Abstract
Background
A nutmeg lung pattern on magnetic resonance imaging (MRI) is an imaging finding associated with pulmonary lymphangiectasia. However, the prognostic value of the nutmeg lung pattern is unknown.
Objective
To evaluate the clinical associations of nutmeg lung indicating lymphangiectasia on fetal lung MRI and its relationship with early mortality in fetuses with primary and secondary lymphangiectasia.
Materials and methods
We retrospectively identified all pregnant patients with a fetal MRI performed for indication of evaluating for pulmonary lymphangiectasia from 2006 to 2019. Two readers evaluated the fetal MRIs and interobserver agreement was calculated. Multivariable logistic regression models were performed to estimate the association of the echocardiographic findings and the presence of nutmeg lung. Kaplan-Meier and Cox regression analyses were performed to evaluate association with mortality in the first 30 days of life. Survival analysis was defined as mortality or orthotopic heart transplant at 30 days of age. P<0.05 was considered significant.
Results
Our sample included 53 fetuses. Forty-seven (89%) had congenital heart disease (CHD) and 6 (11%) were diagnosed postnatally with primary lymphangiectasia. Interobserver agreement was 0.83. Pulmonary vein congestion on echocardiography was the strongest predictor of nutmeg lung (odds ratio [OR]=12.0, P=0.002). Ten fetuses reached the outcome of heart transplantation (n=1) or death (n=9) within the first 30 days of life. In fetuses with CHD, survival of those with nutmeg lung was significantly lower than in those without (P<0.001). Nutmeg lung was an independent risk factor for 30-day mortality (hazard ratio [HR]: 6.1, P=0.01).
Conclusion
Nutmeg lung pattern on fetal MRI is an independent risk factor associated with 30-day mortality in fetuses with CHD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary lymphangiectasia is an abnormal dilation of the lymphatic channels of the lungs [1]. Pulmonary lymphangiectasia can be primary — an inherited lymphatic abnormality — or secondary to congenital heart disease (CHD) [2]. Cardiac malformations that generate elevated left atrial pressure with congestion of the pulmonary veins can cause lymphangiectasia [3]. In particular, fetuses with hypoplastic left heart syndrome (HLHS) with an intact or restrictive atrial septum have a high risk of developing pulmonary lymphangiectasia [3]. In a fetus with HLHS, the lack of adequate communication between the left and right atria restricts left atrial decompression, creates pulmonary venous hypertension and promotes abnormal pulmonary vascular development, including dilation of the lymphatic channels [4,5,6,7].
Fetal magnetic resonance imaging (MRI) allows evaluation of the fetal anatomy with a high degree of resolution. Pulmonary lymphangiectasia has previously been characterized on fetal MRI as a T2-hyperintense heterogeneous lung parenchyma that resembles the mottling pattern of the liver seen in patients with venous congestion — a pattern described as nutmeg lung [8, 9]. MRI has been shown to be superior to ultrasound (US) in evaluating this abnormal pulmonary appearance. Furthermore, MRI has allowed the diagnosis of primary lymphangiectasia, something that could not be done by US [10].
The clinical significance of nutmeg lung on fetal MRI and its usefulness for predicting complications and survival are still poorly understood. The finding of nutmeg lung may prove valuable in prenatal counseling of families, overall management strategies and the development of prenatal therapies for at-risk fetuses. Previous studies suggest nutmeg lung may indicate a poor prognosis in fetuses with HLHS, but survival analyses in fetuses with nutmeg lung are based on relatively small cohorts [3, 11, 12]. The primary goal of this study is to evaluate the clinical associations of the nutmeg lung pattern indicating lymphangiectasia on fetal lung MRI and its relationship with early mortality in fetuses with primary and secondary lymphangiectasia.
Materials and methods
Patient sample
This retrospective study was approved by the institutional review board at the Children’s Hospital of Philadelphia. The requirements for written informed consent were waived. A computer search of our institution’s radiology department database between January 2006 and December 2019 was performed and included pregnant women older than 18 years who had undergone fetal MRI to rule out pulmonary lymphangiectasia in the fetus. At our institution, a fetal MRI is performed in patients in whom pulmonary lymphangiectasia is suspected based on imaging findings in fetal echocardiography, such as pulmonary venous congestion, pleura effusion or intact atrial septum. Patients without CHD were mainly referred for a fetal MRI due to US findings suggestive of a pleural effusion and a suspicion of a primary lymphatic disorder such as chylothorax. In total, 62 fetal MRIs were identified. Nine were excluded given the lack of pre- or postnatal follow-up (Fig. 1). One patient from the Saul et al. [3] and four from the Biko et al. [10] cohorts were included in this sample.
The flowchart shows the inclusion and exclusion criteria used to reach the final sample. Patients were divided based on the etiology of the pulmonary lymphangiectasia (primary or secondary). The number of patients who died or received an orthotopic heart transplant during the first 30 days of life is presented in each group
Fetal magnetic resonance imaging protocol
Fetal MRI was performed with either a 1.5-tesla (T) scanner (Magnetom Avanto; Siemens Healthineers, Erlangen, Germany [n=51]) or two different 3-T scanners (Skyra [n=1] or Verio [n=1], Siemens Healthineers). The fetal lungs were evaluated using a T2-weighted HASTE (half-Fourier single-shot turbo spin echo) with the following parameters: repetition time (TR)/echo time (TE)=1,100/75, flip angle 180°, field of view=280×280, matrix=256×256, slice thickness=3 mm and acquisition time 20–40 s.
Prenatal evaluation
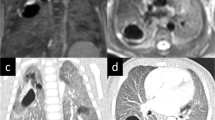
All fetal MRIs were reviewed independently by two pediatric radiologists (T.V., with 13 years of experience, and A.M.J., with 16 years of experience). Disagreements were resolved through consensus. The lungs were evaluated using a T2-weighted single-shot turbo spin echo sequence in the axial and sagittal planes. Nutmeg lung pattern was defined as a heterogeneous appearance of the pulmonary parenchyma with T2-hyperintense linear structures radiating peripherally from the hilum to the pleural surface [9]. The prenatal diagnosis of primary lymphangiectasia was suggested if the lungs were collapsed and demonstrated predominantly T2-hyperintense signal, usually accompanied by frank effusions, in the absence of CHD, and then confirmed postnatally (Fig. 2). Of note, the corresponding prenatal US exams were not evaluated because this study was a dedicated MR study.
Examples of nutmeg lung in three patients on coronal T2-weighted HASTE (half-Fourier single-shot turbo spin echo) imaging. a A 34-year-old woman at 27 weeks of gestation carried a fetus diagnosed with hypoplastic left heart syndrome. b A 30-year-old woman at 30 weeks of gestation carried a fetus diagnosed with Noonan syndrome. c A 32-year-old woman at 34 weeks of gestation carried a fetus diagnosed with double-outlet right ventricle
Pleural effusions were reviewed and grouped as trace (defined as linear T2 hyperintensity along the lung surface) and frank pleural effusion. Cardiac anatomy was determined by a fetal echocardiogram performed on the same day as fetal MRI or earlier during the pregnancy. Pulmonary vein congestion was recorded based on the fetal echocardiogram. Pulmonary vein congestion was defined as either an abnormal pulmonary vein Doppler pattern and significant reversal of flow with atrial contraction, or the anatomical identification of obstructed pulmonary venous return. In fetuses with HLHS, an intact or highly restrictive atrial septum was recorded based on the fetal echocardiogram.
Statistical analysis
Data analysis was performed with SPSS Statistics (v.23.0; IBM, Armonk, NY). Continuous variables were presented as mean±standard deviation (SD) as well as median and range. Categorical variables were presented as percentages and counts. The Mann-Whitney U test was used to compare continuous parameters between fetuses with and without nutmeg lung. Chi-square and Fisher exact tests were used for categorical variables. Interobserver agreement was evaluated using kappa statistics. Agreement was categorized as follows: less than or equal to 0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and equal to or greater than 0.81, almost perfect agreement [13]. Univariate and multivariable logistic regression models were performed to estimate the association of the echocardiographic findings and nutmeg lung. Survival analysis was performed as above, with the end point defined as mortality at 30 days of age or the requirement of orthotopic heart transplant at 30 days of age. Survival without transplant was estimated using the Kaplan-Meier method between fetuses with and without nutmeg lung. Kaplan-Meier curves were made to provide a visual representation of the probability of survival. Univariate and multivariable Cox proportional hazard regression was used to assess the association of nutmeg lung and clinical factors with survival. Cox regression allows the performance of a survival analysis by accounting for other risk factors associated with early mortality, such as intact atrial septum and pulmonary vein congestion. A P-value of <0.05 was considered significant.
Results
Study population
Fifty-three pregnant patients were included in the sample. The median maternal age and median gestational age at fetal imaging were 29 years (interquartile ratio [IQR]=7) and 33 weeks (IQR=7), respectively. One (2%) patient was carrying a twin pregnancy and only one of those fetuses was assessed for nutmeg lung, leaving a total study population of 53. In total, 17 (32%) fetuses were diagnosed with nutmeg lung. Out of the 17 fetuses with nutmeg lung, 12 (71%, 12/17) were diagnosed with CHD and 5 (29%, 5/17) with primary lymphangiectasia postnatally. The most common CHD was HLHS (7/17, 41%). Of 36 fetuses without nutmeg lung, 35 (97%, 35/36) were diagnosed with CHD and 1 (3%, 1/36) with primary lymphangiectasia postnatally. The most common CHD was HLHS (83%, 30/36) (Fig. 1). The distribution of the different CHD between fetuses with and without nutmeg lung is described in Table 1. Six cases (11%) were prenatally diagnosed with hydrothorax or hydrops fetalis (Fig. 1). These six fetuses were diagnosed with a primary lymphatic disorder in the neonatal period. After being diagnosed with nutmeg lung, three fetuses (6%) with HLHS and an intact atrial septum had a US-guided percutaneous placement of a cardiac interatrial stent or a septostomy, of which only one was successful (Table 1). Of the 53 fetuses, 30 (57%) were male and 23 (43%) were female.
On postnatal follow-up, the death of 16 neonates occurred at a median of 8 days of life (mode=0 days, range: 0–5 years). Most patients died shortly after birth regardless of getting standard care. Of the 16 neonates who died, 10 carried a diagnosis of HLHS and 6 had other forms of CHD. One (2%) of the neonates, who was diagnosed with HLHS, had an orthotopic heart transplant at 22 days of life and is alive at the time of this writing. None of the fetuses with a primary lymphatic disorder died by the selected end point of 30 days of life.
Evaluation of nutmeg lung by magnetic resonance imaging
The interobserver agreement for the presence of nutmeg lung between both readers was excellent (κ=0.83, P<0.001). Seventeen fetuses (32%) were deemed to have a nutmeg lung pattern on MRI in consensus: 88% of these (15/17) were bilateral, 12% (2/17) were exclusively in the right lung, and none presented with unilateral left nutmeg lung.
Ten fetuses (19%) had pleural effusions on fetal MRI described as trace (n=4) and frank pleural effusions (n=6). Fetuses with nutmeg lung (9/17, 53%) were more likely to have a pleural effusion compared to those without nutmeg lung (1/36, 1%; P<0.001). Similarly, fetuses diagnosed with primary lymphangiectasia had a higher prevalence of pleural effusion (n=6, trace=1, frank=4) compared to fetuses with CHD (n=47, trace=3, frank=2; P<0.001).
Predictors of nutmeg lung/lymphangiectasia
In fetuses with CHD, 33% (16/47) of fetuses were found to have pulmonary vein congestion on echocardiography. Fetuses with imaging findings of pulmonary vein congestion had a higher proportion of nutmeg lung (75%, 9/12) compared to those without this finding (25%, 3/12; P=0.001). Univariate logistic regression showed that pulmonary vein congestion was a predictor of nutmeg lung (odds ratio [OR]=12.0, 95% confidence interval [CI]: 2.5–56.3; P=0.002) (Table 2).
In fetuses with HLHS, 35% (13/37) showed pulmonary vein congestion on fetal echocardiography and were found to have a higher prevalence of nutmeg lung (P=0.004). Of the 37 fetuses, those with intact atrial septum showed a higher prevalence of nutmeg lung (57%, 4/7) compared to those without an intact atrial septum (43%, 3/7; P=0.01). Univariate logistic regressions for each of these parameters are displayed in Table 2. Multivariate logistic regression shows that pulmonary vein congestion is the only independent predictor of nutmeg lung (OR=13.3, 95% CI: 1.2–143.6; P=0.03) (Table 2).
Survival analysis
One fetus with HLHS with intact atrial septum and nutmeg lung was excluded from the survival analysis as the mother had an intrauterine fetal demise at 28 weeks of gestation. Fifty-two neonates (52/53, 98%) were included in the survival analysis, 10 of whom (19%) reached the combined outcome of heart transplantation (n=1) or death (n=9) within the first 30 days of life. A Kaplan-Meier analysis showed that fetuses with nutmeg lung had a lower survival rate compared to those without nutmeg lung (P=0.002) (Fig. 3). A Cox regression analysis showed that fetuses with nutmeg lung had a higher risk of 30-day mortality or heart transplant than those without nutmeg lung (hazard ratio [HR]: 6.4, 95% CI: 1.6–24.9; P=0.007) (Table 3).
The prognostic differences in patients with and without nutmeg lung. a–c Kaplan-Meier curves show the percentage of those free from death or orthotopic heart transplant within the first 30 days of life in the total sample including primary and secondary lymphangiectasias (n=52) (a), patients with only secondary lymphangiectasia (n=47) (b) and patients with only hypoplastic left heart syndrome (n=36) (c). A Kaplan-Meier curve was not made in patients with primary lymphangiectasia given that only six patients were diagnosed with primary lymphangiectasia and none of them reached the end point (death or heart transplant). The y-axis represents the percentage of patients who reached the end point; the x-axis represents time in days during the first 30 days of life. Kaplan-Meier curves are a visual representation of the probability of death or heart transplant. Each line drop represents one or more patients reaching the end point. The tables below display the number of patients at risk, patients who have not reached the end point, per group (no nutmeg lung versus nutmeg lung) compared to the time that has passed from birth until the first 30 days of life. The days selected in the first row represent the time points at which patients with and without nutmeg lung reached the end point. The sample size of each group shows the number of patients diagnosed prenatally with and without nutmeg lung. Day zero represent the time at birth. If the number of patients at time zero is lower than the sample size, it represents a number of patients who reached the end point soon after birth. The number of patients at risk decreased over time given that some patients reached the end point in both groups
When evaluating all fetuses with CHD, survival of those with nutmeg lung was significantly lower than those without nutmeg lung (P<0.001) (Fig. 3). A multivariate Cox regression analysis showed that nutmeg lung was an independent risk factor for 30-day mortality (HR: 6.1, 95% CI: 1.4–26.3; P=0.01) (Table 3).
When evaluating fetuses with HLHS, survival was significantly lower in fetuses with nutmeg lung (P<0.001) (Fig. 3). A multivariate Cox regression analysis showed that nutmeg lung (HR: 14.7, 95% CI: 1.2–181.1; P=0.03) and an intact atrial septum (HR: 20.0, 95% CI: 1.8–220.4; P=0.01) were independent risk factors associated with 30-day mortality (Table 3).
Discussion
Previous studies have shown that the fetal MRI pulmonary pattern described as nutmeg lung negatively influences neonatal outcomes [3, 12, 14]. However, their study groups were relatively small. The current study includes the largest group of neonates with prenatal findings of lymphangiectasia. We found that in fetuses with CHD, those with nutmeg lung had a lower survival rate and a higher risk of mortality during the first 30 days of life compared to those without nutmeg lung. Similarly, among fetuses with HLHS, those who had MRI findings of nutmeg lung had a lower survival rate compared to those without nutmeg lung, leading to a higher risk of death in the first 30 days of life for those with HLHS and nutmeg lung.
Primary lymphangiectasia is a rare condition consisting of abnormally dilated lymphatic channels without proliferation; its exact etiology is not entirely understood [15]. Usually infants with primary lymphangiectasia die shortly after birth after presenting with respiratory distress syndrome and pleural effusions. However, the outcome of this disease has evolved. We can now prenatally diagnose nutmeg lung using fetal MRI, as suggested in fetuses with partially collapsed lungs that appear T2 hyperintense and heterogeneous, usually with pleural effusions [3, 9]. Further, diagnosis of lymphatic flow anomalies has been made possible by the recent development of the postnatal imaging modality referred to as dynamic contrast-enhanced MR lymphangiography [10]. This modality allows superior visualization of the neonatal anatomy of lymphatic channels, as well as the dynamic distribution of lymphatic flow to the mediastinum and lung tissues. These conditions have been successfully treated with postnatal percutaneous embolization of the abnormal lymphatic channels [16]. Some of these lymphatic channels may become apparent in neonates with HLHS after surgical palliation with the Fontan operation, a procedure that results in systemic venous and lymphatic congestion [10]. Mapping and embolization of abnormal lymphatic channels have also proven successful in treating plastic bronchitis and protein-losing enteropathy, clinical conditions seen in long-term survivors of HLHS [17, 18].
Secondary pulmonary lymphangiectasia is relatively more common than primary lymphangiectasia. It is attributed to chronic pulmonary venous obstruction in fetuses with certain types of CHD, including total anomalous pulmonary venous connection and, more commonly, HLHS with restrictive or intact atrial septum [3, 12, 14, 19]. Chronic pulmonary venous obstruction, particularly in fetuses with an intact atrial septum, leads to increased pulmonary venous pressure and subsequent changes of the pulmonary vasculature, including muscularization of the pulmonary veins and lymphatic channel dilation, with increased pulmonary lymphatic drainage. At imaging, these findings display the described nutmeg lung appearance of dilated lymphovascular channels manifested as linear and tubular T2-hyperintense structures radiating from the hila to the pleural surface [9].
The value of fetal MRI in supplying prognostic information for a fetus with suspicion of pulmonary lymphangiectasia cannot be overstated. In cases of unexplained fetal pleural effusions, an MRI may help determine if the effusions are due to primary lymphangiectasia by demonstrating T2-hyperintense collapsed lungs, rather than the expected hypointense lungs. Less lung volume leads to less retained fetal fluid within the lung parenchyma; whereas in the lymphangiectatic lung, the collapsed lung is hyperintense, presumably secondary to the dilated lymphatic channels and retained fluid. At this time, fetal MRI seems to be more sensitive than US at diagnosing pulmonary lymphangiectasia prenatally and is able to differentiate it from other etiologies of pleural effusions [10].
In fetuses with primary pulmonary lymphangiectasia, nutmeg lung could be used as a diagnostic finding rather than a prognostic factor. None of the fetuses in our sample with primary pulmonary lymphangiectasia died, and all underwent successful neonatal intervention. Fetuses with nutmeg lung on fetal MRI and no evidence of CHD still need to be evaluated in the neonatal period to further characterize if there is an underlying lymphatic disorder, whether a neonatal chylothorax or a central lymphatic flow disorder. Additional imaging findings on fetal MRI, of ascites or body wall edema, may be more suggestive of a central lymphatic flow disorder [10]. However, dynamic contrast-enhanced MR lymphangiography is still helpful to reach a definitive diagnosis. More studies of larger populations are needed to evaluate whether a nutmeg lung pattern or the severity of nutmeg lung appearance is also a prognostic factor in fetuses with primary lymphatic disorders.
In cases of secondary lymphangiectasia, nutmeg lung in fetuses with HLHS has been shown to be an independent risk factor associated with 30-day mortality, regardless of the status of the atrial septum. Some of the previously known risk factors associated with early mortality in fetuses with HLHS include an intact or restrictive atrial septum, severe tricuspid regurgitation and low birth weight [20, 21]. Our results showed that a higher 30-day mortality in fetuses with HLHS could be predicted independently by an intact atrial septum or MRI findings of nutmeg lung. Although an abnormal pulmonary vein Doppler pattern predicts a nutmeg lung pattern and 30-day mortality, pulmonary lymphangiectasia was a stronger predictor of early mortality once we accounted for pulmonary vein congestion [11]. In these cases of secondary lymphangiectasia, a fetal MRI evaluating for nutmeg lung can help the clinical team stratify outcomes and counsel the parents, since this is a strong indicator of poor prognosis. More invasive therapies can then be evaluated, such as enlarging the atrial communication in utero. It would be interesting to evaluate if these interventions release the obstruction and venous backflow, improving lymphatic drainage. Such an assessment could ascertain if the lymphatic alterations are permanent and unalterable at that late point of development. Finally, the advent of 3-T scanners in fetal imaging may improve the diagnostic performance of fetal MRI to detect pulmonary lymphangiectasias. Previous studies have shown that 3-T MRI delivers better image quality in fetal MRI with equivalent specific absorption rate and specific energy dose — surrogates of energy deposition and heating — compared to 1.5-T fetal MRIs [22, 23]. Unfortunately, only two studies were performed using a 3-T scanner, which does not allow sufficient data to statistically evaluate which magnet strength may better identify nutmeg lung.
Our study has several limitations, the first of which is its retrospective nature. Second, the proportion of fetuses with primary lymphangiectasia was relatively low and prevented a proper analysis of this group. Our sample size is also relatively small given the low prevalence of pulmonary lymphangiectasias and that patients with pulmonary lymphangiectasias often die before birth. This limits the accuracy of our analysis and explains the large confidence intervals observed with the hazard ratios. Third, there was a selection bias: at our institution, a fetal MRI is performed in those cases of HLHS with suspected pulmonary venous congestion due to intact or restricted septum as confirmed by echocardiography. Thus, this is a select population and the true incidence of lymphangiectasia in HLHS overall is uncertain. Similarly, very few fetuses with other CHDs receive a fetal MRI to evaluate for nutmeg lung; typically, this occurs only if there is a question of venous obstruction by echocardiography or if there is a suggestion of mild pulmonary heterogeneity seen on US — again, findings that skew evaluation of the true incidence in this population.
Conclusion
A nutmeg lung pattern on fetal MRI may be an independent risk factor associated with 30-day mortality in fetuses with CHD. Fetal MRI should be considered in fetuses with echocardiography findings suggestive of pulmonary vein congestion to rule out a pulmonary lymphangiectasia in order to provide expectant parents with more informed counseling.
References
Faul JL, Berry GJ, Colby TV et al (2000) Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med 161:1037–1046
Esther CR Jr, Barker PM (2004) Pulmonary lymphangiectasia: diagnosis and clinical course. Pediatr Pulmonol 38:308–313
Saul D, Degenhardt K, Iyoob SD et al (2016) Hypoplastic left heart syndrome and the nutmeg lung pattern in utero: a cause and effect relationship or prognostic indicator? Pediatr Radiol 46:483–489
Graziano JN, Heidelberger KP, Ensing GJ et al (2002) The influence of a restrictive atrial septal defect on pulmonary vascular morphology in patients with hypoplastic left heart syndrome. Pediatr Cardiol 23:146–151
Rychik J, Rome JJ, Collins MH et al (1999) The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol 34:554–560
Bellini C, Boccardo F, Campisi C, Bonioli E (2006) Congenital pulmonary lymphangiectasia. Orphanet J Rare Dis 1:43
Barrera CA, Victoria T, Escobar FA et al (2020) Imaging of fetal lymphangiectasias: prenatal and postnatal imaging findings. Pediatr Radiol 50:1872–1880
Li YL, Lee KH, Cheng AK, Yu ML (2018) Nutmeg liver. Abdom Radiol (NY) 43:1275–1276
Victoria T, Andronikou S (2014) The fetal MR appearance of 'nutmeg lung': findings in 8 cases linked to pulmonary lymphangiectasia. Pediatr Radiol 44:1237–1242
Biko DM, Johnstone JA, Dori Y et al (2018) Recognition of neonatal lymphatic flow disorder: fetal MR findings and postnatal MR lymphangiogram correlation. Acad Radiol 25:1446–1450
Serrano RM, Hussain S, Brown B et al (2020) Risk stratification of patients with hypoplastic left heart syndrome and intact atrial septum using fetal MRI and echocardiography. Cardiol Young 30-790-798
Herrmann JL, Irons ML, Mascio CE et al (2017) Congenital pulmonary lymphangiectasia and early mortality after stage 1 reconstruction procedures. Cardiol Young 27:1356–1360
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Seed M, Bradley T, Bourgeois J et al (2009) Antenatal MR imaging of pulmonary lymphangiectasia secondary to hypoplastic left heart syndrome. Pediatr Radiol 39:747–749
Wilson RD, Pawel B, Bebbington M et al (2006) Congenital pulmonary lymphangiectasis sequence: a rare, heterogeneous, and lethal etiology for prenatal pleural effusion. Prenat Diagn 26:1058–1061
Itkin M (2016) Interventional treatment of pulmonary lymphatic anomalies. Tech Vasc Interv Radiol 19:299–304
Dori Y, Keller MS, Rome JJ et al (2016) Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation 133:1160–1170
Itkin M, Piccoli DA, Nadolski G et al (2017) Protein-losing enteropathy in patients with congenital heart disease. J Am Coll Cardiol 69:2929–2937
Lam CZ, Bhamare TA, Gazzaz T et al (2017) Diagnosis of secondary pulmonary lymphangiectasia in congenital heart disease: a novel role for chest ultrasound and prognostic implications. Pediatr Radiol 47:1441–1451
Barron DJ, Kilby MD, Davies B et al (2009) Hypoplastic left heart syndrome. Lancet 374:551–564
Siehr SL, Maeda K, Connolly AA et al (2016) Mitral stenosis and aortic atresia — a risk factor for mortality after the modified Norwood operation in hypoplastic left heart syndrome. Ann Thorac Surg 101:162–167
Barrera CA, Francavilla ML, Serai SD et al (2020) Specific absorption rate and specific energy dose: comparison of 1.5-T versus 3.0-T fetal MRI. Radiology 295:664–674
Victoria T, Johnson AM, Edgar JC et al (2016) Comparison between 1.5-T and 3-T MRI for fetal imaging: is there an advantage to imaging with a higher field strength? AJR Am J Roentgenol 206:195–201
Acknowledgments
We would like to thank Lydia Sheldon for proofreading and constructive criticism of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barrera, C.A., Johnson, A.M., Rychik, J. et al. Prognostic value of the nutmeg lung pattern/lymphangiectasia on fetal magnetic resonance imaging. Pediatr Radiol 51, 1809–1817 (2021). https://doi.org/10.1007/s00247-021-05061-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-021-05061-4