, Co, Dubuque
Reynolds CS (2006) The ecology of phytoplankton. Cambridge University Press, Cambridge
RStudio Team (2015) RStudio: integrated development for R. RStudio, Inc., Boston. http://www.rstudio.com
Schneiders A, Van Daele T, Van Landuyt W, Van Reeth W (2012) Biodiversity and ecosystem services: complementary approaches for ecosystem management? Ecol Indic 21:123–133
Sharma K, Inglett PW, Reddy KR, Ogram AV (2005) Microscopic examination of photoautotrophic and phosphatase-producing organisms in phosphorus-limited Everglades periphyton mats. Limnol Oceanogr 50:2057–2062
Smith VH (2003) Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ Sci Pollut Res 10:126–139
Solorzano L, Sharp JH (1980) Determination of total dissolved P and particulate P in natural waters. Limnol Oceanogr 25:754–758
Stevens DL, Olsen AR (2004) Spatial balanced sampling of natural resources. J Am Stat Assoc 99:262–278
Stevenson RJ, Bahls LL (1999) Periphyton protocols. In: Barbour MT, Gerritsen J, Snyder BD (eds) Rapid bioassessment protocols for use in wadeable streams and rivers: periphyton, benthic macroinvertebrates, and fish, 2nd edn. 6-1-6-22. U.S. Environmental Protection Agency, Office of Water, Washington. EPA 841-B-99-002
Stevenson RJ, McCormick PV, Frydenborg R (2002) Methods for evaluating wetland condition. #11 using algae to assess environmental condition in wetlands. United States Environmental Protection Agency EPA-822-R-02-021
Stockner JG, Rydin E, Hyenstrand P (2000) Cultural oligotrophication: causes and consequences for fisheries resources. Fisheries 25:7–14
Swift DR, Nicholas RB (1987) Periphyton and water quality relationships in the Everglades water conservation areas, 1978–1982. Technical publication 87-2, South Florida Water Management District, West Palm Beach, FL
Teh SY, Deangelis DL, Sternberg LS, Miralles-Wilhelm FR, Smith TJ III, Koh H-L (2008) A simulation model for projecting changes in salinity concentrations and species dominance in the coastal margin habitats of the Everglades. Ecol Model 213:245–265
Trexler JC, Loftus WF (2016) Invertebrates of the Florida Everglades. In: Batzer D, Boix D (eds) Invertebrates in freshwater wetlands: an international perspective on their ecology. Springer, New York, pp 321–356
Vu HD, Wieski K, Pennings SC (2017) Ecosystem engineers drive creek formation in salt marshes. Ecology 98:162–174
Walker MD, Wahrenb HC, Hollisterc RD, Henryd GHR, Ahlquistf LE, Alatalog JM, Bret-Harteh MS, Calefh MP et al (2006) Plant community responses to experimental warming across the tundra biome. Proc Natl Acad Sci USA 103:1342–1346
Wehr JD, Sheath RG, Kociolek JP (eds) (2015) Freshwater algae of North America. Academic Press, San Diego
Williams WJ, Büdel B, Reichenberger H, Rose N (2014) Cyanobacteria in the Australian northern savannah detect the difference between intermittent dry season and wet season rain. Biodivers Conserv 23:1827–1844
Wright JP, Jones CG, Flecker AS (2002) An ecosystem engineer, the beaver, increases species richness at the landscape scale. Oecologia 132:96–101
Zuur A, Ieno EN, Smith GM (2007) Analyzing ecological data. Springer, Berlin
Acknowledgements
The authors thank the South Florida Water Management District and the United States Army Corps of Engineers for funding fieldwork and laboratory analyses, and Florida International University staff for conducting fieldwork and microscope analyses. Joel Trexler led the CERP-MAP sampling program; Viviana Mazzei, John Kominoski, Anson Mackay, Eric Sokol, Mike Rugge, and two anonymous reviewers provided useful comments and advice. This research was developed in collaboration with the Florida Coastal Everglades Long Term Ecological Research program under Cooperative Agreements #DEB-1237517, #DBI-0620409, and #DEB-9910514. This is contribution number 843 from the Southeast Environmental Research Center in the Institute of Water & Environment at Florida International University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bas W. Ibelings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marazzi, L., Gaiser, E.E. & Tobias, F.A.C. Phosphorus scarcity and desiccation stress increase the occurrence of dominant taxa in wetland benthic primary producer communities. Aquat Ecol 51, 571–589 (2017). https://doi.org/10.1007/s10452-017-9637-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-017-9637-0