Abstract
Trichoderma asperellum strain T34 has been reported to control the disease caused by Fusarium oxysporum f.sp. lycopersici (Fol) on tomato plants. To study the importance of iron concentration in the growth media for the activity and competitiveness of T34 and the pathogen, we tested four iron concentrations in the nutrient solution [1, 10, 100, and 1000 µM provided as EDTA/Fe(III)] in a biological control experiment with T34 and Fol in tomato plants. The reduction of the Fusarium-infected shoot by T34 was only significant at 10 µM Fe. We hypothesized that Fe competition is one of the key factors in the biocontrol activity exerted by T34 against Fol, as an increase in Fe concentration over 10 µM would lead to the suppression of T34 siderophore synthesis and thus inhibition of Fe competition with Fol. T34 significantly reduced the populations of Fol at all the doses of Fe assayed. In contrast, Fol enhanced the populations of T34 at 1 and 10 µM Fe. Nevertheless, several plant physiological parameters like net CO2 assimilation (A), stomatal conductance (gs), relative quantum efficiency of PSII (ΦPSII), and efficiency of excitation energy capture by open PSII reactive centers (Fv′/Fm′) demonstrated the protection against Fol damage by treatment with T34 at 100 µM Fe. The first physiological parameter affected by the disease progression was gs. Plant dry weight was decreased by Fe toxicity at 100 and 1,000 µM. T34-treated plants had significantly greater heights and dry weights than control plants at 1,000 µM Fe, even though T34 did not reduce the Fe content in leaves or stems. Furthermore, T34 enhanced plant height even at the optimal Fe concentration (10 µM) compared to control plants. In conclusion, T. asperellum strain T34 protected tomato plants from both biotic (Fusarium wilt disease) and abiotic stress [Fe(III) toxic effects].
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iron is an essential micronutrient for all living organisms. Depending on its solubility, which is affected by soil/substrate properties such as pH, redox potential, and abundance, plants develop strategies to overcome the deficiency and toxicity of this metal [17]. Fe(II) is relatively soluble but unstable under oxidized conditions. The solubility of Fe(III) decreases dramatically with increasing pH. Meanwhile, free Fe(III) is soluble up to 10−6 M at pH 3.3, while this concentration is only 10−17 M Fe(III) at pH 7 [22]. Fe(III) concentration for appropriate plant uptake ranges from 10−4 to 10−8 M Fe [39]. Thus, at the usual Fe concentration found in non-acidic soils, appropriate Fe uptake can be explained only by the presence of mechanisms by which plant roots can uptake soil Fe. Marschner [22] proposed that all higher plants, except Gramineae, improve Fe uptake by excreting protons which acidify the surrounding solution by reducing Fe(III) to Fe(II) and by transporting Fe(II) through the cell membrane by means of Fe transporters. In contrast, Gramineae release phytosiderophores through roots which specifically chelate Fe(III), with even the phytosiderophore–Fe(III) complex taken up by root cells where Fe(III) is reduced to Fe(II). Soil microorganisms release sidorephores, which can also contribute to Fe plant nutrition. In this regard, Arabidopsis plants incorporate Fe–pyoverdine complexes produced by rhizospheric microbes [36]. Synthetic chelates can also be effective in providing Fe to plants, which explains their efficiency in correcting Fe deficiency, e.g., the uptake of metal complexed to ethylenediaminetetraacetic acid (EDTA) by plants can account for much of the total metal uptake when it is supplied in this form. As much as 50% of the total Fe in the xylem of Brassica juncea is present as Fe–EDTA [31]. The uptake of metal–EDTA complexes by Phaseolus vulgaris and Solanum tuberosum and their transport from roots to shoots has also been described [11, 30].
Like plants, fungi can take up Fe before reduction and reduce it before uptake. Many fungi secrete specific hydroxamate siderophores in Fe-deficient media. This strategy is more efficient at low Fe concentrations, but both mechanisms, internal and external reduction, are important in many fungi [14]. In Fusarium graminearum, iron permeases and siderophore transporters have been described [26, 27]. However, permeases do not play a significant role in the pathogenicity of this fungus. Siderophore production by Fusarium venenatum decreased with the addition of 1 µM soluble Fe(III) to the medium and was almost completely repressed by the addition of 7 µM soluble Fe(III). These observations reveal that the activity of this mechanism is decreased at increased Fe concentration in the media [38].
Some Trichoderma atroviride strains isolated from contaminated soil accumulate 4.5 mg g−1 of Fe when the medium is amended with up to 1 g L−1 Fe(III) [18]. Dutta et al. [16] reported that the Trichoderma strains studied (Trichoderma viride, Trichoderma harzianum, and Trichoderma lignorum) were better siderophore producers than the Fusarium strains tested (Fusarium solani and F. oxysporum) and that the optimal siderophore production was found at a concentration of between 1.5 and 21.0 µM Fe(III). Under conditions of Fe deficiency, the culture filtrate of six Trichoderma strains from different species contained the siderophores coprogen, coprogen B, and ferricrocin [5]. In addition, two of them produced siderophores of fusigen type. On the other hand, T. harzianum strain T22 solubilizes insoluble Fe by producing Fe chelates and through redox activity, but not through changes in pH [4].
According to Alabouvette [2], low Fe availability in the substrate can induce siderophore production and competition for Fe, a mechanism used by certain antagonists of Fusarium wilt. The biological control agent (BCA) Trichoderma asperellum strain T34 has been reported to control Fusarium wilt of tomato [12]. Some Trichoderma BCAs produce highly efficient siderophores that chelate Fe and stop the growth of other fungi [9]. In addition, T. harzianum strain T35 controls F. oxysporum by competing for both rhizosphere colonization and nutrients, with biocontrol becoming more effective as the nutrient concentration decreases [34]. However, the mechanisms by which biocontrol agents from the genus Trichoderma control fungal diseases have not been fully defined and are strain-specific [37].
Fe toxicity is a severe growth-limiting factor in flooded rice, as the excess of water-soluble Fe retards crop growth. Fe toxicity is a widespread problem for rice cultivation in India and Southeast Asia [6].
Our aims were: (1) to examine whether the capacity of T. asperellum strain T34 to control F. oxysporum f.sp. lycopersici in tomato plants depends on the Fe concentration in the growth media (perlite) and (2) to study putative competition for Fe (in the plant growth media) between the BCA T. asperellum strain T34 and the plant pathogen F. oxysporum.
Material and Methods
Fungal Strains and Inoculum Preparations
T. asperellum strain T34 (T34) [35] stored in silica gel at 4°C was grown in a liquid medium containing 10 g/L malt for 5 days at 25°C. The medium was agitated in a horizontal shaker at 150 rpm. The culture was filtered through a 50-µm nylon mesh to remove the mycelium and centrifuged at 10,000×g (4°C) in a J-21 C Beckman centrifuge. The pellet was washed twice in sterile distilled water to obtain medium-free conidia. A conidia suspension was prepared in distilled water to inoculate perlite (Europerl, Europerlita Española S. A., Barcelona, Spain) at the final concentration of 104 conidia per milliliter of perlite. Inoculated perlite was kept at 25°C for 2 weeks before beginning the bioassay.
Fusarium oxysporum f. sp. lycopersici (Fol) was grown and prepared as described for T34. A conidia suspension was prepared in water in order to inoculate the plant growth media at 105 conidia per milliliter of perlite.
Plant Material and Bioassay
Plant material was treated as described in Nogues et al. [24] with some modifications. Tomato plants (Lycopersicon esculentum Mill. cv. Roma) from Semillas Fitó (Barcelona, Spain) were grown in a controlled environmental chamber (EGC walk-in chamber). Tomato seeds were first germinated in sterile perlite trays for 10 days, and when the second true leaf appeared, seedlings were transplanted to 80 pots of 0.4 L (four plants/pot).
Microorganism Treatments
Half the pots (40) were filled with perlite that had been previously inoculated with the BCA T34 and the remaining pots were filled with untreated perlite. Next, half of the untreated and T34-treated pots were inoculated with Fol conidial suspension to obtain an inoculum of 105 conidia per milliliter of perlite, thus obtaining four treatments: control, T34, T34 + Fol, and Fol. This point was considered the beginning of the bioassay.
Fe Treatment
At the beginning of the bioassay, the 80 pots were separated into four Fe treatments. During the 21 days of the bioassay, the plants were watered with a modified half-strength Hoagland solution (7 mM NO −3 , 1 mM NH +4 , 1 mM PO4 −x, 3 mM K+, 2 mM Ca++, 0.5 mM Mg++, 0.5 mM SO −24 , 15 µM Mn, 5 µM Zn, 9 µM B, 5 µM Cu, 0.9 µM Mo, and 0.3 µM Co) containing four concentrations of Fe (1, 10, 100, and 1,000 µM) provided as EDTA/Fe(III). Ten micromolars is a standard Fe concentration for plant nutrient solutions. pH was adjusted to 6.0–6.5. These four Fe treatments crossed with the four treatments described above produced a total of 16 combinations with five pots each (each one containing four plants) with a total of 320 plants. The bioassay was performed twice in separate periods of time. Plants received 25 mL of nutrient solution every 3 days during the first week, every 2 days during the second week, and every day during the third week of the bioassay. Plants were watered with distilled water when required. The photosynthetically active photon flux density in the growth chamber was 200 µmol m−2 s−1 during the 16-h photoperiod and the temperature was maintained at 25°C.
Assessment of Disease Severity
Disease symptoms were observed 14 days after Fol inoculation (beginning of the bioassay), and 7 days later, the bioassay was finished. Disease severity was scored as the percentage of infected shoot length by cutting cross-sections of the shoot every 1 cm beginning from the bottom and measuring the length of browned xylem, which indicates the presence of the pathogen [12].
Determination of Fungal Populations
At the end of the experiment, samples from the growth media were serially diluted and plated onto semi-selective media for Trichoderma spp. [10] and Fusarium spp. [20] to determinate the populations of the BCA T34 and the pathogen Fol, respectively.
Leaf Gas Exchange and Fluorescence Analysis
Fourteen days after the beginning of the bioassay (at the onset of disease symptoms), leaf gas exchange measurements and fluorescence analysis were performed using an infrared gas analyzer (model LI-6400, Li-Cor Inc., Lincoln, NE, USA). The youngest fully expanded leaves were used for all measurements. Four plants were randomly selected for each treatment. Net CO2 assimilation (A), stomatal conductance to water vapor (g s), the efficiency of excitation energy capture by open PSII reactive centers (Fv′/Fm′), the maximum quantum efficiency of PSII photochemistry (Fv/Fm), the relative quantum efficiency of PSII electron transport (Φ PSII), and photochemical quenching (q p) were measured. Measurements of Fv/Fm were made after dark adaptation of plants for 15 min [24].
Height and Dry Weight Measurements
At the end of the experiment, we recorded the height and the dry weight of the aerial part of each plant. This part was dried at 60°C until constant weight to measure dry weight.
Fe Content Measurement in Tomato Plants
Fourteen days after the beginning of the bioassay, Fe content was measured in leaves and stems and roots as described previously [33]. Fe determination was performed by inductively coupled plasma optical emission spectrometry using a Perkin-Elmer apparatus, model Optima-3200RL. Three plants per treatment were randomly selected and used as replicates.
Statistical Treatment
The percentage of infected shoot length, A, g s, Fv′/Fm′, Φ PSII, Fv/Fm, q p, plant height, plant dry weight, and Fe content were subjected to analysis of variance (ANOVA) for factor Fe concentration/treatment, for each treatment, or for each Fe concentration. When significant differences were observed (P = 0.05), Duncan’s multiple range test was performed. Data were analyzed by SPSS 11.5 software.
Results
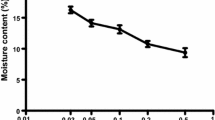
Plants inoculated with Fol (T34 + Fol and Fol treatments) showed browning of the xylem as a result of pathogen colonization of the tissue (Fig. 1). The percentage of infected shoot length was higher as the Fe concentration in the nutrient solution increased; however, the percentage of infected shoot length for Fol-treated plants at 10 and 100 µM Fe were not significantly different and neither were the values for T34 + Fol plants at 1, 10, and 100 µM Fe. Plants in the T34 + Fol treatment showed a lower percentage of infected shoot than those in the Fol treatment; however, the protective effect of T34 was significant (p ≤ 0.05) only at 10 µM Fe. Plants not inoculated with the pathogen did not show signs of disease (Fig. 1).
Infected shoot length (%) of tomato plants at the end of the bioassay (21 days) amended with nutrient solution containing a range of Fe concentrations supplied as EDTA–Fe. Control, non-inoculated control plants; T34, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media; T34 + Fol, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media and F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media; Fol, plants inoculated with F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media. Within each Fe concentration, different lowercase letters represent significant differences. Within each treatment, different capital letters show significant differences across Fe concentrations
Pathogen populations in the growth media of Fol-treated plants at the end of the experiment were higher with increased Fe concentration in the nutrient solution (Fig. 2). In contrast, populations of Fol in the substrate treated with T34 + Fol did not show a significant increase across the Fe treatments (Fig. 2). Accordingly, the T34 + Fol treatment significantly reduced the populations of Fol compared to Fol treatment alone across all the Fe concentrations tested. Substrates not treated with Fol did not contain populations of the pathogen.
F. oxysporum f.sp. lycopersici colony-forming units per milliliter of growth media of tomato plants at the end of the bioassay (21 days) amended with nutrient solution containing a range of Fe concentrations supplied as EDTA–Fe. Control, non-inoculated control plants; T34, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media; T34 + Fol, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media and F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media; Fol, plants inoculated with F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media. Within each Fe concentration, different lowercase letters represent significant differences. Within each treatment, different capital letters show significant differences across Fe concentrations
T34 populations were not significantly affected by the Fe treatments (Fig. 3). However, Fe treatments influenced the way in which Fol affected T34 populations: at 1 and 10 µM Fe, the presence of Fol increased T34 populations, whereas at 100 µM Fe, populations were not affected by Fol, and at 1,000 µM Fe, they were decreased by the pathogen compared with T34-inoculated media without Fol.
T. asperellum strain T34 colony-forming units per milliliter of growth media of tomato plants at the end of the bioassay (21 days) amended with nutrient solution containing a range of Fe concentrations supplied as EDTA–Fe. Control, non-inoculated control plants; T34, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media; T34 + Fol, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media and F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media; Fol, plants inoculated with F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media. Within each Fe concentration, different lowercase letters represent significant differences. Within each treatment, different capital letters show significant differences across Fe concentrations
The presence of Fol significantly affected the growth of the plant, as expected (Figs. 4 and 5). In general, plants treated with Fol were shorter and had lower dry weights than non-infected plants. However, plants in the T34 + Fol treatment had significantly greater heights and weights than those that received Fol alone (except for 1 µM Fe and dry weight).
Height of tomato plants at the end of the bioassay (21 days) amended with nutrient solution containing a range of Fe concentrations supplied as EDTA–Fe. Control, non-inoculated control plants; T34, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media; T34 + Fol, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media and F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media; Fol, plants inoculated with F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media Within each Fe concentration, different lowercase letters represent significant differences. Within each treatment, different capital letters show significant differences across Fe concentrations
Dry weight of tomato plants at the end of the bioassay (21 days) amended with nutrient solution containing a range of Fe concentrations supplied as EDTA–Fe. Control, non-inoculated control plants; T34, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media; T34 + Fol, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media and F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media; Fol, plants inoculated with F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media. Within each Fe concentration, different lowercase letters represent significant differences. Within each treatment, different capital letters show significant differences across Fe concentrations
The optimal concentration of Fe for control plants in terms of plant dry weight was 10 µM (Fig. 5). Plant dry weight was significantly decreased by Fe toxicity at 100 and 1,000 µM and only at 1,000 µM for plant height. However, plants from the T34 treatment were significantly taller (Fig. 4) and showed greater dry weights (Fig. 5) than control plants at 1,000 µM Fe. Furthermore, T34-treated plants were taller than control plants even at the optimal Fe concentration (Fig. 4). At 1,000 µM Fe, most of the plants showed bronzing, consisting of numerous pinpoint spots that start off yellow and quickly turn bronze [3].
In a similar way, both Fol and Fe toxicity affected plant gas exchange parameters (Table 1). The tendencies in A were almost identical to those observed in plant height or weight. However, at 1 µM Fe, no differences were observed between treatments (although plants were diseased), and at 10 µM, no significant differences in A were observed between control, T34, and T34 + Fol-treated plants. At 10, 100, and 1,000 µM Fe, the A values of plants treated with Fol were severely affected. At 100 µM Fe, T34 protected plants against the negative effects induced by Fol on A, even though A values were lower than in control plants. At 1,000 µM, Fe toxicity affected A values in all treatments; however, T34-treated plants were protected to some extent (Table 1). The plant parameter g s showed identical trends as those of A, except that at 1 µM Fe, plants in the T34 + Fol and Fol treatments had lower g s values than control plants (Table 1).
No significant differences between treatments were found in Fv/Fm values (Table 1). Φ PSII and Fv′/Fm′ values had a trend almost identical to that of A (Table 1). q p values were negatively affected by Fe concentration and Fol treatment; however, in this case, T34 treatment protected plants from Fol, but not from high Fe concentrations (Table 1).
Fe content in the leaves and stems of tomato plants rose at increased concentrations of Fe in the nutrient solution in all treatments (Fig. 6). However, the trends differed depending on the treatment. At 1 and 10 µM Fe, plants treated with Fol alone had higher concentrations of Fe than the rest of the plants. Fe content in the roots increased with increasing Fe concentration in the nutrient solution in all treatments, but in higher amounts than in leaves and stems (Fig. 6). At 10 µM Fe, plants treated with Fol alone had higher root Fe content than the rest of the plants, although at 100 µM Fe, the opposite occurred. Interestingly, at 1,000 µM Fe, plants treated with T34 (alone or in combination with Fol) had higher root Fe contents than control or Fol-treated plants (Fig. 6).
Fe concentration in the leaves and stems (a) and roots (b) of tomato plants 14 days after the beginning of the bioassay amended with nutrient solution containing a range of Fe concentrations supplied as EDTA–Fe. Control, non-inoculated control plants; T34, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media; T34 + Fol, plants inoculated with T. asperellum strain T34 at 104 cfu mL−1 growth media and F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media; Fol, plants inoculated with F. oxysporum f.sp. lycopersici at 105 cfu mL−1 growth media. Within each Fe concentration, different lowercase letters represent significant differences. Within each treatment, different capital letters show significant differences across Fe concentrations. N.d. Not determined
Discussion
T. asperellum strain T34 protected tomato plants from both Fusarium wilt disease and the toxic effects of Fe(III) in soilless culture conditions. The reduction of the infected shoot was apparent only at 10 µM Fe. However, at 100 µM Fe, T34 protected several plant physiological parameters (A, gs, ΦPSII, Fv′/Fm′) against the damage induced by Fol. Siderophore production by Trichoderma spp. is higher than in Fusarium spp. and is inhibited at high concentrations of Fe in the media [16, 38]. This observation implies that at 10 µM Fe (which has been shown to be an optimal concentration in the growing media), T34 competes for Fe more efficiently than Fol. We hypothesize that the increment of Fe concentration to 100 µM would lead to the suppression of T34 siderophore synthesis and thus inhibition of Fe competition with Fol. Thus, we propose that Fe competition is one of the key factors in T34 biocontrol activity against Fol. However, in contrast to what is observed in T. harzianum strain T35 [34], strain T34 did not reduce disease under low Fe availability (1 µM). This finding can be attributed to the reduced disease severity detected in Fol-treated plants at 1 µM Fe. According to previous observations, a minimum degree of disease severity is required to obtain a significant reduction by T34 [12]. The low availability of Fe at this concentration could be responsible for the poor performance of both microorganisms. Although we observed that T34 controlled Fol populations even at high Fe concentrations (100 and 1,000 µM), Fe enhanced Fol virulence and the pathogen escaped the biocontrol effect at the two highest Fe concentrations tested. These observations support the notion that T34 controls Fol through Fe competition independently of other fungistatic or fungitoxic mechanisms which regulate Fol populations in the media. It is well established that Fe availability in the soil or growth substrate affects the virulence of Fusarium wilt diseases [8, 40]. Fe, together with other 11 elements (C, H, O, N, P, K, Mg, S, Mn, Mo, Zn), are essential for the normal growth, sporulation, and survival of the fungus [40]. The observation that T34 populations are increased by Fol under 1 and 10 µM Fe suggests the occurrence of mycoparasitism (Fig. 3). Both mycoparasitism and competition for nutrients have been reported as biocontrol mechanisms exerted by Trichoderma strains [7]. The presence of T34 in the perlite during the bioassay significantly reduced the number of Fol colonies compared to Fol alone: by 46% at 1 µM Fe, by 68% at 10 µM Fe, by 66% at 100 µM Fe, and by 73% at 1,000 µM Fe. These findings reinforce the statement that T34 either parasite Fol and/or compete with the pathogen for nutrients, including Fe.
In addition to direct effects on pathogen populations, T34 induces resistance to further pathogen attacks [32].
Another interesting mechanism has been described in the biocontrol of Fol by a strain of Penicillium oxalicum. P. oxalicum-treated plants took up more nutrient solution than non-treated plants when both were infected with Fol. This observation suggests that P. oxalicum partially prevents the blocking or collapse of xylem vessels in infected plants [15]. Furthermore, P. oxalicum treatment protected the plants infected with Fol from losing their cambium, thus favoring the formation of additional secondary xylem while reducing the number of bundles [13].
At symptom onset, the Fv/Fm of tomato plants remained unaffected across all the treatments. Decreases in Fv/Fm are indicative of photo-inhibitory damage in response to stress [23]. Accordingly, Nogues et al. [24] described no changes in the Fv/Fm of tomato plants infected with Fol compared with controls until 31 days after the beginning of a bioassay, similar to that described in the present article (using a standard nutrient solution containing 10 µM Fe). They also described an earlier decrease in Φ PSII, Fv′/Fm′, and q p induced by Fol infection, findings also made in the present work. It has been suggested that the large decreases in A accompanied by more moderate decreases in Fv′/Fm′ indicate that the demand for reductants and ATP have largely decreased in the plant and that this is a major factor in the closure of PSII reaction centers [25]. Interestingly, g s is the first parameter to be affected by disease progression. At low disease severity (1 µM), Fol-treated plants showed a decrease in g s compared to control plants, while the other physiological parameters showed no differences between treatments. As proposed by Nogues et al. [24], leaf water deficit caused by Fol could account for the decreases in gas exchange caused by stomatal closure. It has been widely demonstrated that vascular pathogens increase resistance to water movement as a result of the reduced diameter of conductive elements [1].
T34 also protected plants against Fe toxicity. At the highest Fe concentration assayed, T34 exerted a protective effect, thus yielding higher dry weight, height, A, g s and Fv′/Fm′ than control plants (Table 1, Figs.4 and 5). However, the Fe concentration found in the leaves and stems of the plants at 1,000 µM Fe was similar in all the treatments. This observation indicates that T34 does not affect Fe accumulation in leaves. The concentrations of 100 and 1,000 µM Fe showed toxic effects on plant dry weight. Similarly, Nicotiana plumbaginifolia plants excised from roots treated with 100 µM Fe(III)–EDTA quickly developed a bronzing phenotype [19]. Moreover, Fe overload (500 µM) of Brassica napus causes the accumulation of ascorbate peroxidase transcripts, thus documenting a link between Fe metabolism and oxidative stress [36]. The normal Fe concentration in all tomato plant tissues ranges from 0.03 to 0.3 mg g−1 dry weight [28]. In our bioassays, the Fe concentrations in the leaves and stems of plants at 14 days were within this range, except the control and T34-treated plants at 1,000 µM Fe. Although the Fe concentrations measured in the plants exposed to 1,000 µM Fe were not in the critical range (0.4–1.0 mg g−1 dry weight), typical symptoms of Fe toxicity were observed. When Fe availability is in excess, the accumulation of this metal in plant tissues increases with time [21]. It can be assumed that higher concentrations of Fe would have been found if the analysis had been performed after a longer assay. Although plants treated with 1 µM Fe were under suboptimal growth conditions, they did not show symptoms of Fe deficiency (the concentration of Fe in the leaves and stems was over 50 µg g−1 plant dry weight, which is considered to be the limit for Fe deficiency). In contrast, at 1,000 µM Fe, the dry weight per plant was higher in T34-treated plants than in controls (188% increase); thus, the total amount of Fe accumulated per plant was higher in the former. This observation calls for further studies using heavy metals as it may be relevant in the field of phytoremediation. Fe content in roots rose with increasing Fe concentration in the nutrient solution in all treatments, but more intensely in leaves and stems. Similarly, the concentration of Fe in rice shoots in a range of Fe concentrations was lower than that of root, which is explained by higher Fe accumulation by the latter [6]. It has been reported that Fe toxicity is caused by higher concentration of this metal in the root zone and not by a higher concentration in leaves [29]. However, in the present study, T34-treated plants, which showed greater root Fe at 1,000 µM Fe than control plants, appeared to be healthier. Interestingly, for Fe toxicity to occur, the metal must pass the oxidation barrier of the rhizosphere before it is taken up by the root system. If Fe escapes the oxidative barrier and penetrates the root, part of it is retained in this organ and the remainder may be translocated to the shoots via xylem after passing the barrier of the casparian strip [6].
The higher Fe content found for plants in the T34 and T34 + Fol treatments at 1,000 µM Fe can be explained either by the attachment of T34 mycelia (rich in Fe) to root surfaces or by the increased plant capacity to accumulate Fe induced by the presence of T34.
The higher Fe content found in the leaves and stems of Fol-treated plants compared to the other treatments can be explained by the reduction of biomass produced by the pathogen. However, we cannot exclude the presence of the fungi itself inside the plant tissue, which may contain considerable amounts of the nutrient, or even the production of siderophores by Fol, which could increase nutrient assimilation.
In conclusion, in tomato plants, T34 protects plants against Fusarium wilt disease through Fe competition and also exerts a strong protective effect against Fe(III) toxicity.
References
Aguirreolea J, Irigoyen J, Sanchezdiaz M, Salaverri J (1995) Physiological alterations in pepper during wilt induced by Phytophthora capsici and soil water deficit. Plant Pathol 44:587–596
Alabouvette C (1999) Fusarium wilt suppressive soils: an example of disease-suppressive soils. Australas Plant Pathol 28:57–64
Albano JP, Miller WB, Halbrooks MC (1996) Iron toxicity stress causes bronze speckle, a specific physiological disorder of marigold (Tagetes erecta L). J Am Soc Hortic Sci 121:430–437
Altomare C, Norvell WA, Bjorkman T, Harman GE (1999) Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl Environ Microbiol 65:2926–2933
Anke H, Kinn J, Bergquist KE, Sterner O (1991) Production of siderophores by strains of the genus Trichoderma—isolation and characterization of the new lipophilic coprogen derivative, palmitoylcoprogen. Biol Met 4:176–180
Baruah KK, Das S, Das K (2007) Physiological disorder of rice associated with high levels of iron in growth medium. J Plant Nutr 30:1871–1883
Benitez T, Rincon AM, Limon MC, Codon AC (2004) Biocontrol mechanisms of Trichoderma strains. Int Microbiol 7:249–260
Borrero C, Trillas MI, Ordovas J, Tello JC, Aviles M (2004) Predictive factors for the suppression of Fusarium wilt of tomato in plant growth media. Phytopathology 94:1094–1101
Chet I, Inbar J (1994) Biological control of fungal pathogens. Appl Biochem Biotechnol 48:37–43
Chung YR, Hoitink HAJ (1990) Interactions between thermophilic fungi and Trichoderma hamatum in suppression of Rhizoctonia damping off in a bark compost-amended container medium. Phytopathology 80:73–77
Collins RN, Merrington G, McLaughlin MJ, Knudsen C (2002) Uptake of intact zinc–ethylenediaminetetraacetic acid from soil is dependent on plant species and complex concentration. Environ Toxicol Chem 21:1940–1945
Cotxarrera L, Trillas-Gay MI, Steinberg C, Alabouvette C (2002) Use of sewage sludge compost and Trichoderma asperellum isolates to suppress Fusarium wilt of tomato. Soil Biol Biochem 34:467–476
De Cal A, Garcia-Lepe R, Melgarejo P (2000) Induced resistance by Penicillium oxalicum against Fusarium oxysporum f.sp lycopersici: histological studies of infected and induced tomato stems. Phytopathology 90:260–268
De Luca NG, Wood PM (2000) Iron uptake by fungi: contrasted mechanisms with internal or external reduction. Adv Microb Physiol 43:39–74
DeCal A, Pascual S, Melgarejo P (1997) A rapid laboratory method for assessing the biological control potential of Penicillium oxalicum against Fusarium wilt of tomato. Plant Pathol 46:699–707
Dutta S, Kundu A, Chakraborty M, Ojha S, Chakrabarti J, Chatterejee N (2006) Production and optimization of Fe(III) specific ligand, the siderophore of soil inhabiting and wood rotting fungi as deterrent to plant pathogens. Acta Phytopathol Entomol Hung 41:237–248
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216:541–551
Kacprzak M, Malina G (2005) The tolerance and Zn2+, Ba2+ and Fe3+ accumulation by Trichoderma atroviride and Mortierella exigua isolated from contaminated soil. Can J Soil Sci 85:283–290
Kampfenkel K, Vanmontagu M, Inze D (1995) Effects of iron excess on Nicotiana plumbaginifolia plants—implications to oxidative stress. Plant Physiol 107:725–735
Komada H (1975) Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev Plant Protect Res 8:114–125
Majerus V, Bertin P, Lutts S (2007) Effects of iron toxicity on osmotic potential, osmolytes and polyamines concentrations in the African rice (Oryza glaberrima Steud.). Plant Sci 173:96–105
Marschner H (1995) Mineral nutrition of higher plants, vol 2. Academic, London, UK
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Nogues S, Cotxarrera L, Alegre L, Trillas MI (2002) Limitations to photosynthesis in tomato leaves induced by Fusarium wilt. New Phytol 154:461–470
Nogues S, Baker NR (2000) Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J Exp Bot 51:1309–1317
Park YS, Choi ID, Kang CM, Ham MS, Kim JH, Kim TH, Yun SH, Lee YW, Chang HI, Sung HC, Yun CW (2006) Functional identification of high-affinity iron permeases from Fusarium graminearum. Fungal Genet Biol 43:273–282
Park YS, Kim TH, Chang HI, Sung HC, Yun CW (2006) Cellular iron utilization is regulated by putative siderophore transporter FgSit1 not by free iron transporter in Fusarium graminearum. Biochem Biophys Res Commun 345:1634–1642
Ramadan MAE (2007) Behaviour of trace elements in soil and two varieties of tomato under different rates of fertilizers with and without biofertilizer. J Appl Sci Res 3:1637–1645
Ramirez LMA, Claassen N, Ubiera AA, Werner H, Moawad AM (2002) Effect of phosphorus, potassium and zinc fertilizers on iron toxicity in wetland rice (Oryza sativa L.). Plant Soil 239:197–206
Sarret G, Vangronsveld J, Manceau A, Musso M, D'Haen J, Menthonnex JJ, Hazemann JL (2001) Accumulation forms of Zn and Pb in Phaseolus vulgaris in the presence and absence of EDTA. Environ Sci Technol 35:2854–2859
Schaider LA, Parker DR, Sedlak DL (2006) Uptake of EDTA-complexed Pb, Cd and Fe by solution- and sand-cultured Brassica juncea. Plant Soil 286:377–391
Segarra G, Van der Ent S, Trillas I, Pieterse CMJ (2009) MYB72, a node of convergence in induced systemic resistance triggered by a fungal and a bacterial beneficial microbe. Plant Biol 11:90–96
Segarra G, Casanova E, Borrero C, Aviles M, Trillas I (2007) The suppressive effects of composts used as growth media against Botrytis cinerea in cucumber plants. Eur J Plant Pathol 117:393–402
Tjamos EC, Papavizas GC, Cook RJ (1992) Biological control of plant diseases progress and challenges for the future. Plenum, New York (in cooperation with NATO Scientific Affairs Division)
Trillas I, Cotxarrera L (2003) Substrates containing a Trichoderma asperellum strain for biological control of Fusarium and Rhizoctonia. Patent WO 03/000866 A1
Vansuyt G, Robin A, Briat JF, Curie C, Lemanceau P (2007) Iron acquisition from Fe–pyoverdine by Arabidopsis thaliana. Mol Plant Microbe Interact 20:441–447
Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M (2008) Trichoderma–plant–pathogen interactions. Soil Biol Biochem 40:1–10
Wiebe MG (2002) Siderophore production by Fusarium venenatum A3/5. Biochem Soc Trans 30:696–698
Winkelmann G, Van der Helm D, Neilands JB (1987) Iron transport in microbes, plants, and animals. VCH, Weinheim, Federal Republic of Germany
Woltz SS, Jones JP (1981) Nutritional requirements of Fusarium oxysporum: basis for a disease control system. In: Nelson PE, Tousson TA, Cook RJ (eds) Fusarium: diseases, biology and taxonomy. The Pennsylvania State University Press, University Park, pp 340–349
Acknowledgments
We thank Dr. Antonio Delgado for helpful comments on the manuscript. We also thank the Departament d'Universitats, Recerca i Societat de la Informació of the Government of Catalonia, and the European Social Fund for subsidizing Guillem Segarra’s Ph.D. studentship. This study was supported by the Ministerio de Educación y Ciencia (AGL2005-08137-C03-01), Spain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Segarra, G., Casanova, E., Avilés, M. et al. Trichoderma asperellum Strain T34 Controls Fusarium Wilt Disease in Tomato Plants in Soilless Culture Through Competition for Iron. Microb Ecol 59, 141–149 (2010). https://doi.org/10.1007/s00248-009-9545-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-009-9545-5