Abstract
Background
Contrast-enhanced MRI is often used for diagnosis and follow-up of children with inflammatory bowel disease.
Objective
To compare the accuracy of diffusion-weighted MRI (DWI) to contrast-enhanced MRI in children with known or suspected inflammatory bowel disease.
Materials and methods
This retrospective, consecutive study included 55 children. We used ileo-colonoscopy and histology as the reference standard from the terminal ileum to the rectum, and contrast-enhanced MRI as the reference standard proximal to the terminal ileum. DWI and contrast-enhanced MRI sequences were independently reviewed and compared per patient and per segment to these reference standards and to the follow-up for each child.
Results
We obtained endoscopic data for 340/385 colonic and ileal segments (88%). The rate of agreement per segment between DWI and endoscopy was 64%, and the rate of agreement between contrast-enhanced MRI and endoscopy was 59%. Per patient, sensitivity and specificity of bowel wall abnormalities as compared to the endoscopy were 87% and 100% for DWI, and 70% and 100% for contrast-enhanced MRI, respectively. Positive and negative predictive values were, respectively, 100% and 57% for DWI, and 96% and 41% for contrast-enhanced MRI. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of DWI compare to contrast-enhanced MRI in the segments proximal to the terminal ileum were 90%, 98%, 90%, 98% and 96%, respectively.
Conclusion
The diagnostic performance of DWI is competitive to that of contrast-enhanced MRI in children with known or suspected inflammatory bowel disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several studies have demonstrated the sensitivity of MRI in the detection of inflammatory bowel disease, as well as its capacity in evaluating the extent of the disease and detecting inflammatory activity as well as complications in both the adult and pediatric populations [1,2,3,4,5,6,7]. The MRI protocol for inflammatory bowel disease is traditionally based on contrast-enhanced sequences performed after antiperistaltic agent injection [8, 9]. However this requires intravenous cannula placement, and it increases the length of study, which can cause poorer compliance. Potential side effects of gadolinium (allergy, nephrotoxicity, brain accumulation, nephrogenic systemic fibrosis [10, 11]) and cost must also be considered.
Diffusion-weighted MRI (DWI) has gained wide acceptance in imaging of inflammatory bowel disease [12,13,14,15,16,17,18,19,20,21,22,23]. Recent works have questioned the utility of DWI to replace contrast-enhanced MRI for detecting and assessing activity of inflammatory bowel disease; the results are discordant [14,15,16,17,18,19,20]. Only a few [17,18,19,20] presented results from a pediatric population. The number of pediatric studies remains limited.
Our principal aim was to compare the diagnostic performance (sensitivity, specificity) of DWI versus contrast-enhanced MRI in children by comparing the results to ileo-colonoscopy and histology, considered to be the reference standards from the rectum to the terminal ileum, and by comparing DWI to a reference represented by contrast-enhanced MRI with morphologic sequences for segments proximal to the terminal ileum. Our secondary objective was to determine the concordance of such analysis between junior and senior radiologists.
Our hypothesis was that for children, diffusion-weighted imaging can be used as a replacement for contrast injection in the diagnosis of inflammatory bowel disease.
Materials and methods
Population
We conducted this study in accordance with the Declaration of Helsinki after receiving approval from our hospital ethics committee. All legal representatives of the patients gave written informed consent to undergo the MRI and to be enrolled in the study.
The present study included children younger than 18 years who were diagnosed with or suspected of having inflammatory bowel disease. We enrolled patients at the pediatric radiology and gastroenterology departments at our center. The follow-up was between 23 months and 6 years (median 4 years).
All enrolled children underwent MR enterography and ileo-colonoscopy with biopsies and histological results (reference standard [24, 25]) with a maximum interval of 6 weeks. No treatment specific to inflammatory bowel disease was started between the examinations.
Magnetic resonance enterography
MR enterography was conducted after a 4-h fast. To obtain bowel distention, we asked children to ingest 750–1,000 mL of mannitol 20% (depending on each child’s age) 1 h before the exam. Our institution protocol does not include any spasmolytic drugs or colonic enema. Our standard protocol for all patients includes the following sequences: axial and coronal T2-weighted single-shot turbo spin echo; axial and coronal T2-weighted turbo spin echo; axial and coronal DWI (b=0 and b=1,300); and post-contrast three-dimensional (3-D) T1-weighted spoiled gradient recalled echo with fat saturation acquired 2 min after contrast injection with coronal/axial/sagittal reconstructions (Table 1).
Image analysis
Two pediatric radiologists, one senior (PP) (13 years of experience in pediatric MR enterography) and one junior (ND) (5 years, training resident), retrospectively and independently interpreted the exams on a workstation. Both radiologists were masked to clinical and endoscopic data. To compare the diagnostic performance of DWI to contrast-enhanced MRI, they interpreted the images twice with an interval of 15 days. The following order was applied: the first interpretation concerned morphologic sequences in combination with DWI sequences without the contrast enhancement; the second interpretation included morphologic sequences with contrast-enhanced images without DWI sequences.
MRI scans (Figs. 1, 2 and 3) were interpreted based on bowel segmentation as reported for the endoscopy. The small bowel was divided into five segments according to the four abdominal quadrants in addition to the terminal ileum, corresponding to the last 20 cm of the ileum. The colon was divided into five segments (caecum, ascending colon, transverse colon, descending colon, sigmoid colon) in addition to the rectum [26].
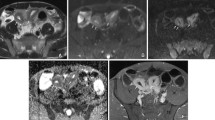
Bowel wall thickening in a 14-year-old boy with Crohn disease. a Axial T2-weighted MR image (repetition time/echo time [TR/TE] = 4.2/2.1 ms) demonstrates the thickening of the terminal ileum (arrowhead). b Axial T1-weighted fat-saturated gadolinium-enhanced sequence ([TR/TE]=4.5/2.1 ms) demonstrates thickening and enhancement of the bowel wall (arrowhead). c Axial diffusion-weighted image (b=1,300) demonstrates the signal hyperintensity of the same segment (arrowhead)
Crohn disease limited to the sigmoid and confirmed by endoscopy in a 12-year-old girl. a Axial T2-weighted sequence (repetition time/echo time [TR/TE] 4.2/2.1 ms) demonstrates discrete wall thickening of the moderately distended sigmoid colon (arrowhead). b Axial T1-weighted fat-saturated contrast-enhanced MR image ([TR/TE]=4.5/2.1 ms) demonstrates the lack of abnormal enhancement of the sigmoid (arrowhead). c Axial diffusion-weighted image (b=1,300) shows clear hyperintensity of the sigmoid (arrowhead). TE echo time, TR repetition time
Severe ulcerative colitis confirmed on endoscopy in a 10-year-old girl. a Coronal T2-weighted sequence (repetition time/echo time [TR/TE] 4.2/2.1 ms) shows thickening of the wall of the transverse colon (arrowheads). b Coronal T1-weighted fat-saturated gadolinium-enhanced sequence (TR/TE) shows no enhancement of the same segment (arrowheads). TE echo time, TR repetition time. c DWI b1,300 sequence demonstrates hyperintensity of this bowel segment. (arrow heads)
For these 11 segments, we evaluated four parameters: the quality of bowel distention and bowel wall thickness on the morphologic sequences (single-shot T2-weighted turbo spin echo), the presence of a bowel wall hyperintensity on either a contrast-enhanced T1-weighted fat-saturated sequence or a DWI sequence, and the suspicion of disease.
We assessed the quality of bowel distention using a 3-point scale (0: poor when the anterior and posterior bowel wall could barely be distinguished; 1: average when the anterior and posterior wall could clearly be distinguished; 2: good when the anterior and posterior wall could clearly be distinguished and the lumen diameter was superior to several millimeters).
Bowel wall thickness was considered pathological if >3 mm [27,28,29,30] and was assessed on a 2-point scale (0: <3 mm; 1: 3–6 mm). If bowel wall thickness exceeded 6 mm, the measurement was expressed in millimeters. We evaluated signal intensity of the bowel wall according to a 3-point scale (0: no hyperintensity; 1: moderate hyperintensity; 2: marked hyperintensity) compared to adjacent muscle. We assigned a score to each segment (0: normal; 1: equivocal 2: pathological). We used “equivocal” when images were not degraded by artefacts and when there was good bowel distention, absence of wall thickening and moderate hyperintensity. We also assigned a score to each examination (0: normal; 1: equivocal 2: pathological). Finally, we collected data regarding inflammatory bowel disease complications including abscesses, fistulae and strictures.
We did not take apparent diffusion coefficient measurements because of the risk of overlap between discrete thickening of the bowel walls and the adjacent tissues.
Reference standards
From the rectum to the terminal ileum
From the rectum to the terminal ileum, we used the results of the endoscopic exploration and those of the histology obtained from the biopsies performed during endoscopy as the reference standards [24, 25]. We obtained endoscopic data by ileo-colonoscopy and reported the results based on the same segmentations applied for MRI. Endoscopic exams were interpreted by a pediatric gastroenterologist blinded to clinical, histological and radiologic data, based on endoscopic reports, available images and pathology reports. Each biopsy was evaluated microscopically and scored on a 3-point scale (0: normal; 1: equivocal; 2: pathological). We evaluated each segment macro- and microscopically based on a 3-point scale (0: normal; 1: equivocal; 2: pathological) and as a result we evaluated the pathological aspect of each segment and of each exam. An equivocal segment would be called equivocal-endoscopy to differentiate it from the ones equivocal at imaging. One single criterion (macro- or microscopic) was sufficient to conclude that the exam or the segment was pathological. A doubtful macroscopic aspect and a doubtful biopsy result did not qualify as adequate for the segment to be determined as pathological.
Above the terminal ileum
Above the terminal ileum, we used MRI including morphologic sequences and contrast-enhanced sequences as the reference standard.
Statistical analysis
The main objective of the study was to evaluate whether a diagnostic difference exists between DWI combined with morphologic sequences and contrast-enhanced MRI combined with morphologic sequences when segmental or global evaluation is performed. The statistical analysis was performed using the Bayesian methodology with Markov chain Monte Carlo simulation. The Bayesian paradigm, which is a system for describing uncertainty using the mathematical language of probability, offers a very different view of hypothesis testing. Specifically, Bayesian approaches allow researchers to incorporate background knowledge into their analysis instead of testing essentially the same null hypothesis over and over again, ignoring the lessons of previous studies. In contrast, statistical methods based on a frequentist (classic) paradigm often involve testing the null hypothesis [31]. By modeling the unknown parameters of the sampling distribution through a probability structure, i.e. by probabilizing uncertainty, the Bayesian approach authorizes a quantitative discourse on these parameters. It also allows prior information to be incorporated in the inferential procedure and allows for the imprecision of this information. Besides, apart from subjective and axiomatic arguments in favor of the Bayesian approach, which is the only system allowing for conditioning on the observations (and thus for an effective implementation of the likelihood principle), frequentist inference, e.g., using P-values and confidence intervals, does not quantify what is known about parameters. Bayesian statistical methods start with existing prior beliefs, and update these using data to give posterior beliefs, which might be used as the basis for inferential decisions. Bayes estimators are also quintessential for the frequentist optimality notions of decision theory.
To test the difference between DWI and contrast-enhanced MRI we studied the difference of distribution between the sequences being tested (DWI) and the current reference standard (contrast-enhanced MRI) based on the interpretation of the senior radiologist. It was considered equivalent in a particular segment if the scores matched. In this way, we evaluated the number of differences between the results of the two sequences. These differences were then run through a set of 100,000 simulations using three Markov chains with a burn-in period of 7,000 iterations. We applied the distribution of Dirichlet on the contingency tables coupled with binomial distribution for the two variables being tested. We also estimated the probabilities of superiority, inferiority and equivalence.
In terms of operator experience, we evaluated the influence of the operators’ experience for accordance and reproducibility of the results. We also evaluated diagnostic performance in terms of sensitivity, specificity, positive predictive value and negative predictive value for each variable [26, 30]. Finally, we compared MRI results with the final diagnostic workup obtained by combining all clinical (including the patient follow-up), biological, endoscopic and histopathological data. MR enterography results were not taken into account.
The children with equivocal endoscopy results were not included in the statistical analysis but were studied in a descriptive table, and we compared the MRI results to the final diagnostic workup in those children.
Analyses were conducted within WinBUGS 1.4 software [32], called from R version 3.0.3 software [33], with R2WinBUGS package [34].
Results
Population
We included 55 patients consecutive children ages 5 to 18 years; all of them had complete MR enterography and endoscopy examinations. Our population included 16 children not affected by inflammatory bowel disease, 25 affected by Crohn disease, 11 affected by ulcerative recto-colitis, 2 with undetermined recto-colitis and 1 without a conclusive diagnosis (Table 2). The time interval between MRI and endoscopy-histopathological exams varied between 0 and 36 days (median 9 days). For the 385 colic and ileal segments (55 × 7 segments) visualized at endoscopy, we obtained endoscopic and histological data for 340 (88%). Among these segments, 53 were equivocal on endoscopy (16%).
Equivalence measurements
We calculated equivalence measurements by evaluating only unequivocal endoscopy segments, accounting for 84% (287) of the 340 available segments. Equivalence rates between DWI and the reference standard, and contrast-enhanced MRI and the reference standard were 64% and 59%, respectively. The difference between these two rates was 0.0455 (95% confidence interval 0.005–0.087). Looking at the confidence limits of the difference, it can be said that there is a tendency for DWI to be at least equivalent to contrast-enhanced MRI and at best better than contrast-enhanced MRI.
Comparison of diagnostic criteria
Considering the analysis per child, sensitivity and specificity of bowel wall abnormalities as compared to the endoscopy and as evaluated on inequivalent endoscopy segments were 87% and 100% for DWI and 70% and 100% for contrast-enhanced MRI, respectively. Positive and negative predictive values were, respectively, 100% and 58% for DWI and 96% and 41% for contrast-enhanced MRI.
In children with Crohn disease, the sensitivity was 100% for DWI and 85% for contrast-enhanced MRI. In ulcerative recto-colitis, the sensitivity was 75% for DWI and 33% for contrast-enhanced MRI. In the group of normal patients, the specificity for both DWI and contrast-enhanced MRI was 100% (Table 3).
Comparing MRI results to those obtained by combining all the clinical (including follow-up), biological, endoscopic and histopathological data, sensitivity and specificity were, respectively, 90% and 82% for DWI and 67% and 71% for contrast-enhanced MRI. Positive and negative predictive values were, respectively, 93% and 78% for DWI and 85% and 46% for contrast-enhanced MRI.
The values of DWI compared to a reference standard of contrast-enhanced MRI in the segments not explored by endoscopy were: sensitivity 90% (95% confidence interval 60–98%), specificity 98% (95% confidence interval 89–100%), positive predictive value 90% (95% confidence interval 60–98%), negative predictive value 98% (95% confidence interval 88–99%) and accuracy 96% (95% confidence interval 88–99%).
On endoscopy 53 segments were equivocal (16%). These segments were distributed as follows on DWI: 59% normal, 9% imaging equivocal and 32% pathological. On contrast-enhanced MRI the segments were: 86% normal, 7% imaging equivocal and 7% pathological. In the global analysis per patient, equivocal endoscopy segments were found in eight studies, corresponding to three equivocal and four normal segments on contrast-enhanced MRI and two equivocal, four normal and two pathological on DWI. DWI was concordant with the final diagnosis in 7/8 cases and contrast-enhanced MRI in 3/8 (Table 4).
MRI showed complications in eight children with Crohn disease, including two abscesses and five ileal stenoses, accounting for a 44% complication rate in children with Crohn disease. An identical response between the junior and the senior radiologists was found in 89% (95% confidence interval 79.5–95.2%) for contrast-enhanced sequences and 84% (95% confidence interval 73.4–91.7%) for DWI. This concordance was significant for all available comparisons.
Discussion
We studied DWI diagnostic criteria and correlated them per segment and per patient to reference standards (endoscopy-relative biopsy samples and MRI with gadolinium injection above the terminal ileum). Our primary goal was to compare the diagnostic performance of DWI and contrast-enhanced MRI in a pediatric population; DWI was found to be superior to contrast-enhanced MRI when compared to endoscopy and to have a 96% accuracy when compared to contrast-enhanced MR for the small bowel not explored by endoscopy.
There is an increasing evidence base supporting the use of DWI as an alternative to gadolinium-enhanced imaging. Shenoy-Bhangle et al. [20] recently reported a cohort of 27 pediatric patients in whom DWI alone showed a lower performance than standard MR enterography for detecting active Crohn disease, but in this series a combination of DWI and MR enterography increased imaging accuracy for disease activity compared with either technique alone. In 44 young adults, Seo et al. [14] found that DWI was non-inferior to contrast-enhanced MR enterography for the evaluation of inflammation in Crohn disease. In a large study of 130 adults and children with Crohn disease, DWI was also found to be of sufficient accuracy to replace gadolinium administration [15]. Some authors have reported that DWI may indeed be superior to contrast-enhanced imaging. Sirin et al. [19] reported that in a cohort of 37 children, DWI revealed lesions not detected with MR enterography performed with gadolinium injection. Similarly, both Dubron et al. [17] and Neubauer et al. [18] reported superior performance of DWI in comparison to gadolinium-enhanced imaging in cohorts of 48 children, and in 33 children and young adults, respectively. For Neubauer, DWI was superior to contrast-enhanced MRI in only 27% of the assessed bowel segments. Their population consisted of 33 children and colonoscopy was available for only two-thirds of them [18]. Our results in a larger population are in accordance with these later publications [18, 19, 21].
In our study, no spasmolytic agents were used. They have been recently considered optional by a consensus statement for pediatric patients [35]. Use of MR enterography without antiperistaltic agents has reached a high diagnostic confidence and excellent agreement with CT enterography for the presence of Crohn disease [36]. If used, antiperistaltic agents need to be administered immediately prior to motion-sensitive sequences (T1-weighted dynamic enhanced sequences, DWI). Dillman et al. [9] found that even if the images obtained with these medications are of better quality than those without, there is no evidence that these agents change the final diagnosis and the children’s therapeutic management. On the other hand, Park et al. [37] reported a decrease in sensitivity and an underestimation of the extent of bowel wall inflammation using DWI without antiperistaltic agents compared to DWI with these agents. If these results are confirmed, our own results would be even better with antiperistaltic agents, but their use requires a venous puncture and increases the length of the exploration. Furthermore, they have frequent side effects [37].
The optimal b values to be used for DWI of the bowel are not clearly defined [38]. In our study, a high b value (1,300) was applied in order to reduce the influence of the T2 shine-through effect. The risk of using such a high b value is to miss an abnormal bowel wall hyperintensity, but it has the advantage of decreasing the risk of false-positive results. In our daily experience and for our MR scanner this high b value is more efficient compared to b=800, but we did not specifically test this and it is beyond the scope of our study. Our protocol based on a portal-phase image acquisition is in accordance with previous published experiences. Sohn et al. [39] showed a better diagnostic performance for portal-phase acquired images as compared to arterial-phase images.
The maximal time interval between endoscopy and MRI in our study was less than 6 weeks (median, 9 days), which is within the range of previous publications (1 week in Sirin et al. [19], 4 in Shenoy-Bhangle et al. [20], 8 in Dubron et al. [17] and 9 in Dillman et al. [40]). Among the 385 studied segments, we had complete endoscopic and histological data in 89% cases because of technical difficulties (insufficient colic preparation), or typical lesions detected in an adjacent segment for which gastro-enterologists did not obtain an additional biopsy.
We introduced in the report the expression “equivocal diagnosis,” which represents the real life of the ordinary clinical practice. Actually, it corresponds to all cases in which a sole technique was not enough to give a definitive diagnosis. Sirin et al. [19] also discussed the doubtful diagnosis. In their study, four patients with doubtful MRI diagnosis were proved to have inflammation on colonoscopy or biopsy. But the term “doubtful diagnosis” was only considered for MRI and not for endoscopy in their study. In our study, when our gold standard endoscopy was rated as doubtful, DWI was more commonly in accordance with the final diagnosis as compared to contrast-enhanced MRI. Diagnostic workup for inflammatory bowel disease is difficult and our paper highlights the importance of follow-up, which significantly modified the concordance rate between MR enterography and final diagnostic response in this study.
Sensitivity rates increased from 87% to 90% with DWI when compared to 70% to 67% for contrast-enhanced MRI. It would be interesting to study the longer-term evolution of these imaging-equivocal segments in order to adapt MRI evaluation. If these segments became pathological during follow-up, it might be necessary to increase the sensitivity. Therefore, we propose to consider as pathological all segments showing DWI high bowel wall signal with good bowel distension, even without bowel wall thickening.
Our study has several limitations. First, it is retrospective. Second, an alternative to our analysis might have been to test the added diagnostic accuracy and diagnostic certainty of the contrast-enhanced sequences over and above the other sequences, including DWI. Third, the percentage of 70% likelihood of disease in our population is high; our results might be influenced by the prevalence of disease in our university teaching hospital’s population. Fourth, we included not only children with Crohn disease but also those affected by other types of inflammatory bowel disease and those clinically suspected to have an inflammatory bowel disease but without final confirmation for the diagnosis. Interestingly enough in our series, the sensitivity of contrast-enhanced MRI was especially low in recto-colitis cases compared to DWI cases. These poor results of contrast-enhanced MRI would have not affected the patient workup because colonoscopy was initially decided by the gastro-enterologist.
The interpretation of MR enterography requires highly skilled radiologists. Previously published studies enrolled highly experienced radiologists (two pediatric radiologists with 9 years and 4 years of experience in Sirin et al. [19], and one radiologist with 9 years of experience in Shenoy-Bhangle et al. [20]). We found good concordance between junior and senior radiologists in all exams. Therefore, after a dedicated formation in inflammatory bowel disease and interpretation of dedicated MR, we believe that a well-trained junior radiologist could reliably read MR enterography studies.
Conclusion
The diagnostic performance of DWI is competitive to that of contrast-enhanced MRI in children with known or suspected inflammatory bowel disease. These results need to be confirmed in a prospective, randomized multi-center study. A simplified protocol including only morphologic (without gadolinium injection) and DWI sequences might be faster, less expensive and less invasive, and with less motion artifacts.
References
Laghi A, Borrelli O, Paolantonio P et al (2003) Contrast-enhanced magnetic resonance imaging of the terminal ileum in children with Crohn’s disease. Gut 52:393–397
Paolantonio P, Ferrari R, Vecchietti F et al (2009) Current status of MR imaging in the evaluation of IBD in a pediatric population of patients. Eur J Radiol 69:418–424
Absah I, Bruining DH, Matsumoto JM et al (2012) MR enterography in pediatric inflammatory bowel disease: retrospective assessment of patient tolerance, image quality, and initial performance estimates. AJR Am J Roentgenol 199:W367–W375
Martin DR, Lauenstein T, Sitaraman SV (2007) Utility of magnetic resonance imaging in small bowel Crohn’s disease. Gastroenterology 133:385–390
Ramalho M, Herédia V, Cardoso C et al (2012) Magnetic resonance imaging of small bowel Crohn’s disease. Acta Medica Port 25:231–240
Maccioni F, Al Ansari N, Mazzamurro F et al (2014) Detection of Crohn disease lesions of the small and large bowel in pediatric patients: diagnostic value of MR enterography versus reference examinations. AJR Am J Roentgenol 203:W533–W542
Makanyanga JC, Taylor SA (2013) Current and future role of MR enterography in the management of Crohn disease. AJR Am J Roentgenol 201:56–64
Mentzel H-J, Reinsch S, Kurzai M, Stenzel M (2014) Magnetic resonance imaging in children and adolescents with chronic inflammatory bowel disease. World J Gastroenterol 20:1180–1191
Dillman JR, Smith EA, Khalatbari S, Strouse PJ (2013) I.V. glucagon use in pediatric MR enterography: effect on image quality, length of examination, and patient tolerance. AJR Am J Roentgenol 201:185–189
Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE (2007) Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 242:647–649
Roberts DR, Holden KR (2016) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38:331–336
Choi SH, Kim KW, Lee JY et al (2016) Diffusion-weighted magnetic resonance enterography for evaluating bowel inflammation in Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis 22:669–679
Oussalah A, Laurent V, Bruot O et al (2010) Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut 59:1056–1065
Seo N, Park SH, Kim K-J et al (2015) MR enterography for the evaluation of small-bowel inflammation in Crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology 278:762–772
Hordonneau C, Buisson A, Scanzi J et al (2014) Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol 109:89–98
Oto A, Zhu F, Kulkarni K et al (2009) Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol 16:597–603
Dubron C, Avni F, Boutry N et al (2016) Prospective evaluation of free-breathing diffusion-weighted imaging for the detection of inflammatory bowel disease with MR enterography in childhood population. Br J Radiol 89:20150840
Neubauer H, Pabst T, Dick A et al (2013) Small-bowel MRI in children and young adults with Crohn disease: retrospective head-to-head comparison of contrast-enhanced and diffusion-weighted MRI. Pediatr Radiol 43:103–114
Sirin S, Kathemann S, Schweiger B et al (2015) Magnetic resonance colonography including diffusion-weighted imaging in children and adolescents with inflammatory bowel disease: do we really need intravenous contrast? Investig Radiol 50:32–39
Shenoy-Bhangle AS, Nimkin K, Aranson T, Gee MS (2016) Value of diffusion-weighted imaging when added to magnetic resonance enterographic evaluation of Crohn disease in children. Pediatr Radiol 46:34–42
Dillman JR, Smith EA, Sanchez R et al (2016) DWI in pediatric small-bowel Crohn disease: are apparent diffusion coefficients surrogates for disease activity in patients receiving infliximab therapy? AJR Am J Roentgenol 207:1002–1008
Oto A, Kayhan A, Williams JTB et al (2011) Active Crohn’s disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging 33:615–624
Kiryu S, Dodanuki K, Takao H et al (2009) Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging 29:880–886
Levine A, Koletzko S, Turner D et al (2014) ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 58:795–806
IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (2005) Inflammatory bowel disease in children and adolescents: recommendations for diagnosis -- the Porto criteria. J Pediatr Gastroenterol Nutr 41:1–7
Lee SS, Kim AY, Yang S-K et al (2009) Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology 251:751–761
Gallego JC, Echarri AI, Porta A, Ollero V (2011) Ileal Crohn’s disease: MRI with endoscopic correlation. Eur J Radiol 80:e8–e12
Chalian M, Ozturk A, Oliva-Hemker M et al (2011) MR enterography findings of inflammatory bowel disease in pediatric patients. AJR Am J Roentgenol 196:W810–W816
Leyendecker JR, Bloomfeld RS, DiSantis DJ et al (2009) MR enterography in the management of patients with Crohn disease. Radiographics 29:1827–1846
Hawass NE (1997) Comparing the sensitivities and specificities of two diagnostic procedures performed on the same group of patients. Br J Radiol 70:360–366
van de Schoot R, Kaplan D, Denissen J et al (2014) A gentle introduction to Bayesian analysis: applications to developmental research. Child Dev 85:842–860
Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS—A Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10:325–337
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria http://wwwR-projectorg. Accessed 26 Apr 2018
Sturtz S, Ligges U, Gelman AE et al (2005) R2WinBUGS: a package for running WinBUGS from R. J Stat Softw 12:1–16
Taylor SA, Avni F, Cronin CG et al (2017) The first joint ESGAR/ESPR consensus statement on the technical performance of cross-sectional small bowel and colonic imaging. Eur Radiol 27:2570–2582
Grand DJ, Beland MD, Machan JT, Mayo-Smith WW (2012) Detection of Crohn’s disease: comparison of CT and MR enterography without anti-peristaltic agents performed on the same day. Eur J Radiol 81:1735–1741
Park SH, Huh J, Park SH et al (2016) Diffusion-weighted MR enterography for evaluating Crohn’s disease: effect of anti-peristaltic agent on the diagnosis of bowel inflammation. Eur Radiol 27:2554–2562
Dohan A, Taylor S, Hoeffel C et al (2016) Diffusion-weighted MRI in Crohn’s disease: current status and recommendations. J Magn Reson Imaging 44:1381–1396
Sohn B, Kim M-J, Koh H et al (2014) Intestinal lesions in pediatric Crohn disease: comparative detectability among pulse sequences at MR enterography. Pediatr Radiol 44:821–830
Dillman JR, Ladino-Torres MF, Adler J et al (2011) Comparison of MR enterography and histopathology in the evaluation of pediatric Crohn disease. Pediatr Radiol 41:1552–1558
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Khachab, F., Loundou, A., Roman, C. et al. Can diffusion weighting replace gadolinium enhancement in magnetic resonance enterography for inflammatory bowel disease in children?. Pediatr Radiol 48, 1432–1440 (2018). https://doi.org/10.1007/s00247-018-4169-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4169-x