Abstract
There is growing interest in the use of contrast-enhanced ultrasound (CEUS) in diagnostic and interventional radiology. CEUS applications in interventional radiology are performed with intravascular or intracavitary administration of microbubble-based US contrast agents to allow for real-time evaluation of their distribution within the vascular bed or in body cavities, respectively, providing additional information beyond gray-scale US alone. The most common interventional-radiology-related CEUS applications in children have been extrapolated from those in adults, and they include the use of CEUS to guide lesion biopsy and to confirm drain placement in pleural effusions and intra-abdominal fluid collections. Other applications are emerging in interventional radiology for use in adults and children, including CEUS to optimize sclerotherapy of vascular malformations, to guide arthrography, and for lymphatic interventions. In this review article we present a wide range of interventional-radiology-related CEUS applications, emphasizing the current and potential uses in children. We highlight the technical parameters of the CEUS examination and discuss the main imaging findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contrast-enhanced ultrasound (CEUS) is increasingly being used as an imaging modality in diagnostic radiology, with a wide number of applications. In recent years, the use of CEUS has expanded to interventional radiology (IR) as well, and it is important for the interventional radiologist to be familiar with the techniques, applications and interpretation of findings on CEUS.
Pediatric interventional radiologists are often adept at using US for guidance during procedures, and in certain IR applications, agitated saline is already used as an inexpensive medium to generate a temporary contrast effect with US [1, 2]. With the development of commercial ultrasound contrast agents (UCAs), however, stabilized inert gas-filled microbubbles have enabled improvement in the contrast resolution and in the duration of the contrast effect. As a result, there has been a rapid growth in the use of CEUS as an adjunct or problem-solving tool in both adult and pediatric IR. Similar to diagnostic CEUS applications, microbubbles are injected through an intravenous (IV) line or administered percutaneously into cavities through needles or catheters.
Contrast-enhanced US examinations with IV administration of microbubbles provides dynamic assessment of the enhancement patterns of different tissues and lesions. In adult IR, common IV CEUS applications include guiding biopsy of lesions that are inconspicuous or poorly visible on gray-scale US and evaluating residual enhancing tumor after percutaneous ablation or embolization [3,4,5,6,7,8]. Other highly technical procedures utilizing IV CEUS include its use in guiding endoleak repair, arteriovenous fistula intervention, and prostatic artery embolization [3, 7, 9,10,11].
Contrast-enhanced US examination with intracavitary UCA administration in adults is performed to confirm the correct placement of tubes and drains within physiological body cavities such as pleural, biliary or gastrointestinal locations; or non-physiological cavities such as abscesses or fluid collections; and to assess for possible communications or fistulous tracts between regions [12,13,14].
The adoption of CEUS in pediatric IR, however, has been slower, and barriers for more widespread use include a lack of user experience and available equipment, off-label use of US contrast agents, a different cohort of common interventional procedures in children, and potential costs relative to gray-scale US. Common IR CEUS applications in children have been adapted from their use in adults and include CEUS to guide lesion biopsy and to confirm the correct position of drains [15]. Other IR CEUS applications are only recently being developed in children, including adjunctive guidance during sclerotherapy of vascular malformations and during arthrography, and confirmation of the intra-nodal position of needles for subsequent magnetic resonance lymphangiography [16].
The benefits of CEUS for pediatric diagnostic imaging are mirrored in IR and include the lack of ionizing radiation, high temporal and spatial resolution, increased lesion conspicuity, bedside applications, and a low adverse event profile from the UCA. Although the cost of CEUS examination is greater than that of US alone, the overall cost-effectiveness of a CEUS program in a pediatric radiology department has been demonstrated [17].
In this review article, we present a wide range of IR CEUS applications with an emphasis on children. We highlight the technical aspects of the CEUS examination and interpretation of the main imaging findings. Typical pediatric case examples, in which CEUS can impact the success of the interventional procedure and patient management, are discussed.
Intravenous contrast-enhanced ultrasound technique for interventional applications
The United States Food and Drug Administration (FDA) approved sulfur hexafluoride lipid-type A microspheres (known as Lumason from Bracco Diagnostics, Monroe Township, NJ; and SonoVue from Bracco, Milan, Italy) for three pediatric applications: characterization of liver lesions and echocardiography following IV injection, and vesicoureteral reflux detection following intravesical administration. All other applications in the field of pediatric diagnostic radiology or IR are performed off-label. More off-label uses are gradually being instituted in pediatric diagnostic and interventional US as their clinical value becomes clearer.
The dose of Lumason for IR-related IV CEUS applications in children is 0.03 mL/kg, the same as for diagnostic imaging, up to a maximum of 2.4 mL per injection. The total dose administered, however, is adjusted based on the patient size and the organ being imaged, as well as the choice of transducer and image settings. If high-resolution linear transducers are needed, the dose might need to be increased up to two-fold.
A nurse or technologist administers the contrast agent while the interventional radiologist performs the procedure. It is important to discuss with the staff providing sedation or anesthesia that IV access might be temporarily occupied while the contrast agent is given. The contrast agent is injected slowly at a rate of 1 mL/s through a peripheral IV catheter, preferably 22-gauge or larger, or a central venous catheter, and is followed by a 10-mL saline flush. To decrease microbubble destruction, the contrast agent should be administered without the use of a hemostatic cap on the catheter. If a 3-way stopcock is used, the contrast agent should be injected through the port in-line (180°) with the IV cannula. Central venous catheters can be used for UCA administration if aseptic technique is used, and contrast arrival times are usually shorter than for peripheral injection. However, the use of central venous catheters is discouraged if peripheral venous access can be easily achieved.
Following bolus injection, the echogenic effect of the circulating microbubbles peaks within about 1 min and lasts for approximately 5 min. To prolong enhancement compared to single-dose bolus injection, continuous infusion of diluted UCA via gravity drip or a dedicated power pump can be used, as shown in studies in adults; for Lumason, an infusion rate of 0.5–1.0 mL/min has been reported [18].
When planning a procedure, such as for tissue biopsy, repeat dosing can be performed as needed [3]. In this setting, one IV UCA dose is injected after the child is sedated/anesthetized and before he or she is draped to identify the lesion and nearby landmarks, determine the optimal sonographic window, plan needle trajectory, and optimize imaging parameters. Then either the biopsy is guided by the findings of this pre-biopsy CEUS scan, or a second dose is injected just before deploying the biopsy instrument during the procedure. The latter approach is particularly helpful while targeting small lesions [3]. A dual-display mode, where both the gray-scale and contrast-enhanced images are displayed side by side, allows for simultaneous anatomical orientation and accurate lesion identification. If necessary, a third UCA dose can be administered after the procedure to confirm that the biopsy tract traversed the enhancing target lesion or to assess for post-procedural complications, such as bleeding or other vascular injuries.
In general, about 10-min intervals between bolus injections usually suffice for intravascular clearance. Alternatively, to accelerate microbubble destruction between injections, switching to color Doppler mode or applying flash technique is used to deliver a high mechanical index insonation that bursts the microbubbles.
Intracavitary contrast-enhanced ultrasound technique for interventional applications
Prior to the procedure, the UCA is reconstituted and drawn into a syringe for sterile administration into a cavity through a needle or catheter placed percutaneously. In general, when the UCA is administered within a cavity, it should be substantially diluted compared to intravascular administration because the fluid volume confined within the cavity is much lower than the circulating blood volume [10]. If the contrast agent is too concentrated, a strong posterior acoustic shadow might appear and limit far-field visualization. If this occurs, it might be necessary to either burst the microbubbles within the cavity by sonication or withdraw the contrast agent from the cavity and then re-administer a more diluted solution. Once within a cavity, UCA might have little or no movement compared to the constant flow with the vascular circulation, which further lends to agent stability [15, 19].
Aside from intravesical administration where a 0.2% solution is utilized, there are no manufacturer dose recommendations for intracavitary UCA use. Depending on the indication, the dilution index can range from a few drops of contrast agent (0.1–0.2 mL) to 1–2 mL in 10 mL or more of normal saline [7, 10, 13, 15]. The dilution index can then be titrated to optimize visualization of the cavity as needed. In vitro studies have shown that SonoVue/Lumason is stable in a diluted state and provides sufficient backscatter echoes that last up to 20–30 min [19].
Contrast-enhanced ultrasound imaging findings in interventional applications
Aspiration and drainage
Aspiration and drainage of intra-abdominal fluid collections and pleural effusions are among the most common procedures in IR. On US, the imaging features of an abscess vary depending on the stage of its evolution, but it is often visualized as a poorly defined isoechoic or anechoic lesion surrounded by an irregular, typically hyperemic outer rim. CEUS can accurately confirm the presence of an abscess and assess its size and morphology. On IV CEUS, an abscess appears as a central non-enhanced area with a rim of peripheral enhancement [20].
Once an abscess is detected, fluid aspiration and drain insertion are usually performed under direct US visualization. In this setting, the diluted UCA can be administered through the drainage catheter to confirm appropriate access to the collection, wherein UCA will disperse within but remain confined by the abscess cavity. Intracavitary CEUS can be useful to identify potential communication between loculations in a complex collection because the UCA instilled into one region should opacify all connected areas [7, 9, 13].
The utility of CEUS for drainage is demonstrated in the management of appendicitis and complicated pneumonia, both of which are frequently encountered in pediatric IR. Appendiceal perforation occurs in up to 30% of children with appendicitis and can result in abdominopelvic abscesses [21, 22]. When drainage is indicated in children with multiple collections, intracavitary administration of diluted UCA through the initial drain placed can reveal whether the abscess is loculated or a single cavity. If multiple loculations are present without robust communication between them, additional drainage sites might be needed (Fig. 1).
Complex fluid collections in a 10-year-old girl presenting with vomiting and abdominal pain following appendectomy for appendicitis. a Contrast-enhanced CT of the pelvis, coronal reformat, shows complex fluid collections in the right lower quadrant (upper arrow) and pelvis (lower arrow) with a possible thin connecting channel extending between them (arrowheads). b Intravenous-contrast-enhanced axial CT of the pelvis shows a complex fluid collection within the pelvis (arrows), with a peripherally enhancing wall located anterior to the rectum and extending anteriorly (arrowheads). c Contrast-enhanced ultrasound (CEUS) performed following the placement of a transrectal drainage catheter into the pelvic fluid collection component. Ultrasound contrast agent solution was injected through the transrectal drainage catheter to assess whether the components of the collection connected with each other. Axial CEUS in contrast-only mode shows the contrast agent microbubbles fill the pelvic collection (asterisk) in a configuration mirroring that on the axial CT image (b). The microbubbles failed to communicate with the right lower quadrant collection (not shown). In this case, CEUS was used as a problem-solving tool, changing the management by prompting an additional drain placement
Pneumonia is considered complicated when it is associated with a parapneumonic effusion, empyema or lung necrosis progressing to a parenchymal abscess. It is estimated that parapneumonic effusion or empyema is present in 1–10% of community-acquired pneumonia cases [23]. In the setting of a superficial lung abscess, IV CEUS helps to differentiate the consolidated lung parenchyma that enhances uniformly from the non-enhancing regions associated with a pulmonary abscess, which can be difficult to discern from each other on gray-scale and Doppler imaging in the early stages of infection. Therefore, by use of CEUS, the interventional radiologist can decrease the amount of normal lung traversed during drain placement (Fig. 2) [10, 24].
Lung abscess in a 1-year-old girl presenting with fever and respiratory symptoms that did not respond to antibiotic therapy. a Contrast-enhanced CT of the chest, coronal reformat, shows a large thick-walled multiloculated rim-enhancing fluid collection (arrow) occupying the right upper lobe. It is predominantly hypoenhancing and also contains a few tiny foci of gas, compatible with a lung abscess. b Contrast-enhanced ultrasound (CEUS) was performed with intravenous administration of ultrasound contrast agent prior to abscess drainage. Simultaneous display of gray-scale (left) and contrast-enhanced (right) modes in the coronal plane shows the necrotic components of the abscess as non-enhancing areas (asterisks), while there is enhancement of the adjacent consolidated lung (arrows). In this case, CEUS was an adjunct tool that enabled accurate differentiation between the abscess cavities and the consolidated lung. This information was important for precise placement of the percutaneous drainage catheter
Similar techniques are adopted for post-procedural drain management. For example, when the output of an indwelling drain is unexpectedly low, bedside intracavitary instillation of UCA solution through the catheter can both (1) assess the position of the drain within the thoracic or abdominal collection and (2) evaluate for persistent loculations that require fibrinolytic agents to facilitate drainage [24]. When the drain is ready to be removed, CEUS can be used to identify any residual collection and assess for any fistulae that would preclude catheter removal.
Biopsy
A target lesion for a biopsy procedure that is well-seen on MRI or CT might be inconspicuous on US, making a US-guided procedure technically challenging and increasing the potential for a non-diagnostic result. In this setting, CEUS can improve detection of the lesion and guide tissue sampling (Fig. 3) [3, 5, 10].
Biopsy in a 13-year-old girl with a history of osteosarcoma and medulloblastoma who presented with new liver lesions detected on screening whole-body MRI. a Axial fat-saturated T2-weighted MRI shows a T2-hyperintense lesion in liver Segment III (arrow) near the stomach (asterisk). b Axial gray-scale US shows no visible mass in the region adjacent to the stomach (asterisk). c Axial contrast-enhanced ultrasound (CEUS) performed with intravenous contrast administration at the time of biopsy identifies a hyperenhancing lesion (arrow) corresponding to the MRI findings. In this case, CEUS increased the operator’s confidence and directed the biopsy needle into a lesion that was not visible on gray-scale US. The pathology was diagnostic of focal nodular hyperplasia
The capacity of CEUS to provide differential enhancement between the targeted lesion and the surrounding tissue enables better delineation of the lesion’s size and borders and of intralesional features. Specifically, CEUS readily distinguishes between the enhancing tumor and non-enhancing intralesional elements, such as cystic or necrotic areas and hemorrhage. For larger or heterogeneous tumors with varying degrees of central necrosis, IV CEUS can accurately demonstrate the portions of the tumor that are solid or more vascular, which correspond to areas of viability not evident on gray-scale or color Doppler imaging (Fig. 4; Online Supplementary Material 1) [25]. This information helps to direct biopsy toward the viable region, which is important not only for obtaining a histological diagnosis but also for providing the specimens needed for advanced pathological analysis, such as molecular profiling, next-generation sequencing and biobanking. At the same time, IV CEUS can minimize procedural morbidity by elucidating blood vessels or adjacent structures that should be avoided during biopsy.
Biopsy in a 15-year-old boy with large liver mass (same boy depicted in Online Supplementary Material 1). a Axial fat-saturated T2-weighted MRI shows a large heterogeneous mass occupying part of the right hepatic lobe (arrowheads). b Axial color Doppler US shows the mass as diffusely hyperechoic with no appreciable internal flow signals. Doppler flow is noted within the adjacent normal liver parenchyma. Discrimination between viable soft tissue and areas of necrosis is limited based on the gray-scale and Doppler US findings. c Contrast-enhanced ultrasound (CEUS) with intravenous contrast agent and simultaneous display of gray-scale (left) and contrast (right) modes in the axial plane. CEUS examination was performed immediately prior to firing the biopsy needle. A large non-enhancing area is seen centrally within the mass, in keeping with tumor necrosis; however, CEUS clearly identifies nodular enhancing tissue in the periphery of the mass (arrowheads) that corresponds to viable tissue. Of note, a track (asterisk) is seen within the non-enhancing component of the tumor from a non-diagnostic biopsy performed 2 days prior without CEUS guidance. In this case, CEUS was crucial for accurate planning of the biopsy trajectory, and sufficient viable tissue was obtained for the diagnosis of undifferentiated embryonal sarcoma
Studies have shown CEUS is beneficial for biopsy of liver, pancreatic, renal, soft-tissue and peripheral lung lesions [5, 6, 26, 27]. Specifically, the use of real-time CEUS for guiding liver lesion biopsy increased diagnostic accuracy in small lesions (≤2 cm) and increased the diagnostic yield 10–15% by decreasing the false-negative rate [3, 28].
Genitourinary system
Percutaneous nephrostomy placement is also a common procedure in adult and pediatric IR and can be performed in both dilated and non-dilated systems. The main indications for nephrostomy tube insertion are to relieve urinary tract obstruction, to provide urinary tract diversion (e.g., following ureteral injury or hemorrhagic cystitis), and to gain access to the urinary tract for subsequent intervention (e.g., stone removal, dilation/stenting of a ureteral stricture) [29,30,31]. Elements of this procedure are, by convention, performed with fluoroscopic guidance; however, some of these can be modified by CEUS, thereby decreasing ionizing radiation exposure [32].
For example, CEUS can be used to aid in accessing the collecting system. For dilated systems, a small amount of diluted UCA is placed within the access needle, and once it enters the collecting system, the contained UCA can be seen refluxing out of the needle because of the increased back pressure of urine within the obstructed system, indicating successful access. As the UCA is gently instilled into the renal pelvis, it should flow away from the needle tip. Conversely, amorphous pooling of contrast agent outside the collecting system margins can indicate misplacement [12].
In certain circumstances, percutaneous nephrostomy is required in non-dilated or minimally dilated collecting systems to create an access route (e.g., for subsequent stone removal) [32]. This procedure can be technically challenging because the calyces might be inconspicuous from the adjacent renal parenchyma. Because microbubbles administered intravenously are not excreted into the collecting system, IV CEUS can be used to differentiate the enhancing renal parenchyma from the non-enhancing collecting system and help direct correct placement of the nephrostomy tube [9, 32].
Following successful percutaneous nephrostomy insertion, an antegrade nephrostogram can be performed with direct administration of diluted UCA through the nephrostomy catheter. This technique is useful for evaluating the passage of contrast agent from the renal collecting system and into the bladder and for identifying any points of obstruction [12, 32, 33]. Similarly, this can be performed to assess the patency of a ureteric stent by ensuring free passage of contrast agent into the bladder and unobstructed ureteric drainage. If bilateral nephrostograms are necessary to evaluate the patency of both the right and left collecting systems, then complete drainage of the bladder or bursting of microbubbles by sonication is necessary before examining the contralateral side. Compared to fluoroscopic assessment alone, CEUS is more sensitive at assessing ureteral patency, which can enable an earlier capping trial or drain removal [32]. CEUS also accurately identifies possible complications, such as urine leak or uretero-enteric fistulae, with high spatial and temporal resolution [12].
Children with simple renal cysts might be referred for percutaneous sclerotherapy because of persistent flank pain; however, these cysts can be difficult to differentiate from calyceal diverticula. When other imaging modalities are unable to make this distinction prior to the procedure, CEUS can be useful for further characterization. In this setting, the UCA is injected through the percutaneous puncture directly into the cystic lesion to clarify the anatomy and reveal any communication with the collecting system or bladder (Online Supplementary Material 2). This clarification is crucial for subsequent management because sclerotherapy can proceed when a renal cyst is identified. Management options differ for a calyceal diverticulum because communication with the renal collecting system would be a contraindication to the administration of a sclerosing agent.
Hepatobiliary system
Evaluation of the biliary tree is usually performed by either magnetic resonance cholangiopancreatography or fluoroscopically guided percutaneous transhepatic cholangiography [34]. Similar to accessing a non-dilated renal collecting system, intraductal CEUS can be useful for accessing decompressed biliary systems, which might be encountered in the setting of a split liver transplant, where duct dilation is less apparent, or in the setting of bile leakage, primary biliary cirrhosis, and primary sclerosing cholangitis [35]. After the needle is thought to be positioned in the bile duct, the UCA is injected to confirm intraductal access, whereby contrast agent flows toward the liver hilum. CEUS has the benefit that if microbubble extravasation develops from extra-ductal administration, it can easily be eliminated by sonication, allowing for immediate repeat US-guided puncture to be performed.
In critically ill children with cholecystitis who cannot be transported to the IR suite, bedside percutaneous cholecystostomy tube insertion under US guidance can be performed. In this setting, intracavitary CEUS can confirm the correct catheter placement by demonstrating UCA collecting in the gallbladder lumen without extravasation. Similarly, cystic duct patency can be evaluated by monitoring the contrast flow from the cystic and common bile ducts to the duodenum (Fig. 5) [36].
Contrast-enhanced ultrasound in a 12-year-old girl with a history of acute myeloid leukemia status post percutaneous cholecystostomy drain placement with persistently elevated bilirubin levels that were suspicious for drain occlusion or common bile duct obstruction. a Fluoroscopic image from the cholangiogram performed shortly after placement by direct administration of iodinated contrast medium via the gallbladder drain. The gallbladder is opacified with iodinated contrast agent, and there is obstruction of the cystic duct caused by numerous filling defects, in keeping with obstructing sludge (circle). No contrast agent passed into the duodenum. b Axial contrast-enhanced ultrasound (CEUS) performed with direct administration of the US contrast agent suspension through the cholecystectomy drain a week after placement. Echogenic microbubbles delineate the gallbladder (asterisk), which appears normal in size with homogeneous opacification of its lumen. There is contrast agent progression from the gallbladder to the duodenum (arrowhead), confirming the drain and cystic duct patency, and the common bile duct obstruction. In this case, CEUS provided accurate diagnostic information, sparing the further use of fluoroscopy
Gastrointestinal system
Penetrating Crohn disease, marked by abscess and fistulae, occurs in 20–40% of affected patients during the course of the disease [37]. In this setting, the interventional radiologist can perform CEUS to guide abscess drainage and to evaluate for fistulous communications. Following CEUS-guided abscess drainage, diluted UCA is injected through the catheter into the abscess cavity. When a fistula is present, contrast microbubbles will flow in a duct-like structure between the abscess cavity and into the neighboring bowel. Fistulae located in the deep pelvis or retroperitoneal locations, however, might have limited visibility on CEUS [38, 39]. Enteric fistulae are often difficult to visualize on US, and confirming the presence and the location of a fistulous tract and delineating its anatomical features can greatly inform management decisions.
Intracavitary CEUS can also be used to assess the position of a gastrostomy tube after dislodgement and replacement. Instead of confirming with fluoroscopy prior to using the tube, diluted UCA can be administered into the tube at the bedside to confirm appropriate placement; if the tube has been replaced appropriately, CEUS shows contrast agent within the gastric lumen without extraluminal extravasation [13].
Vascular malformations and lymphatic interventions
Vascular malformations are complex congenital lesions comprising an abnormal network of interconnecting vascular channels. These lesions can affect any tissue or organ and can be infiltrative in nature, usually involving multiple tissue planes [40, 41]. Gray-scale US coupled with color/power Doppler is useful for diagnosis and allows the delineation between slow-flow lesions (e.g., often venous and lymphatic malformations) and high-flow lesions (e.g., arteriovenous malformations). While MRI is the imaging modality of choice for assessing the size and degree of these lesions, CEUS can provide further definition of lesions that are more difficult to visualize with gray-scale and color/power Doppler imaging. IR plays an important role in the management of these malformations, with treatment options such as embolization and sclerotherapy, and CEUS is emerging as an adjunct guidance modality during intervention [42, 43].
During sclerotherapy, US is used to guide percutaneous access into the vascular channels of a venous malformation. Here, UCA can be directly injected through the needle to achieve intralesional opacification. The ability of CEUS to image in multiple planes, and its superior spatial resolution over fluoroscopy, might improve visualization of an outflow vein and subsequent occlusion, thereby potentially decreasing the risk of non-target sclerotherapy. In the absence of robust venous outflow, UCA can be directly injected into the intended treatment area to determine the volume of the lesion being treated. Areas of the lesion identified on gray-scale US and other imaging modalities that do not fill with contrast agent would require separate access points for treatment (Fig. 6). Importantly, CEUS should be performed before foam-based sclerotherapy because the presence of air limits sonographic evaluation following injection.
Treatment in a 14-year-old girl with a microcystic lymphatic malformation in the posterior right deltoid muscle. a Axial fat-saturated T2-weighted MRI shows the oval configuration of the lesion (arrow), which comprises multiple small cysts. b Contrast-enhanced ultrasound (CEUS) performed with direct administration of US contrast agent through the puncture needle into the lesion following percutaneous access. Contrast-only mode in the axial plane shows microbubbles filling a small, eccentrically located part of the lesion (outline and asterisk). The shape and volume of this space do not seem to correspond to the overall shape and volume of the lesion as it was depicted on MRI. c An additional access point was made in a location lateral to the first, and another axial CEUS examination was performed by injecting US contrast agent through the second puncture needle. A larger part of the lesion is now opacified that does not communicate with the previous one (outline and asterisk). The combined volume of these two spaces corresponds to the entire volume of the lesion. In this case, CEUS findings prompted additional points of access and confirmed complete lesion coverage, ensuring successful treatment outcome
Similar to the evaluation in venous malformations, CEUS can be performed prior to sclerotherapy of lymphatic malformations. After direct percutaneous access into a cyst of the lymphatic malformation with a needle or catheter, microbubbles are directly instilled to assess for communication between the cystic spaces. Importantly, if there are large cysts, fluid aspiration is performed prior to UCA administration to avoid overfilling or rupture, as well as to determine the volume of the lesion prior to sclerosis. This pre-treatment information is valuable because non-communicating cysts require additional, separate access to ensure complete treatment of the malformation. Furthermore, UCA can be mixed with a non-foamed sclerosing agent to delineate the distribution of this mixture in real-time and to monitor for possible extravasation into normal soft tissues. Aside from the lack of ionizing radiation, CEUS has the advantage of evaluating the proximity of the lymphatic malformations to adjacent critical structures, such as the airway.
Recently, interventional radiologists have also used quantitative analysis of IV microbubble flow in the capillary microcirculation to estimate therapy outcomes, specifically by monitoring induced capillary changes of vascular malformations after percutaneous treatment [44, 45].
Additional applications in interventional radiology
Interventional radiologists are often consulted to guide management decisions in children potentially requiring image-guided intervention. For example, if there is a concern for active bleeding after trauma or iatrogenic injury, IV CEUS offers superior temporal resolution and can be performed over the area of concern to evaluate for arterial contrast extravasation (which would prompt angiography and embolization), slow venous bleeding or the presence of a pseudoaneurysm [46]. Similarly, if the child continued to demonstrate a downtrend in hemoglobin levels, a repeat CEUS should be considered as an alternative to serial CT scanning because CEUS might be logistically easier and avoid the additional radiation exposure.
Intravenous CEUS can also be used to quickly triage patients when there is a concern for venous thrombosis. For example, slow flow in the portal vein is known to mimic portal vein thrombosis on color/power Doppler imaging. Instead of transporting the child for a contrast-enhanced CT, clinicians could easily and quickly perform CEUS concomitant with the liver US to confirm findings prior to any other intervention (Fig. 7).
Assessment in a 5-year-old girl with a history of embryonal rhabdomyosarcoma who presented with fever and acute liver failure, prompting hepatic ultrasound. a Axial power Doppler US of the liver shows no-flow signal within the main portal vein (arrowheads). b Contrast-enhanced ultrasound (CEUS) was performed with intravenous administration of ultrasound contrast agent at the bedside in the critical care unit. Contrast-only mode in the axial plane shows echogenic microbubbles within the main- and right-branch portal veins without filling defects to indicate thrombus. In this case, CEUS was used as a problem-solving tool and a portal vein thrombectomy was avoided
Emerging interventional radiology applications
Additional CEUS applications include those used in adult IR that have not been fully translated to children, such as guidance during oncological interventions, arthrography, and diagnostic and therapeutic lymphangiography [8].
Percutaneous ablation of solid abdominal tumors is an oncological intervention that involves the placement of ablation probes into the tumor under image guidance [7]. Ablation modalities, such as radiofrequency ablation, microwave ablation and cryoablation, all require correct positioning of an ablation needle within a lesion for treatment. In this setting, IV CEUS can be performed prior to the ablation to evaluate the size and morphological features of the tumor as well as to guide appropriate placement of the ablation probe during the procedure. After treatment, CEUS can be used to evaluate the ablation zone and assess for the absence of nodular enhancement along the ablation margin. If persistent enhancement is seen, immediate repeat ablation can be performed in the same session [47].
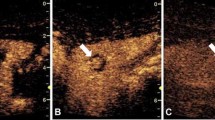
Contrast-enhanced US provides an option for arthrography in children. Joint injections can be guided using a variety of methods, including US, MRI and fluoroscopy, depending on the preference of the interventional radiologist and the available modality. When a US-guided approach is used, the addition of CEUS can confirm the correct position of the needle in the joint space. When the needle is extra-articular, injection of diluted UCA results in contrast pooling or infiltration of the surrounding soft tissues, as opposed to when the needle is correctly positioned where echogenic microbubbles exit the needle and quickly fill the joint space without resistance [48]. UCA instilled via the needle should flow freely in the joint space to confirm tip position prior to instillation of steroid or MRI contrast agent (Fig. 8).
Intra-articular injection in a 13-year-old boy with history of juvenile idiopathic arthritis who presented with hip pain refractory to therapy. Contrast-enhanced ultrasound (CEUS) was performed following the intra-articular injection of ultrasound contrast agent (UCA) solution into the left hip joint. Simultaneous display of gray-scale (left) and contrast-enhanced (right) modes in the coronal plane. Under US guidance, a 21-gauge needle was placed into the hip joint. The correct position of the needle was confirmed with direct injection of UCA. Echogenic microbubbles (arrowheads) are diffusely seen within the joint space, without clustering or clumping at the needle tip (arrow). In this case, CEUS was used as an alternative to fluoroscopy to confirm the position of the needle into the joint and proceed with intra-articular corticosteroid injection
Pediatric lymphatic interventions involve direct access of the lymphatic system, as well as nodal access for diagnostic lymphangiography with fluoroscopy or dynamic MRI. A recent clinical study showed that, prior to performing dynamic contrast-enhanced MR lymphangiography, CEUS can confirm the intranodal position of the injection needles [16]. Under US guidance, the needle is inserted into an accessible inguinal node and then the UCA is injected through the needle. If the needle is appropriately placed into the lymph node, contrast microbubbles flow into the efferent lymphatic duct and the central lymphatic structures in the pelvis. Nodal CEUS is performed outside the MRI scanner and provides excellent spatial and temporal resolution while avoiding the fluoroscopy room time and cost and the ionizing radiation exposure.
Contrast-enhanced US also holds promise in assessing the patency of the thoracic duct, potentially decreasing fluoroscopic time. The thoracic duct drains into the systemic circulation, usually at the junction of the left subclavian and internal jugular veins [49]. To evaluate patency of the thoracic duct, iodinated contrast material is injected into the lymphatic system under fluoroscopic guidance after obtaining percutaneous access into the cisterna chyli. As an alternative, UCA can be administered via the transabdominal microcatheter while the venous angle is interrogated with US (Fig. 9). Efflux of microbubbles into the subclavian vein confirms patency of the thoracic duct. Early experience suggests that CEUS might provide high sensitivity in assessing the patency of the thoracic duct because of its superior spatial and temporal resolution compared to fluoroscopic lymphangiography.
Treatment in an 11-year-old boy with a history of a Fontan creation for hypoplastic left heart who presented with chylothorax. a Contrast-enhanced ultrasound (CEUS) was performed following embolization of abnormal perihilar lung lymphatics and the injection of US contrast agent into the thoracic duct. Simultaneous display of contrast mode (left) and gray-scale mode (right) in the axial plane shows microbubbles through the venous angle with communication into the left subclavian vein (arrowheads). In this case, CEUS was used to determine the patency of the thoracic duct at the venous angle. b Corresponding anteroposterior spot fluoroscopic image obtained after intervention demonstrates the thoracic duct (arrows) filled with iodinated contrast agent. Contrast agent was seen entering into the subclavian vein during real-time fluoroscopy
Conclusion
Sonography is already well established as a modality for guiding IR procedures. The introduction of UCA expands the potential of US to improve procedural guidance and direct periprocedure management. Current IR-related CEUS applications in children are predominantly extrapolated from adults. Advantages of CEUS include its superior temporal and spatial resolution combined with real-time information on enhancement and flow dynamics. Other advantages include the ability to perform CEUS in multiple locations (e.g., bedside, IR suite, operating room, emergency department), its lack of ionizing radiation, its robust diagnostic utility and its high safety profile. Thus, CEUS can serve as a valuable alternative or adjunctive technique to fluoroscopy or CT for a variety of procedures.
References
Raymond-Martimbeau P (2009) Transient adverse events positively associated with patent foramen ovale after ultrasound-guided foam sclerotherapy. Phlebology 24:114–119
Wen M, Stock K, Heemann U et al (2014) Agitated saline bubble-enhanced transthoracic echocardiography: a novel method to visualize the position of central venous catheter. Crit Care Med 42:e231–e233
Nolsoe CP, Nolsoe AB, Klubien J et al (2018) Use of ultrasound contrast agents in relation to percutaneous interventional procedures: a systematic review and pictorial essay. J Ultrasound Med 37:1305–1324
Sparchez Z, Radu P, Zaharia T et al (2011) Usefulness of contrast enhanced ultrasound guidance in percutaneous biopsies of liver tumors. J Gastrointestin Liver Dis 20:191–196
Yoon SH, Lee KH, Kim SY et al (2010) Real-time contrast-enhanced ultrasound-guided biopsy of focal hepatic lesions not localised on B-mode ultrasound. Eur Radiol 20:2047–2056
Sparchez Z, Radu P, Zaharia T et al (2010) Contrast enhanced ultrasound guidance: a new tool to improve accuracy in percutaneous biopsies. Med Ultrason 12:133–138
Huang DY, Yusuf GT, Daneshi M et al (2018) Contrast-enhanced ultrasound (CEUS) in abdominal intervention. Abdom Radiol 43:960–976
Gummadi S, Eisenbrey JR, Lyshchik A (2018) Contrast-enhanced ultrasonography in interventional oncology. Abdom Radiol 43:3166–3175
Huang DY, Yusuf GT, Daneshi M et al (2017) Contrast-enhanced US-guided interventions: improving success rate and avoiding complications using US contrast agents. Radiographics 37:652–664
Kessner R, Nakamoto DA, Kondray V et al (2019) Contrast-enhanced ultrasound guidance for interventional procedures. J Ultrasound Med 38:2541–2557
Lorentzen T, Nolsoe CP, Ewertsen C et al (2015) EFSUMB guidelines on interventional ultrasound (INVUS), Part I. General aspects (short version). Ultraschall Med 36:464–472
Daneshi M, Yusuf GT, Fang C et al (2019) Contrast-enhanced ultrasound (CEUS) nephrostogram: utility and accuracy as an alternative to fluoroscopic imaging of the urinary tract. Clin Radiol 74:167.e9–167.e16
Yusuf GT, Fang C, Huang DY et al (2018) Endocavitary contrast enhanced ultrasound (CEUS): a novel problem solving technique. Insights Imaging 9:303–311
Muller T, Blank W, Leitlein J et al (2015) Endocavitary contrast-enhanced ultrasound: a technique whose time has come? J Clin Ultrasound 43:71–80
Kljucevsek D, Riccabona M, Ording Muller LS et al (2020) Intracavitary contrast-enhanced ultrasonography in children: review with procedural recommendations and clinical applications from the European Society of Paediatric Radiology abdominal imaging task force. Pediatr Radiol 50:596–606
Nadolski GJ, Ponce-Dorrego MD, Darge K et al (2018) Validation of the position of injection needles with contrast-enhanced ultrasound for dynamic contract-enhanced MR lymphangiography. J Vasc Interv Radiol 29:1028–1030
Yusuf GT, Sellars ME, Deganello A et al (2017) Retrospective analysis of the safety and cost implications of pediatric contrast-enhanced ultrasound at a single center. AJR Am J Roentgenol 208:446–452
Quaia E, Gennari AG, Angileri R et al (2016) Bolus versus continuous infusion of microbubble contrast agent for liver ultrasound by using an automatic power injector in humans: a pilot study. J Clin Ultrasound 44:136–142
Goddi A, Novario R, Tanzi F et al (2004) In vitro analysis of ultrasound second generation contrast agent diluted in saline solution. Radiol Med 107:569–579
Popescu A, Sporea I, Sirli R et al (2015) Does contrast enhanced ultrasound improve the management of liver abscesses? A single centre experience. Med Ultrason 17:451–455
Kosloske AM, Love CL, Rohrer JE et al (2004) The diagnosis of appendicitis in children: outcomes of a strategy based on pediatric surgical evaluation. Pediatrics 113:29–34
Smink DS, Finkelstein JA, Garcia Pena BM et al (2004) Diagnosis of acute appendicitis in children using a clinical practice guideline. J Pediatr Surg 39:458–463
Tracy MC, Mathew R (2018) Complicated pneumonia: current concepts and state of the art. Curr Opin Pediatr 30:384–392
Deganello A, Rafailidis V, Sellars ME et al (2017) Intravenous and intracavitary use of contrast-enhanced ultrasound in the evaluation and management of complicated pediatric pneumonia. J Ultrasound Med 36:1943–1954
Mao F, Dong Y, Ji Z et al (2017) Contrast-enhanced ultrasound guided biopsy of undetermined abdominal lesions: a multidisciplinary decision-making approach. Biomed Res Int 2017:8791259
Dong Y, Mao F, Wang WP et al (2015) Value of contrast-enhanced ultrasound in guidance of percutaneous biopsy in peripheral pulmonary lesions. Biomed Res Int 2015:531507
Wei Y, Yu XL, Liang P et al (2015) Guiding and controlling percutaneous pancreas biopsies with contrast-enhanced ultrasound: target lesions are not localized on B-mode ultrasound. Ultrasound Med Biol 41:1561–1569
Wu W, Chen MH, Yin SS et al (2006) The role of contrast-enhanced sonography of focal liver lesions before percutaneous biopsy. AJR Am J Roentgenol 187:752–761
Kljucevsek T, Pirnovar V, Kljucevsek D (2020) Percutaneous nephrostomy in the neonatal period: indications, complications, and outcome — a single centre experience. Cardiovasc Intervent Radiol 43:1323–1328
Dagli M, Ramchandani P (2011) Percutaneous nephrostomy: technical aspects and indications. Semin Intervent Radiol 28:424–437
Hwang JY, Shin JH, Lee YJ et al (2018) Percutaneous nephrostomy placement in infants and young children. Diagn Interv Imaging 99:157–162
Liu BX, Huang GL, Xie XH et al (2017) Contrast-enhanced US-assisted percutaneous nephrostomy: a technique to increase success rate for patients with nondilated renal collecting system. Radiology 285:293–301
Chi T, Usawachintachit M, Weinstein S et al (2017) Contrast enhanced ultrasound as a radiation-free alternative to fluoroscopic nephrostogram for evaluating ureteral patency. J Urol 198:1367–1373
Anupindi SA, Chauvin NA, Khwaja A et al (2016) Magnetic resonance imaging of pancreaticobiliary diseases in children: from technique to practice. Pediatr Radiol 46:778–790
Lee W, Kim GC, Kim JY et al (2008) Ultrasound and fluoroscopy guided percutaneous transhepatic biliary drainage in patients with nondilated bile ducts. Abdom Imaging 33:555–559
Ignee A, Baum U, Schuessler G et al (2009) Contrast-enhanced ultrasound-guided percutaneous cholangiography and cholangiodrainage (CEUS-PTCD). Endoscopy 41:725–726
Feuerstein JD, Cheifetz AS (2017) Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc 92:1088–1103
Quaia E (2013) Contrast-enhanced ultrasound of the small bowel in Crohn's disease. Abdom Imaging 38:1005–1013
Mao R, Chen YJ, Chen BL et al (2018) Intra-cavitary contrast-enhanced ultrasound: a novel radiation-free method to detect abscess associated penetrating disease in Crohn's disease. J Crohns Colitis 13:593–599
McCuaig CC (2017) Update on classification and diagnosis of vascular malformations. Curr Opin Pediatr 29:448–454
Cahill AM, Nijs EL (2011) Pediatric vascular malformations: pathophysiology, diagnosis, and the role of interventional radiology. Cardiovasc Intervent Radiol 34:691–704
Acord M, Srinivasan AS, Cahill AM (2016) Percutaneous treatment of lymphatic malformations. Tech Vasc Interv Radiol 19:305–311
Dubois J, Thomas-Chausse F, Soulez G (2019) Common (cystic) lymphatic malformations: current knowledge and management. Tech Vasc Interv Radiol 22:100631
Wiesinger I, Schreml S, Wohlgemuth WA et al (2015) Perfusion quantification of vascular malformations using contrast-enhanced ultrasound (CEUS) with time intensity curve analysis before and after treatment: first results. Clin Hemorheol Microcirc 62:283–290
Wiesinger I, Jung W, Zausig N et al (2018) Evaluation of dynamic effects of therapy-induced changes in microcirculation after percutaneous treatment of vascular malformations using contrast-enhanced ultrasound (CEUS) and time intensity curve (TIC) analyses. Clin Hemorheol Microcirc 69:45–57
Lv F, Tang J, Luo Y et al (2012) Percutaneous treatment of blunt hepatic and splenic trauma under contrast-enhanced ultrasound guidance. Clin Imaging 36:191–198
Minami Y, Kudo M (2011) Review of dynamic contrast-enhanced ultrasound guidance in ablation therapy for hepatocellular carcinoma. World J Gastroenterol 17:4952–4959
Fermand M, Hassen CS, Ariche L et al (2000) Ultrasound investigation of the rotator cuff after computed arthrotomography coupled to bursography. Joint Bone Spine 67:310–314
Capobianco SM, Fahmy MW, Sicari V (2020) Anatomy, thorax, subclavian veins. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK532885/. Accessed 29 Aug 2020
Acknowledgments
Figure 8 courtesy of Yoav Dori, MD, Children’s Hospital of Philadelphia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
A 15-year-old boy presenting for biopsy of a large hepatic mass. Same boy as depicted in Fig. 3. Axial intravenous-contrast-enhanced ultrasound (CEUS) was performed to plan the biopsy trajectory. CEUS reveals an extensive area of necrosis centrally within the mass; however, peripherally, there are areas of viable tumor, which were subsequently targeted for biopsy. Sufficient viable tissue was obtained to provide a diagnosis of undifferentiated embryonal sarcoma (MP4 31484 kb)
A 12-year-old girl presenting with large symptomatic left renal cystic lesion for sclerotherapy injection. Coronal contrast-enhanced ultrasound was performed with direct administration of ultrasound contrast agent through the puncture needle into the cyst following percutaneous access. No communication of the cyst with the renal pelvis was identified, and therefore sclerotherapy proceeded without concern that the sclerosant material would harm the renal collecting system (MP4 17888 kb)
Rights and permissions
About this article
Cite this article
Acord, M.R., Cahill, A.M., Durand, R. et al. Contrast-enhanced ultrasound in pediatric interventional radiology. Pediatr Radiol 51, 2396–2407 (2021). https://doi.org/10.1007/s00247-020-04853-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-020-04853-4