Abstract
Lymphangiectasias are lymphatic malformations characterized by the abnormal dilation and morphology of the lymphatic channels. The classification and treatment of these disorders can be challenging given the limited amount of literature available in children. Various imaging modalities are used to confirm suspected diagnosis, plan the most appropriate treatment, and estimate a prognosis. Prenatal evaluation is performed using both prenatal US imaging and fetal MRI. These modalities are paramount for appropriate parental counseling and planning of perinatal care. During the neonatal period, chest US imaging is a useful modality to evaluate pulmonary lymphangiectasia because other modalities such as conventional radiography and CT display nonspecific findings. Finally, the recent breakthroughs in lymphatic imaging with MRI have allowed us to better classify lymphatic disorders. Dynamic contrast-enhanced lymphangiography, conventional lymphangiography and percutaneous lymphatic procedures offer static and dynamic evaluation of the central conducting lymphatics in children, with excellent spatial resolution and the possibility to provide treatment. The purpose of this review is to discuss the normal and abnormal development of the fetal lymphatic system and how to best depict it by imaging during the prenatal and postnatal life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lymphangiectasia denotes lymphatic malformations characterized by the abnormal dilation and morphology of the lymphatic channels [1]. The incidence of these anomalies is still unknown, but autopsy reports in the perinatal period have estimated that 0.5–1% of feto-neonatal deaths can be attributed to congenital pulmonary lymphangiectasias [2]. Most cases tend to manifest spontaneously, with only a few cases showing a familial predisposition [3, 4]. However, more severe and diffuse forms of the disease are more frequently associated with a genetic condition [5]. Lymphangiectasia can present as a challenging diagnosis given that it can easily be misidentified as other lymphatic-type disorders such as lymphatic malformation (dilated and dysplastic lymphatic channels) and lymphangiomatosis (progressive multifocal proliferation and dilation of the lymphatics) [6]. Most of these lesions can be differentiated by the age of presentation and the histological examination [1].
Pre- and postnatal imaging of the lymphatic system has improved considerably in the last decade. The high resolution of fetal MRI and prenatal US imaging has allowed radiologists and maternal–fetal medicine specialists to detect lymphatic disorders and prepare for a multidisciplinary delivery [7, 8]. Neonatal US and MR imaging have played a relevant role in the diagnosis, treatment and prognosis of these children as well. In particular, new techniques such as dynamic contrast-enhanced MR lymphangiography and conventional lymphangiography provide high-resolution images of the central conducting lymphatics and allow us to provide groundbreaking treatments that have shown promising results [9].
Our knowledge on the diverse forms of lymphangiectasias is limited by the low incidence and high mortality of these lesions diagnosed in fetuses and neonates [10]. The clinical manifestations of lymphangiectasias are nonspecific and clinicians have to rely on the imaging findings to reach a definitive diagnosis [1, 11]. Further, the amount of literature available on fetal lymphangiectasias is scarce. It is imperative to know the progression of the different imaging findings of lymphangiectasias from the prenatal to the immediate postnatal life in order to provide appropriate parental counseling. In the present article, we discuss the normal and abnormal development of the fetal lymphatic system and how to best depict it by imaging during the prenatal and postnatal life.
Clinical classification and associated conditions
The International Society for the Study of Vascular Anomalies (ISSVA) classifies lymphatic malformation into five groups: common lymphatic malformation (sub-divided into macrocystic, microcystic and mixed cystic); generalized lymphatic anomaly; Gorham–Stout disease; channel-type lymphatic malformation; and primary lymphedema [12, 13]. Each category encompasses different diseases that vary across age groups and underlying diagnoses. Neonatal lymphatic flow disorders compose one known group that is classified as a channel-type lymphatic malformation and can be detected prenatally as hydrops fetalis, chylous ascites or chylothorax [14]. Postnatally, dynamic contrast-enhanced MR lymphangiography aids in dividing neonatal lymphatic flow disorders into three types: pulmonary lymphatic perfusion syndrome, central lymphatic flow disorder (also called congenital lymphatic dysplasia) and congenital chylous ascites [14]. Fetal lymphangiectasia is considered a neonatal lymphatic flow disorder; however, subsequent postnatal classification depends on the degree of involvement because it can manifest locally (e.g., isolated chylothorax) or diffusely (e.g., hydrops fetalis) in the fetus [6]. For example, a localized pulmonary lymphangiectasia might be considered a pulmonary lymphatic perfusion disease; however, diffuse compromise of the lymphatic channels suggests a central lymphatic flow disorder rather than a pulmonary lymphatic perfusion syndrome (Fig. 1).
Fetal lymphangiectasia can occur in any organ with lymphatic drainage. The lungs tend to be more frequently affected and are almost invariably involved in the diffuse forms (i.e. more than one organ) [6]. Noonan et al. [15] in 1970 proposed to organize neonatal pulmonary lymphangiectasias into three groups: (1) as part of a generalized lymphangiectasia, (2) secondary to pulmonary obstruction and (3) as a primary developmental disorder. More recently, most authors classify pulmonary lymphangiectasia as primary and secondary for simplicity [16, 17]. Primary lymphangiectasia is considered when the inherent developmental abnormality is in the lymphatic system — either as an isolated malformation or associated with a genetic syndrome such as Turner syndrome [16]. Secondary lymphangiectasia is usually a consequence of a congenital heart disease with significant obstructive cardiac dysfunction that leads to an elevated systemic venous pressure and lymphatic fluid accumulation (e.g., hypoplastic left heart syndrome) [18].
Normal and abnormal development of the lymphatic system
The embryology of the lymphatic system is poorly understood. However, the advent of molecular biology and the discovery of specific markers have brought with them a better perspective about the ontogeny of the lymphatics. The lymphatic channels are thought to arise from venous precursor cells after the heart and the major vascular primitive structures have developed, as follows. First, by vasculogenesis, a cluster of mesodermal cells in the embryo differentiates into endothelial precursor cells (angioblasts) and forms the first blood vessels, the aorta, and the cardinal venous structures [19, 20]. Then, the blood circulation is established and stimulates the development of the primary capillary plexi into the well-known hierarchical network of blood vessels — arteries, arterioles, capillaries, venules and veins — through a process known as angiogenesis [19, 21]. At the end of the fifth week of gestation, a cluster of endothelial cells in the primitive veins starts to differentiate into lymphatic endothelial cells, which sprout out from the primitive veins and form the first lymphatic sacs [20, 22, 23]. These sacs further develop throughout the body and give rise to the lymphatic system. The jugular lymphatic sacs appear at about the sixth week of development and drain the lymphatic vessels of the upper limbs, upper trunk, head and neck. The retroperitoneal lymphatic sac, cisterna chyli, and posterior lymphatic sacs appear and drain the lymph of the trunk and lower extremities [24]. Finally, the primitive lymphatic vessels branch out and form a hierarchy similar to the blood vessels — from lymphatic capillaries to collecting ducts. The blind-ending lymphatics come together and form the main collecting vessels (the thoracic duct and right lymphatic duct) that drain into the central veins [19, 20].
The etiology of primary lymphangiectasia is not completely elucidated. On histological examination of samples from patients with congenital pulmonary lymphangiectasias, the lung displays large cystic spaces in the subpleural, interlobular, perivascular and peribronchial areas [16]. However, it is not clear whether these aberrant lymphatic channels are the result of a failed normal regression of the pulmonary connective tissue during the 16th week of pregnancy or an uncontrolled proliferation of the lymphatics or an inability to remodel into a mature lymphatic structure. Secondary lymphangiectasia, on the other hand, seems to be related to the timing between the development of the cardiovascular system and the lymphatic channels. The heart and blood vessels develop earlier in gestation compared to the lymphatic channels. A possible congenital anomaly or erroneous signaling event might trigger a cascade of events that can lead to the dilation of the lymphatics [24, 25]. For instance, a high retrograde pressure from the left atrium to the pulmonary veins and pulmonary lymphatics, as happens in hypoplastic left heart syndrome with intact or restrictive atrial septum, might lead to pulmonary lymphangiectasia [26]. Congenital and acquired lymphatic maldevelopment is frequent among patients with obstructive left-side heart conditions such as hypoplastic left heart syndrome with restrictive atrial septum and total anomalous pulmonary venous return [27,28,29]. Similarly, congenital heart diseases associated with increased pulmonary blood flow — such as atrial and ventricular septal defect — lead to a high capillary filtration of protein-poor fluid into the interstitial space and an increased lymphatic fluid clearance [30]. This chronic high pulmonary blood flow subsequently leads to lymphatic endothelial dysfunction, decreased clearance and lymphatic malfunction [31].
Prenatal imaging
The most frequent findings on prenatal US imaging in pulmonary lymphangiectasias include a heterogeneous appearance of the lung parenchyma and the presence of pleural effusions that might raise concerns for an underlying pulmonary lymphatic lung abnormality [26]. Similarly, prenatal US imaging plays a pivotal role as the first step for early identification of congenital heart disorders and their hemodynamic repercussions [32]. Hypoplastic left heart syndrome can be diagnosed on prenatal US imaging, which can also exquisitely evaluate the characteristics of interatrial communication [32]. However, US imaging might not be as sensitive in finding pulmonary lymphangiectasia as MRI.
Pulmonary lymphangiectasia in fetal MRI manifests as a heterogeneous appearance of the lung parenchyma with T2-hyperintense thin and branching tubular structures extending from the hila to the pleural surface — an appearance known as the nutmeg lung [33]. Correlation between histological evaluation and the MR images suggests that these tubular areas of T2 hyperintensity reflect dilated lymphatic channels associated with pleural and interlobular septal thickening [26]. The enlarged lymphatic and blood vessels can increase the lung size and yield a larger lung volume expected for gestational age [33]. This appearance can also be identified on prenatal US examination, although it is less conspicuous compared to MRI [34] (Fig. 2). The nutmeg lung appearance on MRI has also been linked to a poor clinical evolution and higher mortality in infants with hypoplastic left heart disease [26]. Pulmonary lymphangiectasia secondary to congenital heart disease is associated with thickening of elastin layers (arterialization), which might reflect an irreversible damage of the lymphatic wall that leads to poor prognosis [26].
Pre- and postnatal findings of pulmonary lymphangiectasia. a Coronal T2-weighted MR image at 32 weeks of gestation shows bilateral T2-hyperintense heterogeneous appearance of the lung parenchyma with thin and branching tubular structures (arrows) extending from the hila to the pleural surface, consistent with nutmeg lung and pulmonary lymphangiectasia. b Axial prenatal US imaging performed at 36 weeks of gestation displays the same pattern in the lung (arrows). c Anteroposterior postnatal chest radiograph at 1 day of age depicts diffuse coarse interstitial markings bilaterally. The girl was subsequently diagnosed with hypoplastic left heart syndrome
Encountering additional fetal anomalies helps to elucidate the etiology of the pulmonary lymphangiectasia. A previous study showed that prenatal body wall edema was present in a fetus that subsequently was diagnosed with a central lymphatic flow disorder, whereas fetuses whose only finding was nutmeg lung were diagnosed with neonatal chylothorax [34]. This suggests that imaging findings such as ascites, body wall edema and hydrops fetalis (presence of fluid in at least two fetal compartments) might reflect a more severe lymphatic flow abnormality compared to those with isolated nutmeg lung [34].
Fetal US and MR imaging are of paramount importance for appropriate parental counseling and perinatal care planning [35]. Identification of nutmeg lung can be used for risk stratification of prenatal surgical interventions such as percutaneous catheter-directed balloon dilation or atrial septectomy in the fetus with hypoplastic left heart syndrome. These procedures are associated with better early survival and improved clinical parameters (such as oxygen requirements) compared to immediate neonatal interventions [36].
Postnatal imaging
Chest ultrasonography
During the neonatal period, chest radiograph and CT findings in patients with pulmonary lymphangiectasia include subpleural and perivascular interlobular septal thickening. However, CT is not the best imaging modality to diagnose pulmonary lymphangiectasia because some imaging findings can be confused with interstitial edema — especially in the setting of congenital heart disease — and other interstitial lung diseases [37]. Chest US imaging has proved to be a useful imaging modality for identifying pulmonary lymphangiectasia in neonates with chronic venous obstruction secondary to a congenital heart disease [38]. Lung surface irregularity and subpleural cystic-appearing structures are the most frequent findings in these patients. The irregular lung surface seems to display the interface between air-filled lung and the dilated lymphatics, whereas the subpleural cystic-appearing structures reflect the dilated lymphatics in the subpleural space. However, only the presence of both these findings yields the highest diagnostic performance in neonates with pulmonary lymphangiectasia secondary to congenital heart disease, and they might be related to a worse prognosis [38] (Fig. 3). Other US findings, such as vertical-ring-down artifacts, are not specific for pulmonary lymphangiectasia and should be interpreted within the appropriate clinical context [39].
Primary lymphangiectasia. a Coronal prenatal T2-weighted MRI in a 30-week fetus with bilateral hypoplastic lungs shows associated abnormal T2 signal consistent with nutmeg lung (arrow), associated with a pleural effusion. He was born via cesarean section at 36 weeks of gestation and intubated at birth. b Axial postnatal chest US image obtained at 1 month of age in the same boy shows irregular lung surface (solid arrows) with subpleural cystic-appearing structures (dashed arrows) and multiple vertical-ring-down artifacts. c Coronal post-contrast T1-weighted MR lymphangiogram shows abnormal and diffuse lymphatic perfusion to both lungs (arrows). d At 2 months old, the boy underwent an intranodal lymphangiogram with ethiodized oil to fill the central lymphatic system. A follow-up anteroposterior chest radiograph shows ethiodized oil in both lungs
Dynamic contrast-enhanced lymphangiography
Recent breakthroughs in the imaging of the lymphatic system with MRI have allowed us to better diagnose and treat lymphatic malformations. Dynamic contrast-enhanced MR lymphangiography is a novel technique that permits the static and dynamic evaluation of the central conducting lymphatics in children [14]. With US imaging, both inguinal lymph nodes can be identified and directly accessed using a 25-gauge needle and a short connecting tube. The intranodal position of the needle tip can be confirmed by fluoroscopy using a small volume of iodinated contrast agent or oil-based contrast agent. The infant is subsequently moved to the MRI scanner, avoiding any movements to reduce the chances of needle dislodgment. A routine macrocyclic gadolinium-based contrast agent is injected into the inguinal nodes at a standard dose (0.1–0.2 mmol/kg) for evaluating the lymphatic channels [40]. The dynamic contrast-enhanced MR lymphangiography protocol consists of the following sequences: (1) T2-weighted MR images and (2) pre- and post-contrast T1-weighted dynamic MR images [9]. Heavily T2-weighted sequences are excellent to visualize the lymphatic structures and are used to delineate the lymphatic channels. Post-contrast 3-D spoiled gradient-recalled echo T1-weighted sequences with fat suppression are acquired repeatedly at approximately 30–60 s [9]. These images permit evaluation of the flow of contrast agent throughout the central conducting lymphatics. Respiratory- and electrocardiography-triggered protocols can improve visualization of the lymphatic channels with respect to blood vessels. Normal dynamic contrast-enhanced MR lymphangiography following intranodal injection implies passage of the contrast agent first to the inguinal and iliac lymphatics, then to the retroperitoneal lymphatics and cisterna chyli, and subsequently to the thoracic duct, ending in the left venous angle (Fig. 4) [14]. Peripheral lymphatic channels should not enhance given that the lymphatic flow is unidirectional because of the lymphatic valves [9]. Retrograde lymph flow to any organ (e.g., lungs, liver, soft tissues) is considered abnormal.
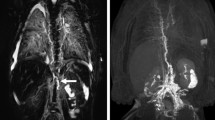
Coronal post-contrast T1-weighted (a) and maximum-intensity projection (b) images in a 3-year-old girl with a history of protein-losing enteropathy and a normal dynamic contrast-enhanced MR lymphangiogram. After the intranodal injection, contrast agent is visible first in the inguinal and iliac lymphatics, then in the retroperitoneal lymphatics at the aortic bifurcation and cisterna chyli, and subsequently in the thoracic duct, ending in the left venous angle. A normal central lymphatic system does not display any lymphatic flow to the lungs or any dilation or strictures of the thoracic duct
Intrahepatic dynamic contrast-enhanced MR lymphangiography is a variation of the standard dynamic contrast-enhanced MR lymphangiography for evaluating the hepatic lymphatic system. This technique relies on percutaneous access to a lymphatic branch in the vicinity of the portal vein under US guidance, through which gadolinium-based contrast agent is administered. A standard dynamic contrast-enhanced MR lymphangiography protocol is then performed [41]. A previous study showed that intrahepatic dynamic contrast-enhanced MR lymphangiography is useful for visualizing the lymphatic drainage pattern and that it is more sensitive than standard inguinal intranodal dynamic contrast-enhanced MR lymphangiography for identifying contrast leakages in the duodenum (Fig. 5) [41]. Intrahepatic dynamic contrast-enhanced MR lymphangiography should be considered in children with high suspicion for a central lymphatic flow disorder because it can help to identify lymphatic disorders that might not be visualized on an inguinal intranodal dynamic contrast-enhanced MR lymphangiography study.
MR lymphangiography in a 2-month-old boy with a history of bilateral pleural effusions and congenital chylothorax. a–d Coronal post-contrast T1-weighted (a, b [posterior to a]) and maximum-intensity projection (c, d [posterior to c]) images of intrahepatic dynamic contrast-enhanced MR lymphangiography show a leak of contrast agent into the peritoneum (dashed arrows) and subsequent abnormal lymphatic perfusion to both lungs (solid arrows). The black artifact in the right inferior quadrant corresponds to the intrahepatic needle or the respiratory navigator
The postnatal evaluation of the central conducting lymphatics in children with known fetal lymphangiectasia has significant implications regarding the intervention and clinical outcome [42]. Prenatal imaging findings on fetal MRI seem to correlate with postnatal findings on dynamic contrast-enhanced MR lymphangiography [34]. The nutmeg lung appearance on fetal MRI likely corresponds to abnormal postnatal pulmonary lymphatic perfusion, whereas fetal body wall edema reflects retrograde lymph flow to the subcutaneous tissue seen on postnatal dynamic contrast-enhanced MR lymphangiography, findings known as dermal backflow [34]. It is important to determine in dynamic contrast-enhanced MR lymphangiography whether contrast agent is passing to one or more body compartments in the neonate. Children with neonatal chylothorax usually present with only abnormal pulmonary lymphatic perfusion, whereas those with a central lymphatic flow disorder present with contrast passage to other compartments (Fig. 6). Fetal lymphangiectasia secondary to genetic syndromes usually presents with a broader involvement of the central conducting lymphatics. A previous study in a cohort of people with Noonan syndrome showed that frequent findings on dynamic contrast-enhanced MR lymphangiography were retrograde intercostal lymphatic flow, pulmonary lymphatic perfusion, and agenesis or dysgenesis of the central conducting lymphatics [42].
Comparison between neonatal chylothorax (pulmonary lymphatic perfusion syndrome) and central lymphatic flow disorder. a Coronal post-contrast T1-weighted MR image in a 25-day-old girl prenatally diagnosed with bilateral pleural effusions shows abnormal lymphatic lung perfusion bilaterally (arrows). b Coronal post-contrast T1-weighted MR image in a 4-month-old boy with a history of transposition of the great arteries demonstrates abnormal lymphatic perfusion in both lungs (arrows) and dermal backflow in the left inguinal and right supraclavicular regions (circle). The black artifact in the mid-abdomen corresponds to the intrahepatic needle or the respiratory navigator
Conventional lymphangiography and percutaneous lymphatic procedures
Percutaneous lymphatic procedures such as thoracic duct embolization are well-known alternatives to surgical interventions. These procedures are divided into two steps: conventional intranodal lymphangiography and lymphatic/thoracic duct embolization. During intranodal lymphangiography, the inguinal lymph nodes are directly accessed under US guidance. Then, an oil-based contrast agent (ethiodized oil) is injected in the lymph nodes until opacification of the lymphatic vessels is observed under fluoroscopy [43]. The goal of this phase is to visualize the abdominopelvic lymphatics, cisterna chyli, and thoracic duct to identify the target lymphatic vessels [43]. Subsequently, the lymphatic system is accessed through a contributing lymphatic feeder by using a transabdominal approach. Contrast agent is injected under fluoroscopy to identify the target vessels and image-guided embolization is performed [44].
Conventional lymphangiography has been described in different clinical settings and in a very heterogeneous group of people and has the advantage of allowing percutaneous intervention if needed. The correlation between dynamic contrast-enhanced MR lymphangiography and conventional lymphangiography seems to be excellent in various conditions; however, dynamic contrast-enhanced MR lymphangiography is more sensitive to showing the degree of lymphatic perfusion of the lungs and mediastinum [34, 42, 45].
Treatment
There is no standardized treatment for patients with fetal lymphangiectasias. Treatment is patient-specific and depends mainly on the fetus’s age and the extension of the disease. Prenatal treatments such as intrauterine thoracentesis [46] and thoracoamniotic shunting [47] have proved to be beneficial in fetuses with hydro- and chylothorax; however, the utility of these interventions in fetuses with lymphangiectasias is unknown. Postnatal treatments can be divided into conservative, pharmaceutical and percutaneous/surgical treatments. Neonates with mild symptoms and extension of the disease can be managed with conservative treatment consisting of a low-oral-fat diet with medium-chain triglycerides, with the goal of reducing lymphatic production [48]. Similarly, octreotide [49] and sirolimus [50] are two well-known drugs that have been shown to improve symptoms in the short term by mechanisms not fully understood. Finally, percutaneous interventions with conventional lymphangiography can be considered the cornerstone of treatment in neonates with lymphangiectasia. Most interventions in neonates with lymphangiectasia include embolization with ethiodized oil, N-butyl cyanoacrylate glue, or coils (Fig. 7) [34, 40, 42, 51]. The goal of lymphatic embolization is to reduce the lymph flow from the thoracic duct to the lung parenchyma or abnormally perfused organ (video in Supplemental Online Material). Neonates with mild to moderate symptoms might require only selective embolization, whereas those with more global involvement might not respond favorably to percutaneous interventions [40]. The patency of the thoracic duct is paramount, and infants with an occluded thoracic duct might benefit more from a surgical lymphovenous anastomosis to reconstitute the normal drainage.
Conventional lymphangiography with percutaneous embolization with ethiodized oil in a 2-month-old boy with a history of bilateral pleural effusions and congenital chylothorax (same boy as Fig. 5). a Anteroposterior (AP) image from percutaneous bilateral inguinal lymphatic access (arrow) is obtained with US guidance and confirmed. b AP fluoroscopic image of the chest shows embolization of the abnormal pulmonary lymphatic network (arrows) is performed by injecting ethiodized oil into the inguinal nodes bilaterally and pushing it to the abnormal pulmonary lymphatics by manual compression. c Follow-up AP chest radiograph displays the ethiodized oil in both lungs as expected. A video of this procedure can be found as Online Supplemental Material
Conclusion
Fetal lymphangiectasia is a rare condition with variable degrees of involvement of the lymphatic system. Prenatal imaging with US and fetal MR imaging is essential for appropriate parental counseling and perinatal treatment planning. Postnatal imaging with dynamic contrast-enhanced MR lymphangiography and conventional lymphangiography is paramount to further classify and evaluate lymphatic maldevelopment. Treatment is patient-specific and, as of yet, not standardized, with the manner of treatment depending mainly on the degree of involvement of the lymphatic flow disorder.
References
Faul JL, Berry GJ, Colby TV et al (2000) Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med 161:1037–1046
Laurence KM (1959) Congenital pulmonary lymphangiectasis. J Clin Pathol 12:62–69
Bellini C, Mazzella M, Campisi C et al (2004) Multimodal imaging in the congenital pulmonary lymphangiectasia–congenital chylothorax–hydrops fetalis continuum. Lymphology 37:22–30
Jacquemont S, Barbarot S, Boceno M et al (2000) Familial congenital pulmonary lymphangectasia, non-immune hydrops fetalis, facial and lower limb lymphedema: confirmation of Njolstad's report. Am J Med Genet 93:264–268
Blei F (2008) Congenital lymphatic malformations. Ann N Y Acad Sci 1131:185–194
Malone LJ, Fenton LZ, Weinman JP et al (2015) Pediatric lymphangiectasia: an imaging spectrum. Pediatr Radiol 45:562–569
Victoria T, Johnson AM, Edgar JC et al (2016) Comparison between 1.5-T and 3-T MRI for fetal imaging: is there an advantage to imaging with a higher field strength? AJR Am J Roentgenol 206:195–201
Pugash D, Brugger PC, Bettelheim D, Prayer D (2008) Prenatal ultrasound and fetal MRI: the comparative value of each modality in prenatal diagnosis. Eur J Radiol 68:214–226
Shaikh R, Biko DM, Lee EY (2019) MR imaging evaluation of pediatric lymphatics:: overview of techniques and imaging findings. Magn Reson Imaging Clin N Am 27:373–385
Bouchard S, Di Lorenzo M, Youssef S et al (2000) Pulmonary lymphangiectasia revisited. J Pediatr Surg 35:796–800
Yuan SM (2017) Congenital pulmonary lymphangiectasia. J Perinat Med 45:1023–1030
Marugan V, Lopez-Gutierrez J (2016) Primary intestinal lymphangiectasia and its association with generalized lymphatic anomaly: case series and review. J Pediatr Rev 4
International Society for the Study of Vascular Anomalies (2018) ISSVA classification for vascular anomalies. https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf. Accessed 7 Mar 2020
Chavhan GB, Amaral JG, Temple M, Itkin M (2017) MR lymphangiography in children: technique and potential applications. Radiographics 37:1775–1790
Noonan JA, Walters LR, Reeves JT (1970) Congenital pulmonary lymphangiectasis. Am J Dis Child 120:314–319
Reiterer F, Grossauer K, Morris N et al (2014) Congenital pulmonary lymphangiectasis. Paediatr Respir Rev 15:275–280
Wagenaar SS, Swierenga J, Wagenvoort CA (1978) Late presentation of primary pulmonary lymphangiectasis. Thorax 33:791–795
Esther CR Jr, Barker PM (2004) Pulmonary lymphangiectasia: diagnosis and clinical course. Pediatr Pulmonol 38:308–313
Oliver G (2004) Lymphatic vasculature development. Nat Rev Immunol 4:35–45
Adams RH, Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8:464–478
Patan S (2004) Vasculogenesis and angiogenesis. Cancer Treat Res 117:3–32
Wigle JT, Oliver G (1999) Prox1 function is required for the development of the murine lymphatic system. Cell 98:769–778
Cueni LN, Detmar M (2006) New insights into the molecular control of the lymphatic vascular system and its role in disease. J Invest Dermatol 126:2167–2177
Schoenwolf GC, Bleyl SB, Brauer PR, Francis-West PH (2015) Development of the vasculature. In: Bleyl SB, Brauer PR, Francis-West PH, Larsen WJ (eds) Larsen’s human embryology. Elsevier, Philadelphia, pp 304–340
Butler MG, Isogai S, Weinstein BM (2009) Lymphatic development. Birth Defects Res C Embryo Today 87:222–231
Saul D, Degenhardt K, Iyoob SD et al (2016) Hypoplastic left heart syndrome and the nutmeg lung pattern in utero: a cause and effect relationship or prognostic indicator? Pediatr Radiol 46:483–489
Graziano JN, Heidelberger KP, Ensing GJ et al (2002) The influence of a restrictive atrial septal defect on pulmonary vascular morphology in patients with hypoplastic left heart syndrome. Pediatr Cardiol 23:146–151
Rychik J, Rome JJ, Collins MH et al (1999) The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol 34:554–560
Bellini C, Boccardo F, Campisi C, Bonioli E (2006) Congenital pulmonary lymphangiectasia. Orphanet J Rare Dis 1:43
Feltes TF, Hansen TN (1989) Effects of an aorticopulmonary shunt on lung fluid balance in the young lamb. Pediatr Res 26:94–97
Datar SA, Johnson EG, Oishi PE et al (2012) Altered lymphatics in an ovine model of congenital heart disease with increased pulmonary blood flow. Am J Physiol Lung Cell Mol Physiol 302:L530–L540
Chitra N, Vijayalakshmi IB (2017) Fetal echocardiography for early detection of congenital heart diseases. J Echocardiogr 15:13–17
Victoria T, Andronikou S (2014) The fetal MR appearance of ‘nutmeg lung’: findings in 8 cases linked to pulmonary lymphangiectasia. Pediatr Radiol 44:1237–1242
Biko DM, Johnstone JA, Dori Y et al (2018) Recognition of neonatal lymphatic flow disorder: fetal MR findings and postnatal MR lymphangiogram correlation. Acad Radiol 25:1446–1450
Seed M, Bradley T, Bourgeois J et al (2009) Antenatal MR imaging of pulmonary lymphangiectasia secondary to hypoplastic left heart syndrome. Pediatr Radiol 39:747–749
McElhinney DB, Tworetzky W, Lock JE (2010) Current status of fetal cardiac intervention. Circulation 121:1256–1263
Nobre LF, Muller NL, de Souza Junior AS et al (2004) Congenital pulmonary lymphangiectasia: CT and pathologic findings. J Thorac Imaging 19:56–59
Lam CZ, Bhamare TA, Gazzaz T et al (2017) Diagnosis of secondary pulmonary lymphangiectasia in congenital heart disease: a novel role for chest ultrasound and prognostic implications. Pediatr Radiol 47:1441–1451
Trinavarat P, Riccabona M (2014) Potential of ultrasound in the pediatric chest. Eur J Radiol 83:1507–1518
Dori Y (2016) Novel lymphatic imaging techniques. Tech Vasc Interv Radiol 19:255–261
Biko DM, Smith CL, Otero HJ et al (2019) Intrahepatic dynamic contrast MR lymphangiography: initial experience with a new technique for the assessment of liver lymphatics. Eur Radiol 29:5190–5196
Biko DM, Reisen B, Otero HJ et al (2019) Imaging of central lymphatic abnormalities in Noonan syndrome. Pediatr Radiol 49:586–592
Nadolski G, Itkin M (2013) Thoracic duct embolization for the management of chylothoraces. Curr Opin Pulm Med 19:380–386
Chen E, Itkin M (2011) Thoracic duct embolization for chylous leaks. Semin Intervent Radiol 28:63–74
Dori Y, Keller MS, Rome JJ et al (2016) Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation 133:1160–1170
Mon RA, Treadwell MC, Berman DR et al (2018) Outcomes of fetuses with primary hydrothorax that undergo prenatal intervention (prenatal intervention for hydrothorax). J Surg Res 221:121–127
Carr BD, Sampang L, Church JT et al (2018) Fetal intervention for congenital chylothorax is associated with improved outcomes in early life. J Surg Res 231:361–365
Desai AP, Guvenc BH, Carachi R (2009) Evidence for medium chain triglycerides in the treatment of primary intestinal lymphangiectasia. Eur J Pediatr Surg 19:241–245
Kuroiwa G, Takayama T, Sato Y et al (2001) Primary intestinal lymphangiectasia successfully treated with octreotide. J Gastroenterol 36:129–132
Ozeki M, Hori T, Kanda K et al (2016) Everolimus for primary intestinal lymphangiectasia with protein-losing enteropathy. Pediatrics 137:e20152562
Gray M, Kovatis KZ, Stuart T et al (2014) Treatment of congenital pulmonary lymphangiectasia using ethiodized oil lymphangiography. J Perinatol 34:720–722
Acknowledgments
The authors would like to thank Dr. Yoav Dori, MD, PhD, director of the Jill and Mark Fishman Center for Lymphatic Disorders, for his contributions and mentorship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(MP4 5,261 kb)
Rights and permissions
About this article
Cite this article
Barrera, C.A., Victoria, T., Escobar, F.A. et al. Imaging of fetal lymphangiectasias: prenatal and postnatal imaging findings. Pediatr Radiol 50, 1872–1880 (2020). https://doi.org/10.1007/s00247-020-04673-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-020-04673-6