Abstract
Microorganisms mediate the decomposition of leaf-litter through the release of extracellular enzymes. The surfaces of decomposing leaves are both chemically and physically heterogeneous, and spatial patterns in microbial enzyme activity on the litter surface should provide insights into fine-scale patterns of leaf-litter decomposition. Platanus occidentalis leaves were collected from the floodplain of a third-order stream in northern Mississippi, enclosed in individual litter bags, and placed in the stream channel and in the floodplain. Replicate leaves were collected approximately monthly over a 9-month period and assayed for spatial variation in microbial extracellular enzyme activity and rates of organic matter (OM) decomposition. Spatial variation in enzyme activity was measured by sampling 96 small discs (5-mm diameter) cut from each leaf. Discs were assayed for the activity of enzymes involved in lignin (oxidative enzymes) and cellulose (β-glucosidase, cellobiohydrolase) degradation. Rates of OM loss were greater in the stream than the floodplain. Activities of all enzymes displayed high variability in both environments, with severalfold differences across individual leaves, and replicate leaves varied greatly in their distribution of activities. Geostatistical analysis revealed no clear patterns in spatial distribution of activity over time or among replicates, and replicate leaves were highly variable. These results show that fine-scale spatial heterogeneity occurs on decomposing leaves, but the level of spatial variability varies among individual leaves at the measured spatial scales. This study is the first to use geostatistical analyses to analyze landscape patterns of microbial activity on decomposing leaf litter and in conjunction with studies of the microbial community composition and/or substrate characteristics, should provide key insights into the function of these processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Leaf litter is the main source of carbon and energy in most forested, low-order streams as light availability is often restricted by the canopy and primary productivity is low [36, 50]. Litter decomposition has been extensively studied in stream ecosystems, with research focusing on determining rates of litter processing as well as the invertebrate and microbial assemblages responsible [2, 17, 49]. At the microbial level, the decomposition of leaf litter is mediated primarily by the activity of microbial extracellular enzymes such as lignin and cellulose-degrading enzymes [46]. These extracellular enzymes are required for the remineralization of leaf nutrients and the breakdown of structural macromolecules into molecules more readily transported across the microbial cell membrane [9, 51]. The release of extracellular enzymes occurs in response to fine-scale interactions between microorganisms and their immediate environment [1, 18], and interactions among microbial populations or between microorganisms and their environment are an integral component of biogeochemical processes [7, 41, 43].
The surface of living leaves, or phyllosphere, is a highly heterogeneous surface and supports diverse and spatially variable assemblages of bacteria and fungi [32]. Diverse microbial communities are also found colonizing the surfaces of decaying leaves, and fungal species have been observed to have variable spatial distributions across the surface of decaying leaves [8, 45]. Attachment of fungal spores has also been shown to be spatially variable on decomposing leaves [10, 24], and successional patterns of fungal and bacterial communities have been described on decaying leaves, suggesting that the spatial distribution of microorganisms may change over time [11, 40]. However, studies of spatial or temporal variation in the activity of microbial communities on decomposing leaf litter at fine scales are lacking. The majority of studies that have looked at spatial variation in leaf litter decomposition have focused on large-scale patterns, such as differences in decomposition rates between streams [3, 34] or between different locations within the same system [25–27]. Other studies have compared decomposition rates of different plant species [16, 37, 44] or how combinations of different leaf species affect decomposition rates [15, 28, 31]. Little is known about the variability in leaf litter decomposition at scales less than an individual leaf.
While litter decomposition rates would be difficult to determine for different parts of an individual leaf, microbial activity could be measured at these scales. Rates of leaf litter and particulate organic matter (POM) decomposition have been linked to microbial extracellular enzyme activity in a number of studies [20, 21, 48], and measuring fine scale patterns in microbial extracellular enzyme activity may allow us to detect fine-scale patterns in leaf litter decomposition. In this study, we measured lignocellulase activity of microbial communities associated with the surface of individual leaves as they decomposed in a stream and its floodplain. Geostatistical analyses were used to assess patterns in the variability and spatial distribution of microbial activity across the leaf surface. We hypothesized that the spatial distribution of enzyme activity would become more homogeneous over the course of decomposition, as greater areas of the leaves became colonized and that this homogenization would occur faster in the stream channel (where leaves were submerged) than the floodplain.
Methods
Study Site and Sample Collection
American Sycamore (Platanus occidentalis) leaves were collected in January 2007 from the floor of a hardwood forest in the floodplain of Cypress Creek, an oligotrophic, temperate, third-order stream in Holly Springs National Forest, northern Mississippi. P. occidentalis is a native species to the region [6]. Leaves were air dried for 5 days at 22°C and weighed. Ten air-dried leaves were oven dried (65°C, 24 h), reweighed, and used as a reference to calculate the actual initial dry mass of each leaf (i.e., to convert air-dried mass to oven-dried mass). This step circumvented the need to oven dry the leaves used in the decomposition study [5]. These same ten leaves were ashed (500°C, 2 h), reweighed, and used as a reference for calculating the initial organic content (as ash free dry mass; AFDM) of other leaves. Remaining air-dried leaves were placed individually in litter bags constructed from 0.5 mm fiberglass mesh. Litter bags were spread out so as not to overlap each other (naturally occurring leaf litter at the study site is thinly dispersed) and anchored in the stream channel and floodplain. Litterbags were sampled after 2 days in both the stream and floodplain to provide an initial sample point to correct for losses associated with handling and leaching. Bags were then sampled after 16 days, and then monthly for a period of 9 months. Four replicate litterbag samples were collected from both the floodplain and the stream on each sampling date and assayed for enzymatic activity and the amount of AFDM (i.e., organic matter) remaining. HOBO Water Temp Pro [H20-001] data loggers were attached in both the creek and floodplain to get a continuous measure of the temperature over the study period.
Enzyme Assays
Thirty-two small circular discs (5 mm diameter) were cut at random from across the surface of the leaf for measurement of one of the three extracellular enzymes (β-glucosidase, cellobiohydrolase (CBH), and oxidative enzymes—a combination of phenol oxidase and peroxidase). Two discs within 1 cm of each of those 32 discs were then taken for measuring the activity of the other two enzymes, for a total of 96 discs per leaf. Digital images of the remaining leaf (minus discs) were taken to identify the location of sample points for subsequent geostatistical analysis. Leaf discs were incubated with 300 μl of artificial substrate. The substrates used for measurements of β-glucosidase and CBH activity were 5 mM p-nitrophenyl (pNP)-β-d-glucopyranoside and 2 mM pNP-cellobioside, respectively. The substrate for oxidative enzyme activity was 5 mM l-3,4-dihydroxyphenylalanine (l-DOPA), with the addition of 15 μl 0.3% hydrogen peroxide. All substrates were dissolved in pH 5.0 acetate buffer [22]. Duplicate sample controls for each leaf consisted of a leaf disc amended with acetate buffer. Duplicate substrate controls for each enzyme consisted of the appropriate substrate without the leaf disc.
Leaf discs were incubated for 1–3 h, and 150 μl of the reaction mixture was transferred to a microplate well. Transferred volumes from β-glucosidase and cellobiohydrolase assays received 150 μl of 0.066 M NaOH, and absorbance was measured at 405 nm [4]. Transferred volumes from the oxidative enzyme reactions were mixed with 150 μl of water and absorbance determined at 450 nm [30]. Activity was calculated by dividing the adjusted absorbance (measured absorbance − sample control − substrate control + empty well absorbance) by 22.98 for β-glucosidase and CBH (the absorbance of 1 μmol p-nitrophenol under these specific assay conditions) or 4.28 for oxidative enzymes (the absorbance of 1 μmol completely oxidized l-DOPA under these conditions). Final activity for all enzymes was expressed as μmol substrate consumed h−1 cm−2 of leaf surface.
Decomposition Rates
After enzyme assays were complete, leaf discs were recombined with their respective leaf, oven dried (65°C, 24 h), and weighed to determine dry weight remaining. Dry leaves were ashed (500°C, 2 h) and weighed to measure the amount of AFDM remaining. This value was compared to the estimated initial AFDM in the original air-dried leaf in order to determine the rate of organic matter decomposition. Decomposition rates were determined following a linear decay model (regression of percent AFDM remaining over time).
Spatial Analysis of Microbial Activity
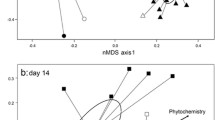
Semivariograms were created for each enzyme on each individual leaf using a linear-sill model with VESPER software [35]. To assess differences in spatial variability, the range and sill were calculated from each semivariogram [14]. The range is the distance beyond which variance is no longer a function of spatial separation, and the sill is the semivariance at which the variogram levels off [19]. Both are useful measures in determining the spatial dependence of a system, and an example of how the two measures are calculated is shown in Fig. 1a. More advanced geostatistical approaches (interpolation) were used to map microbial enzyme activity over the leaf surface using SADA software (Spatial Analysis and Decision Assistance, Version 4.1.50, The University of Tennessee). An example of the kriging analyses is displayed in Fig. 1b.
Example of analyses used to assess spatial patterns and structure in microbial activity on the surface of decomposing leaves. a Kriging map displaying the spatial distribution of β-glucosidase activity across the surface of a single leaf with activity interpolated between sample points. b Semivariogram measuring the spatial autocorrelation of β-glucosidase activity across the surface of the same leaf, showing the range and the sill
Results
Leaf litter generally followed a linear decay model for both the stream and the floodplain (Fig. 2). The decomposition rate was almost three times faster in the stream (0.11% day−1, R 2 = 0.91) than in the floodplain (0.04% day−1, R 2 = 0.76), (analysis of covariance, p < 0.001). After 9 months, there was a mean of 68.1% original AFDM remaining for leaves in the stream compared with 87.8% for leaves in the floodplain (Fig. 2).
Mean activities of β-glucosidase and CBH showed similar temporal patterns in each environment, until the final sampling date when activities of CBH and oxidative enzymes on litter in the stream were reduced (Fig. 3). Oxidative enzyme activity, however, was near the lower limit of detection for the first several months on litter in the floodplain and was an order of magnitude greater on litter in the stream than the floodplain over the remainder of the study period. Correlations between mean daily temperature and activity on litter ranged from weak (oxidative enzymes, R = 0.38) to moderate (β-glucosidase, R = 0.43, and CBH, R = 0.57) in the floodplain and from no correlation (β-glucosidase, R = −0.05) to weak (CBH, R = 0.39) to moderately strong (oxidative enzymes, R = 0.70) on leaf litter in the stream channel. Temperatures ranged from 1.9°C to 36.3°C in the stream compared to −9.5°C to 58.0°C in the floodplain, and correlations among daily low and high temperatures were no greater than daily mean temperature.
Temporal patterns in microbial extracellular enzyme activity on decomposing sycamore leaves in a stream (a, c, e) and its floodplain (b, d, f). Values represent the mean (±SE) of activity of four leaves at each point. Activity for each leaf was determined from the mean activity measured at 32 separate points on each leaf. Enzymes measured were β-glucosidase (a, b), cellobiohydrolase (c, d), and oxidative enzymes (a combination of phenol oxidase and peroxidase; e, f). Dates are 2007
At whole leaf scales, mean activities of β-glucosidase and CBH were correlated in both the stream and floodplain on individual leaves collected over the entire study period (Table 1). Correlations between mean CBH activity and oxidative enzyme activity were less strong, ranging from weak (R = 0.37, n = 36) on litter in the stream to moderate (R = 0.63, n = 36) in the floodplain. β-glucosidase activity did not correlate with oxidative enzyme activity at this scale on litter in the stream (R = −0.06, n = 36), but the two were moderately correlated (R = 0.53, n = 36) on leaf litter in the floodplain. To investigate correlations between enzymes at finer scales, we measured the correlation between enzyme activities at sample points located within 1 cm of each other over all of the leaves collected throughout the entire study period. At finer scales, correlations between β-glucosidase and CBH activities still existed (Table 1). Correlations between CBH and oxidative enzyme activities at fine scales were weaker than those observed at larger scales, with correlation coefficients of 0.24 (n = 1152) and 0.44 (n = 1152) in the stream and floodplain, respectively. As with the larger scale patterns, β-glucosidase and oxidative enzyme activity were not correlated at fine scales on leaf litter in the stream, and were only weakly correlated on litter in the floodplain (R = 0.34, n = 1152).
Spatial autocorrelation was measured for each enzyme on each leaf by measuring the range and sill from the constructed semivariograms (e.g., Fig. 1a). Spatial autocorrelation was highly variable among replicate leaves in both the stream and floodplain over the entire study period, with some replicates showing patterns of spatial autocorrelation at the measured scale and others showing no spatial patterns (Table 2). This variability among replicate leaves obscured the ability to distinguish any patterns over time and between the two sites.
Differences among replicate leaves were also apparent from kriging maps (an example is shown in Fig. 1b), which display microbial activity across the surface of individual leaves with values interpolated between sampled points to estimate activity at unmeasured locations. While activity was highly variable across the leaf surface, from these data, there were no clear spatial patterns over time for any of the three enzymes tested in either location or even among replicate leaves. The distribution of activity across the surface of replicate leaves were quite different, with some leaves characterized by a few spots of high activity and a replicate leaf characterized by a more homogeneous distribution.
Discussion
Our results showed that measurement of microbial extracellular enzyme activity can be used to assess the activity of microbial communities at fine spatial scales. However, the spatial structure in microbial extracellular enzyme activity at the centimeter scale was not as we expected in both the stream and floodplain. We expected to see a patchy distribution of activity initially (i.e., high values of range and sill as neighboring points begin to influence each other), as microorganisms capable of degrading the leaf became associated with the leaf surface. Activity was then expected to homogenize over time (i.e., lower values of range and sill as more efficient decomposers spread across the surface and sample points near each other become dependent upon each other). We predicted that this homogenization would occur more rapidly in the stream as substrata introduced to aquatic systems become colonized quite rapidly [24, 29]. Also, water availability may limit microbial growth over much of the surface of dead leaves in terrestrial environments (the leaf bags in the floodplain were never submerged during the study period), which should lead to a decrease in the area of leaf surface available for bacteria and fungi to colonize and increase patchiness [12]. Instead, we found a lack of spatial structure in both environments.
Microbial community structure has been found to be spatially structured at the centimeter scale in a homogeneous agricultural field [14] and at the centimeter scale in unvegetated salt marsh sediments [13]. However, no spatial structure for fungal and bacterial biomass was found in hardwood forest soils at the 1- to 10-cm scale [38], suggesting that spatial structure may only be evident in more homogeneous environments. The surface of decomposing leaves may be a highly heterogeneous environment at the centimeter scale. The phyllosphere of living leaves supports a wide array of microenvironments, varying greatly in water, O2, light availability, and nutrient content [32]. It is likely that similar microenvironments exist on decomposing leaf litter, though no studies have attempted to measure them. In the case of P. occidentalis leaves, pubescent areas and cuticular deposits on the leaf surface are also likely to create a range of microenvironments that alter the structure of colonizing microbial communities.
There are also larger structures on the leaf surface (e.g., veins) which likely influence the spatial distribution of microbial species and affect microenvironmental conditions, though no evidence for differences in activity at these sites were clear from comparisons of kriging maps (such as Fig. 1b), which display the spatial distribution of enzymatic activity across the surface of individual leaves, and digital images of the leaves, which show the location of such leaf surface structures (data not shown).
At the scale of the individual leaf, overall enzyme activities on replicate leaves were fairly similar. Mean β-glucosidase and CBH activity were correlated in both the stream and floodplain. These two enzymes are involved in cellulose degradation and are typically correlated in environmental samples [20]. Mean oxidative enzyme activity varied in its correlation to both cellulases depending on the environment, and oxidative activity may be a better indicator of decomposition than the other two enzymes measured. Decomposition was more rapid in the stream where oxidative enzyme activity was usually an order of magnitude greater than in the floodplain, whereas mean activity of both β-glucosidase and CBH were generally similar at the two sites, although activity for both was initially higher on leaves in the stream channel. This initial lag in activity on leaves in the floodplain may be because of more rapid colonization of the leaf surface in the channel [23, 29] or to increased activity with the availability of water as the floodplain was never inundated with water [22, 33]. It may also be due in part to temperature effects, which typically correlate with extracellular enzyme activity [47], though correlations between activity and temperature varied by enzyme.
Based on previous decomposition studies of P. occidentalis [42] and the decomposition rates determined in this study, P. occidentalis appears to be a rather recalcitrant leaf. Invertebrate decomposers may break down the leaf structure and allow fungi and bacteria to gain access, although even with the addition of invertebrates, P. occidentalis is often one of the slower leaves to decompose [39]. The inherent resistance of the leaf type to microbial degradation may have prevented a homogenization of enzymatic activity or may have just slowed the spread of activity. Fungal species have been shown to follow successional patterns in species composition from the leaf still being attached to the plant through different stages of decomposition [40]. If the leaf surface is more resistant to degradation, this may slow the successional process. The lack of detectable spatial structure or homogenization over time may be the result of simply failing to follow the process for a long enough period of time. The leaves studied here may still be in the early stages of decomposition, with more capable decomposer populations still attempting to colonize or spread across the leaf surface. However, given that the leaves used were collected from the forest floor, it is also quite likely that these leaves had undergone some leaching and initial decomposition prior to the study. Leaves were air dried initially to limit the disturbance of the existing microbial populations and allow observations of more naturally occurring processes, and linear models of decomposition fit the mass loss data well, suggesting that leaching and any initial loss of rapidly utilizable material had already occurred.
The study presented here is the first to measure spatial patterns in microbial activity on decomposing leaf litter at scales less than that of an individual leaf. The results show that although there was no spatial dependence of microbial enzyme activity at these scales, there was a tremendous amount of variability in activity across the leaf surface. Microbial activity may be even more heterogeneous at even finer scales and studies focused at these scales may provide greater insight into the functioning of these microbial communities. A diameter of 5 mm was chosen for the leaf discs, as we were unsure of the level of microbial activity at these scales over the study period and wanted to be certain we were able to measure activity on the discs. From our findings, these methods appear to be capable of measuring activity at scales finer than those studied here, allowing smaller areas to be assayed and thus allowing for more samples to be taken from across an individual leaf. Landscape studies of microbial activity by themselves may show some interesting findings, but it is likely that these analyses in combination with studies of the community diversity or biomass, or the structure and composition of the underlying substrate, should provide some very important insights into microbial interactions in these systems. By connecting interactions among and between microbial communities with their landscape level activity, a clearer picture of the processes involved in leaf litter decomposition should begin to emerge.
References
Aro N, Pakula T, Penttilä M (2005) Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol Rev 29:719–739
Baldy V, Gobert V, Guerold F, Chauvet E, Lambrigot D, Charcosset JY (2007) Leaf litter breakdown budgets in streams of various trophic status: effects of dissolved inorganic nutrients on microorganisms and invertebrates. Freshw Biol 52:1322–1335
Bergfur J, Johnson RK, Sandin L, Goedkoop W, Nygren K (2007) Effects of nutrient enrichment on boreal streams: invertebrates, fungi and leaf-litter breakdown. Freshw Biol 52:1618–1633
Blum DL, Kataeva IA, Li XL, Ljungdahl LG (2000) Feruloyl esterase activity of the Clostridium thermocellum cellulosome can be attributed to previously unknown domains of XynY and XynZ. J Bacteriol 182:1346–1351
Boulton AJ, Boon PI (1991) A review of methodology used to measure leaf litter decomposition in lotic environments: time to turn over an old leaf? Austr J Mar Freshw Res 42:1–43
Brewer JS (2001) Current and presettlement tree species composition of some upland forests in northern Mississippi. J Torrey Botanical Soc 128:332–349
Cardinale BJ, Palmer MA, Swan CM, Brooks S, Poff L (2002) The influence of substrate heterogeneity on biofilm metabolism in a stream ecosystem. Ecology 83:412–422
Chamier AC, Dixon PA, Archer SA (1984) The spatial distribution of fungi on decomposing alder leaves in a freshwater stream. Oecologia 64:92–103
Chróst RJ (1990) Microbial ectoenzymes in aquatic environments. In: Overbeck J, Chróst RJ (eds) Aquatic microbial ecology: biochemical and molecular approaches. pp, Springer-Verlag, pp 47–78
Dang CK, Gessner MO, Chauvet E (2007) Influence of conidial traits and leaf structure on attachment success of aquatic hyphomycetes on leaf litter. Mycologia 99:24–32
Das M, Royer TV, Leff LG (2007) Diversity of fungi, bacteria, and Actinomycetes on leaves decomposing in a stream. Appl Environ Microbiol 73:756–767
Dulla G, Lindow SE (2008) Quorum size of Pseudomonas syringae is small and dictated by water availability on the leaf surface. Proc Natl Acad Sci USA 105:3082–3087
Franklin RB, Blum LK, McComb AC, Mills AL (2002) A geostatistical analysis of small-scale spatial variability in bacterial abundance and community structure in salt marsh creek bank sediments. FEMS Microbiol Ecol 42:71–80
Franklin RB, Mills AL (2003) Multi-scale variation in spatial heterogeneity for microbial community structure in an eastern Virginia agricultural field. FEMS Microbiol Ecol 44:335–346
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–246
Gessner MO, Chauvet E (1994) Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75:1807–1817
Graca MAS, Canhoto C (2006) Leaf litter processing in low order streams. Limnetica 25:1–10
Han SO, Yukawa H, Inui M, Doi RH (2003) Regulation of expression of cellulosomal cellulase and hemicellulase genes in Clostridium cellulovorans. J Bacteriol 185:6067–6075
Isaaks EH, Srivastava RM (1989) An introduction to applied geostatistics. Oxford University Press
Jackson CR, Foreman CM, Sinsabaugh RL (1995) Microbial enzyme activities as indicators of organic matter processing rates in a Lake Erie coastal wetland. Freshw Biol 34:329–342
Jackson CR, Vallaire SC (2007) Microbial activity and decomposition of fine particulate organic matter in a Louisiana cypress swamp. J North Am Benth Soc 26:743–753
Jackson EF, Echlin HL, Jackson CR (2006) Changes in the phyllosphere community of the resurrection fern, Polypodium polypodioides, associated with rainfall and wetting. FEMS Microbiol Ecol 58:236–246
Jones PR, Cottrell MT, Kirchman DL, Dexter SC (2007) Bacterial community structure of biofilms on artificial surfaces in an estuary. Microb Ecol 53:153–162
Kearns SG, Bärlocher F (2008) Leaf surface roughness influences colonization success of aquatic hyphomycete conidia. Fungal Ecol 1:13–18
Kobayashi S, Kagaya T (2004) Litter patch types determine macroinvertebrate assemblages in pools of a Japanese headwater stream. J North Am Benth Soc 23:78–89
Kobayashi S, Kagaya T (2005) Hot spots of leaf breakdown within a headwater stream reach: comparing breakdown rates among litter patch types with different macroinvertebrate assemblages. Freshw Biol 50:921–929
Koetsier P, McArthur JV, Leff LG (1997) Spatial and temporal response of stream bacteria to sources of dissolved organic carbon in a blackwater stream system. Freshw Biol 37:79–89
Kominoski JS, Pringle CM, Ball BA, Radford MA, Coleman DC, Hall DB, Hunter MD (2007) Nonadditive effects of leaf litter species diversity on breakdown dynamics in a detritus-based stream. Ecology 88:1167–1176
Kröpfla K, Vladára P, Szabób K, Ácsc E, Borsodib AK, Szikorad S, Carolie S, Záray G (2006) Chemical and biological characterisation of biofilms formed on different substrata in Tisza river (Hungary). Environ Pollution 144:626–631
Larson JL, Zak DR, Sinsabaugh RL (2002) Extracellular enzyme activity beneath temperate trees growing under elevated carbon dioxide and ozone. Soil Sci Soc Am J 66:1848–1856
Lecerf A, Risnoveanu G, Popescu C, Gessner MO, Chauvet E (2007) Decomposition of diverse litter mixtures in streams. Ecology 88:219–227
Lindow SE, Brandl MT (2003) Microbiology of the phyllosphere. Appl Environ Microbiol 69:1875–1883
Lund V, Goksoyr J (1980) Effects of water fluctuations on microbial biomass and activity in soil. Microb Ecol 6:115–123
Mckie BG, Petrin Z, Malmqvist B (2006) Mitigation or disturbance? Effects of liming on macroinvertebrate assemblage structure and leaf-litter decomposition in the humic streams of northern Sweden. J Applied Ecology 43:780–791
Minasny B, McBratney AB, Whelan BM (2005) VESPER version 1.62. Australian Centre for Precision Agriculture, McMillan Building A05, The University of Sydney, SW 2006. (http://www.usyd.edu.au/su/agric/acpa)
Minshall GW, Peterson RC, Cummins KW, Bott TL, Sedell JR, Cushing CE, Vannote RL (1983) Interbiome comparison of stream ecosystem dynamics. Ecol Monogr 53:1–25
Moretti M, Goncalves JF, Callisto M (2007) Leaf breakdown in two tropical streams: differences between single and mixed species packs. Limnologica 37:250–258
Morris SJ (1999) Spatial distribution of fungal and bacterial biomass in southern Ohio hardwood forest soils: fine scale variability and microscale patterns. Soil Biol Biochem 31:1375–1386
Neatrour MA, Webster JR, Benfield EF (2004) The role of floods in particulate organic matter dynamics of a southern Appalachian river–floodplain ecosystem. J North Am Benth Soc 23:198–213
Osono T (2005) Colonization and succession of fungi during decomposition of Swida controversa leaf litter. Mycologia 97:589–597
Paerl HW, Pinckney JL (1996) A mini-review of microbial consortia: their roles in aquatic production and biogeochemical cycling. Microb Ecol 31:225–247
Paul RW, Benfield EF, Cairns J (1983) Dynamics of leaf processing in a medium-sized river. In: Fontaine TD, Bartell SM (eds) Dynamics of lotic systems. Ann Arbor Science, Ann Arbor, MI, pp 402–423
Romaní AM, Fischer H, Mille-Lindblom C, Tranvik LJ (2006) Interactions of bacteria and fungi on decomposing litter: differential extracellular enzyme activities. Ecology 87:2559–2569
Rueda-Delgado G, Wantzen KM, Tolosa MB (2006) Leaf-litter decomposition in an Amazonian floodplain stream: effects of seasonal hydrological changes. J North Am Benth Soc 25:233–249
Shearer CA, Lane LC (1983) Comparison of three techniques for the study of aquatic hyphomycete communities (Fungi). Mycologia 75:498–508
Sinsabaugh RL, Antibus RK, Linkins AE (1991) An enzymic approach to the analysis of microbial activity during plant litter decomposition. Agr Ecosys Environ 34:43–54
Sinsabaugh RL (1994) Enzymic analysis of microbial pattern and process. Biol Fertil Soils 17:69–74
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24
Webster JR, Benfield EF (1986) Vascular plant breakdown in freshwater ecosystems. Annu Rev Ecol Syst 17:567–594
Webster JR, Meyer JL (1997) Organic matter budgets for streams: a synthesis. J North Am Benth Soc 16:141–161
Wetzel RG (1991) Extracellular enzymatic interactions: storage, redistribution, and interspecific communication. In: Chróst RJ (ed) Microbial enzymes in aquatic environments. pp, Springer-Verlag, pp 6–28
Acknowledgements
We thank Hsuanhua Smart for her assistance in the lab and field. We would also like to thank Drs. Steve Brewer and Cliff Ochs for their helpful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smart, K.A., Jackson, C.R. Fine Scale Patterns in Microbial Extracellular Enzyme Activity during Leaf Litter Decomposition in a Stream and its Floodplain. Microb Ecol 58, 591–598 (2009). https://doi.org/10.1007/s00248-009-9512-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-009-9512-1