Abstract
Aim
To evaluate the role of diffusion-weighted MRI (DW-MRI) in detecting and differentiating acute from chronic bowel inflammation in patients with Crohn’s disease (CD).
Materials and methods
MR-enteroclysis examinations with DW-MRI were reviewed from 24 patients with histologically proven CD. Segments of bowel were evaluated for acute and chronic inflammation in three different reviews of the MRI images: T2w alone, T2w + DWI, and T2w + CET1w. Mean ADC values of normal bowel segments, as well as bowel segments with acute and chronic inflammation were calculated and compared. Analyses of receiver-operating characteristic (ROC) curve were performed.
Results
Hundred and forty four bowel segments in total were reviewed. Inflammation was present in 45 segments. Acute inflammation was present in 31 segments, chronic inflammation in 14. 98 bowel segments showed no inflammatory activity. Sensitivity and specificity for differentiation between normal and inflamed bowel segments was 0.6, 0.67, and 0.80 on T2w, T2w + DWI, and T2w + CET1w datasets, respectively. Specificities for differentiation between normal and inflamed bowel segments were 0.96, 0.96, and 0.98. Sensitivities for differentiation between acute and chronically inflamed bowel segments were 0.85, 0.91, and 0.96, and specificities were 0.88, 0.89, and 1.0, respectively. The mean ADC value of normal bowel (2.18 ± 0.37 × 10−3 mm2/s) was statistically significantly greater than the mean value of inflamed bowel segments (p < 0.001). The mean ADC value of acutely inflamed bowel segments was statistically significantly lower than that of chronically inflamed bowel segments (1.09 ± 0.18 × 10−3 vs. 1.55 ± 0.21 × 10−3 mm2/s) (p < 0.001). Estimated area under the ROC curve for the diagnosis of acute vs. chronic inflammation was 0.950. A threshold of ADC value of 1.41 × 10−3 mm2/s was optimal for calculation of sensitivity and specificity.
Conclusion
DW-MRI improves detection and differentiation of acute vs. chronic inflammatory changes of the bowel in patients with CD compared to T2w-images alone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Crohn’s disease (CD) is a chronic, inflammatory condition characterized by episodes of remission and relapse. The disease involves the entire gastrointestinal tract and is complicated by abscess and fistulae in approximately 10% of patients [1]. Accurate assessment of the extent of disease, degree of inflammation, and monitoring of treatment response are crucial for appropriate management and monitoring.
Several imaging modalities such as ultrasound, contrast-enhanced computed tomography, and MRI are used for diagnosing CD and to differentiate between acute and chronic inflammation/fibrosis. Although MR-enteroclysis (MRE) requires on active bowel distension, the excellent soft tissue contrast of MRI enables visualization of morphological bowel alterations in CD. Thus, MRE has become an established diagnostic procedure in inflammatory bowel diseases, particularly in adult and pediatric patients with CD [2–5]. A further advantage of MRI is the lack of ionizing radiation. MRI findings suggesting inflammation include wall thickening, submucosal edema, mesenteric fat stranding, contrast enhancement of local lymph nodes, and increased mesenteric vascularity [6]. However, chronic fibrotic changes are sometimes difficult to differentiate from acute inflammation [7–9].
DW-MRI has been increasingly used in the abdomen [10, 11], providing qualitative and quantitative information reflecting tissue cellularity and cell membrane integrity. Inflammatory changes are associated with increased tissue cellularity and cell density which results in restricted diffusion, manifesting as high signal on high b-value diffusion-weighted MR images and low corresponding ADC values. Several studies have shown that DW-MRI enables detection of inflamed bowel loops and that ADC values of inflamed bowel segments are decreased [12, 13]. However, it remains unclear if diffusion-weighted MRI can accurately differentiate acute vs. chronic bowel inflammatory changes.
Therefore, the purpose of this study is to evaluate the role of diffusion-weighted MRI in the detection and differentiation of acute vs. chronic bowel inflammation and fibrosis in patients with CD.
Materials and methods
Patients
Between August 2011 and August 2013, 24 patients (7 female, 17 male; mean age 34.8 ± 12.1 years) with a diagnosis of CD underwent MRE including DW-MRI. Inclusion criteria for this retrospective study were that the patients had active or chronic disease diagnosed histopathologically (endoscopically or surgically) within 7 weeks of the MR examination.
Histopathologic findings accepted as being indicative of active disease included the presence of crypt abscesses, mucosal ulceration, neutrophilic infiltration, and edema. Furthermore, acute inflammation is defined as the presence of neutrophil granulocytes, e.g., in crypt abscesses, but also diffuse or concentrated in mucosa, submucosa, or deeper parts of the bowel wall. Typical features of chronic inflammation are plasmocytosis of basal mucosal stroma, spot-like lymphoplasmacellular infiltrates of the lamina propria, flanked by less specific findings as atrophy of villi and crypts, loss of goblet cells, follicular lymphatic hyperplasia, stroma fibrosis, as well as granulomas and a lipomatous outgrowth of the serosa.
Institutional review board approval was obtained. Written and oral informed consent was obtained both for the study as a whole prior to enrollment and for each MR examination prior to imaging. The study was compliant with the Health Insurance Portability and Accountability Act.
MR imaging
The bowel preparation was identical to that utilized for the diagnostic colonoscopies. A nasojejunal tube (the probe tip positioned beyond the ligament of Treitz) was positioned under fluoroscopic guidance. The MRE was performed with the patients lying supine in a 1.5 T MRI system (Aera, Sonata, Siemens Healthcare, Erlangen, Germany). The bowel was filled with 2.5 L of 0.5%-methylcellulose solution under MR-fluoroscopic guidance until uniform and complete distension of bowel was achieved. Then 20–60 g Butylscopolamin was administered for bowel peristalsis. The MR-examination protocol included T2-weighted, half-Fourier single-shot fast-spin-echo sequences, steady-state-free precession sequences, T1-weighted 2D- and 3D-spoiled gradient-echo sequences with fat saturation before and after intravenous contrast media administration, and diffusion-weighted images (b-values: 50 and 800 s/mm2). Standard doses of Gd-DTPA (Magnevist*, Bayer-Schering Pharma, Germany) were utilized for post-contrast imaging. The detailed parameters are listed in Table 1.
At our institution, diffusion-weighted imaging (DWI) is typically performed using 50 s/mm2 for the low b-value and 800 s/mm2 for the high b-value sequence for calculating ADC values in abdominal MRI studies. The lower b-value sequences are typically reviewed to better identify anatomy, while the high b-value sequences are utilized to detect bowel segments with restricted diffusion.
Image evaluation
All MRI data were independently reviewed by two radiologists (with 10 and 7 years of experience in body MRI) who were aware of the patients’ diagnosis of CD but blinded to disease acuity, location of inflammatory changes, and any extraluminal disease manifestations.
Qualitative analysis
For the purpose of image analysis, the gastrointestinal tract was divided into six segments: jejunum, ileum, ascending colon, transverse colon, descending colon, and rectosigmoid colon. The MR images were reviewed for the number and location of abnormal bowel segments.
The MRI data were reviewed in three different sessions based on the review of T2-weighted images alone (T2w alone), T2-weighted and DWI images (T2w + DWI), and T2-weighted and contrast-enhanced T1-weighted images (T2w + CET1w). The different review sessions were separated by 3 week intervals and conducted in randomized order to avoid recall/learning bias.
Each segment was evaluated for the presence of inflammation and acute vs. chronic changes.
Based on T2w-datasets, a segment of bowel was classified to be acutely inflamed if the wall showed wall thickening greater than 4 mm diameter [5]. Additional criteria for acute inflammatory processes were bowel wall edema with high mucosal/submucosal or transmural signal intensity on T2w-datasets, a multilayered appearance of the wall, fibro-fatty proliferation (stranding and retraction of mesenteric fat), enlarged lymph nodes (>1 cm), and the presence of increased blood flow in the vasa recta of a bowel segment (“comb sign”) [6].
The presence of bowel wall fibrosis was considered to be a major criterion for chronic inflammation/changes. Furthermore, chronic inflammation was defined as having moderate (<4 mm) or no bowel wall thickening on T2w-images and a lack of bowel wall edema/submucosal edema, fatty proliferation, or lymphadenopathy [14].
DWI findings indicative of acute inflammation included significant diffusion restriction [13], multilayered appearance of bowel wall, and enlarged lymph nodes with high signal on DWI-datasets. If the segments demonstrated no or moderate diffusion restriction and no or moderate wall thickening, then the segment of bowel was considered chronically inflamed. Images with b-values of 50 and 800 s/mm2 were both reviewed to detect bowel segments with restricted diffusion. DWIs were also evaluated in consensus for the presence of artifacts limiting evaluation of the bowel segments. This was accomplished by means of a 4-point scale as follows: 0 = no artifact; 1 = minimal artifact (no substantial effect on evaluation); 2 = moderate artifact (major effect on evaluation); 3 = severe artifact (nondiagnostic).
The contrast-enhanced T1w images were evaluated to identify thickened bowel loops and increased enhancement compared to adjacent loops. Contrast-enhanced T1w image findings of acute inflammation included increased mesenteric vascularity, lymphadenopathy, abscesses/fistulas, and proliferation of mesenteric fat. Increased wall enhancement without significant bowel wall thickening, mesenteric vascularity, lymphadenopathy, proliferation of mesenteric fat or presence of complications were criteria for chronic inflammation [6]. Examples of patients with acute and chronic inflammatory changes are given in Figs. 1, 2, 3, and 4.
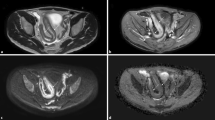
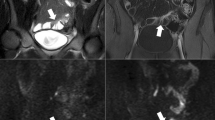
41-year-old man with acute inflammatory changes in the terminal ileum. The axial T2-weighted single-shot fast-spin-echo image (A) shows bowel wall thickening in the terminal ileum with intramural edema and mesenteric fat stranding, indicating acute inflammation. The ileum demonstrates hyperintensity on DW-MRI with b-values = 50 s/mm2 (B) and 800 s/mm2 (C) with corresponding low ADC values (D) indicating restricted diffusion. An axial post-contrast fat-suppressed T1-weighted gradient-echo image (E) reveals intense enhancement of the inflamed ileal segments (short arrows)
35-year-old woman with acute inflammatory changes in the terminal ileum The axial T2-weighted single-shot fast-spin-echo image (A) shows wall thickening in the terminal ileum with intramural edema and mesenteric fat stranding, indicating active inflammation. The ileum demonstrates hyperintensity on DW-MRI with b-values of 50 s/mm2 (B) and 800 s/mm2 (C) and corresponding low ADC values (D) indicating restricted diffusion. An axial post-contrast fat-suppressed T1-weighted gradient-echo image (E) reveals intense enhancement of the inflamed ileal segments (short arrows)
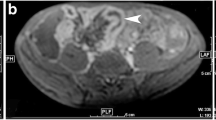
56-year-old man with chronic inflammatory changes in the ileum. The axial T2-weighted single-shot fast-spin-echo image (A) shows moderate wall thickening in the terminal ileum without intramural edema or mesenteric fat stranding. Findings are typical of chronic inflammation. The ileal wall demonstrates bright signal on DW-MR images with b-values of 50 s/mm2 (B) and 800 s/mm2 (C) and corresponding dark signal on ADC maps (D) indicating restricted diffusion. Axial post-contrast fat-suppressed T1-weighted gradient-echo image (E): moderate enhancement of the inflamed wall of the terminal ileum (short arrows)
43-year-old man with chronic inflammatory changes in the ascending colon. Axial T2-weighted single-shot fast-spin-echo image demonstrates (A) moderate wall thickening in the ascending colon without intramural edema. Findings are typical of chronic inflammation. The ascending colon wall demonstrates hyperintensity on DW-MR images with b-values of 50 s/mm2 (B) and 800 s/mm2 (C), and corresponding low ADC values (D) indicating restricted diffusion. An axial post-contrast fat-suppressed T1-weighted gradient-echo image (E) reveals moderate enhancement of the inflamed wall of the ascending colon (short arrows)
Whisker-plot diagram of ADC values of normal vs. acute and chronic bowel inflammation. Despite the substantial overlap between the ADC values of normal (1), acutely inflamed (2), and chronically inflamed (3) bowel segments, the mean ADC value of normal bowel segments (2.18 ± 0.37 × 10−3 mm2/s) was statistically significantly lower than that of the inflamed bowel segments (p < 0.001). The mean ADC value of acutely inflamed bowel segments was statistically significantly lower than that of chronically inflamed bowel segments (1.09 ± 0.18 × 10−3 vs. 1.55 ± 0.21 × 10−3 mm2/s) (p < 0.001)
Quantitative analysis
ADC measurements were performed for each bowel segment by two different radiologists who were blinded to endoscopic and histologic results. For the ADC measurements, the images were magnified, and-spin-echo oval ROIs were drawn. The ROIs were made as large as possible encompassing the area of the brightest signal within the bowel wall seen on the DW image with high b-value. The mean of the two radiologists’ ADC value measurements were accepted as the ADC value of the respective bowel segment. For ADC measurements, T2w-images also were used to correlate inflammatory changes.
There were no statistically significant differences between ADC values of small bowel and colon regardless of whether the bowel was inflamed or not (p > 0.05).
Reference standard
Clinical and surgical records were collected by a third radiologist (with 2 years of experience in body MRI). After the two reviewing sessions, the three radiologists reviewed all the images together with the clinical records of each patient. For proximal disease (i.e., in the jejunum), the diagnosis was based on imaging findings on all sequences and follow-up examinations.
Statistical analysis
For all statistical analyses, a standard software package (SPSS 15.0 for Windows; SPSS inc., Chicago) was used. For each session, sensitivity, specificity, positive predictive values (PPVs), and negative predictive values (NPVs) for detection of inflamed bowel loops and for differentiation between acutely and chronically inflamed bowel segments were calculated by means of cross tabulation. Mean ADC values of normal bowel segments as well as ADC values of the acute and chronically inflamed bowel segments were calculated and compared using an unpaired two-tailed Student t test with a significance level of 0.05. The mean ADC values of small and large bowels were also compared using an unpaired two-tailed Student t test with a significance level of 0.05. Interobserver variability was assessed for ADC measurements using Cronbachs’ Alpha, which provides a test score reflecting an internal consistency estimate.
Sensitivity of the presence of inflammation and differentiation between acute and chronic inflammation for each review session were compared using Mc Nemar’s test with a significance level of 0.05. In sensitivity and specificity analyses, the calculation of 95% confidence intervals was incorporated to determine statistically significant differences, which were presumed when the intervals of two tests being compared did not overlap.
Significance levels of sensitivity and specificity of each review session were calculated using a McNemar-Chi2 Test.
Results
Reference standard
Histopathology from 144 bowel segments was reviewed. Inflammation was present in 45 segments. 31 segments demonstrated acute inflammation, 14 segments demonstrated chronic inflammation, and 98 segments demonstrated no inflammatory activity. In the 23 patients who had acute inflammatory changes, the ileum (n = 17) was most frequently involved followed by the ascending (n = 5), transverse (n = 2), descending (n = 3), and rectosigmoid colon (n = 4). Chronic inflammation was diagnosed histologically in eight patients. In total, 14 bowel segments showed inflammatory changes involving the ileum (n = 4), ascending (n = 3), transverse (n = 2), descending (n = 3), and rectosigmoid colon (n = 2).
In all 24 patients, with a total of 120 endoscopically accessible segments per patient (ileum, ascending, transverse, descending colon, rectosigmoid), the diagnosis was based on histopathologic findings after endoscopy or operation. Four of the patients had surgery with pathological correlation of the resected bowel. All of these patients also had histopathological correlation with endoscopy prior to the operation. Results of surgical and endoscopically acquired histopathology were concordant.
The mean time interval between the MR examination and histopathology was 10 days (±14). The range of time between the MR examination and the pathologic confirmation was 0–50 days.
Three patients (n = 3; 50, 40, and 38 days, respectively) with delayed pathologic confirmation after MR imaging (≥4 weeks) were untreated in the interval, and according to the clinical records, the clinical condition of those patients remained unchanged.
Qualitative analysis
Table 2 displays the statistical parameters of diagnostic accuracy for the respective reading sessions.
In 11 patients, DWI quality was rated as excellent without artifacts. In 11 patients, minimal artifacts were observed. In two patients, moderate artifacts were identified.
Diagnostic accuracy: normal vs. inflamed bowel segments
With T2w-images alone, the sensitivity for detection and differentiation of normal and inflamed (both acute and chronic) bowel segments was 0.6, the specificity 0.96, the PPV 0.87, and the NPV 0.84. For the T2w + DWI session, sensitivity, specificity, PPV, and NPV were 0.67, 0.96, 0.88, and 0.86, respectively. For the T2w + CET1w session, sensitivity, specificity, PPV, and NPV were 0.80, 0.98, 0.95, and 0.92, respectively.
Applying a McNemar Chi2 test, sensitivity of T2w + CET1w was significantly higher compared to T2w and T2w + DWI (p = 0.01). Sensitivity of T2w + DWI was significantly (p = 0.02) higher than that of T2w alone. Sensitivity for the detection and differentiation of normal vs. inflamed bowel segments was nonsignificantly (p = 0.08) greater for T2w + DWI compared to T2w datasets alone. We did not detect any statistically significant difference in specificities between the groups (T2w vs. T2w + DWI: p = 1.0; T2w vs. T2w + CET1w: p = 0.15; T2w + DWI vs. T2w + CET1w p = 0.15).
Diagnostic accuracy: acute vs. chronic inflammatory changes
Both acute and chronically inflamed bowel segments demonstrated restricted diffusion as defined by high signal intensity on DWI and low ADC values.
The sensitivity for differentiation between acute and chronically inflamed bowel segments was 0.85, the specificity 0.88, the PPV 0.94, and the NPV 0.7 on T2w-images. For the T2w + DWI session, sensitivity, specificity, PPV, and NPV were 0.91, 0.89, 0.95, and 0.8, respectively. For the T2w + CET1w session, sensitivity, specificity, PPV, and NPV were 0.96, 1, 1, and 0.92, respectively. Sensitivity for the differentiation of normal vs. inflamed bowel segments was greater with T2w + DWI compared to T2w-datasets alone.
The above-mentioned parameters of diagnostic accuracy were tested for statistical significance comparing their confidence intervals. T2w yielded a sensitivity of 0.85 (95% CI 0.69, 1.01) and specificity of 0.88 (95% CI 0.65, 1.05) for the differentiation between acute and chronic inflammatory changes. T2w + DWI exhibited a sensitivity of 0.91 (95% CI 0.80–1.03) and a specificity of 0.89 (95% CI 0.68–1.09). T2w + CET1w images yielded a sensitivity of 0.96 (95% CI 0.89–1.04) and specificity of 100% (95% CI 1.00–1.00). As the confidence intervals did overlap, there were no significant differences with regard to sensitivity and specificity between the different reading sessions.
Quantitative analysis
The mean ADC value of normal bowel (2.18 ± 0.37 × 10−3 mm2/s) was statistically significantly higher than that of inflamed bowel segments (p < 0.001). The mean ADC value of acutely inflamed bowel segments was statistically significantly lower than that of the chronically inflamed bowel segments (1.09 ± 0.18 × 10−3 vs. 1.55 ± 0.21 × 10−3 mm2/s) (p < 0.001) (Fig. 5). The mean ADC values for each segment are listed in Table 3.
The internal consistency of ADC measurements was good (Cronbachs’ Alpha of 0.884). The mean ROI area of normal bowel was 31.93 (±13.19) mm2. This was comparable to the area of the ROIs utilized for assessment acutely inflamed bowel segments 35.26 mm2 (±17.49). The mean ROI in chronically inflamed segments was 26.6 mm2 (±14.88).
The estimated area under the ROC curve (Fig. 6) for the diagnosis of acute vs. chronic bowel inflammation with DWI was 0.950 with sensitivity of 0.93 and specificity of 0.75. These results were obtained using a threshold ADC value of 1.41 × 10−3 mm2/s.
There were no statistically significant differences between ADC values of small bowel and colon regardless of whether the bowel was inflamed or not (p > 0.05).
Discussion
Our results indicate that MR enteroclysis with DW-MRI allows the differentiation between acute and chronically inflamed bowel segments in patients with CD using both qualitative analysis and quantitative measurements.
The ability to distinguish between acute and chronic inflammatory changes and to monitor disease activity is crucial for the treatment of the patients. Recently, patients with acute inflammation have been treated with immunomodulation by antibodies to tumor necrosis factor. However, due to the potential for adverse side effects with this therapy, it should be reserved only for use in patients with acute bowel inflammation.
There are multiple imaging modalities for assessing suspected intestinal inflammatory changes in patients with CD. Endoscopy based techniques are highly sensitive, but do not allow visualization of the deep gut layers nor of extraluminal complications [15]. MR provides cross-sectional information with high soft tissue contrast. Further advantages include the lack of radiation and the safety profile of intravenous contrast media. MR enterography and MR enteroclysis are the two techniques most commonly performed. With enterography, the contrast media is administered orally, whereby in enteroclysis, a nasoenteric tube is utilized for instillation of material to distend the bowel. There is ongoing controversy as to whether MR enteroclysis is superior to MR enterography. The advantages of MR enteroclysis over MR enterography are contested by some authors: Negaard et al. and Schreyer et al. found similar sensitivities of both techniques for the detection of active inflammation in CD [16–18]. However, a study by Maselli et al. showed MR enteroclysis to provide superior depiction of mucosal abnormalities [19]. As of now, it remains unclear if greater bowel distension with enteroclysis improves image quality or diagnostic accuracy.
DW-MRI allows assessment of changes in water mobility caused by interactions of the water molecules with cell membranes, macromolecules, and alterations of the tissue environment [20] and therefore enables evaluation of diffusion processes in vivo [10, 21]. In neuroimaging, DW-MRI is an established means for diagnosing acute stroke and is frequently used for the diagnosis of intracranial infectious processes (such as brain abscess, cerebritis, and subdural or intraventricular empyema) [22–27]. Recent technical advances have facilitated the application of DW-MRI in the abdomen. Beyond the use of DW-MRI for detection and characterization of tumors, DW-MRI is increasingly used for detection of inflammation in the abdomen and pelvis. There are several studies evaluating the role of DW-MRI in detecting inflamed bowel segments in patients with CD. All of them demonstrated diffusion restriction associated with bowel wall inflammation and, when measured, decreased ADC values of inflamed relative to normal bowel wall [7, 12, 13, 28–31]. In a study by Oto et al., the mean ADC value of inflamed bowel segments was 1.59 ± 0.45 × 10−3 mm2/s compared to a significantly higher mean ADC value of normal bowel segments (2.74 ± 0.45 × 10−3 mm2/s) [12]. Kiryu et al. showed that the ADC values in areas of active CD were statistically significantly lower than those in areas of inactive disease within the small and large bowels (1.61 ± 0.44 × 10−3 vs. 2.56 ± 0.51 × 10−3 mm2/s in small bowel and 1.52 ± 0.43 × 10−3 vs. 2.31 ± 0.59 × 10−3 mm2/s in large bowel, respectively) [28].
In the present study, we demonstrated restricted diffusion in actively inflamed bowel loops with correspondingly decreased ADC values compared to normal bowel wall, similar to the results of Oto et al. and Kiryu et al. [12, 28]. Kiryu et al. also found significantly lower ADC values in the large bowel than that in the small bowel [28]. Mechanisms for this may relate to viscosity differences of the intestinal contents between small and large bowels. However, in the present study, no statistically significant differences between ADC values of small bowel and colon were identified, regardless of whether the bowel was inflamed or not. This may be the result of differences in the study design. In distinction to Kiryu et al. [28], in this study, the bowel was fully prepared ahead of time and prior to the MR scan and was subsequently filled with methylcellulose to uniform and complete distension. This ensured that there were no viscosity differences in the intestinal contents between small and large bowels.
In our study, acutely inflamed bowel segments demonstrated significantly lower ADC values compared to chronically inflamed/fibrotic bowel segments. Thus, DW-MRI may allow for the differentiation between acute and chronic/fibrotic inflammatory changes. However, the exact mechanism for restricted diffusion in acutely inflamed bowel remains elusive, and further studies are thus needed. Mechanisms for restricted diffusion in acutely inflamed bowel segments may include lymphocyte aggregation, dilatation of lymphatic ducts, hypertrophy of neuronal tissue, and the presence of granulomas within the intestinal wall occupying and increasing the extracellular space [12, 21]. No other studies to date have evaluated the role of DW-MRI in chronic/fibrotic changes of bowel segments in patients with CD. However, fibrosis also seems to be associated with restricted diffusion and decreased ADC values. This is not unexpected as several studies demonstrate a decrease in hepatic ADC values in liver cirrhosis [32–37]. Similar to the liver, we found restricted diffusion in chronically inflamed/fibrotic bowel segments. Diffusion restriction in chronically inflamed bowel may be related to a predominance of connective tissue within the bowel wall and decreased blood flow [32, 38].
According to our results, assessing T2w-datasets alone yielded satisfactory diagnostic accuracies both for the detection and differentiation of normal from inflamed bowel segments and for the differentiation of acute and chronically inflamed segments. When utilized in combination with DWI, these results were markedly improved. Appending additional sequences adds to the total acquisition time for the comprehensive abdominal MRI protocol. However, the additional acquisition time of 3 min for the DW-MRI sequence used in the present study can be considered a reasonable compromise with regard to its additional diagnostic value.
In the present study, T2w + CET1w demonstrated the highest sensitivity for the differentiation between acute and chronic inflammatory small bowel changes, followed by T2w + DW-MRI. Therefore, T2w + CET1w allows for the most reliable imaging differentiation of acute and chronic inflammation. However, sensitivity of T2w + DW-MRI was not significantly lower than for T2w + CET1w (0.91 vs. 0.96). Thus, T2w + DWI-MRI may represent a comparably sensitive alternative to T2w + CET1w and may therefore be employed in patients with contraindications for gadolinium chelates (i.e., renal failure with an estimated glomerular filtration rate <30 mL/min and known hypersensitivity without the possibility of premedication) or in pediatric patients. In addition, we found a higher sensitivity for T2w + DW-MRI compared to T2w alone. Although the difference in sensitivity between these two sequences proved only borderline-significant (p = 0.08), the present study results suggest that DW-MRI may augment the sensitivity of T2w alone.
As described previously, DWI allows a fast assessment of inflammatory changes in patients with Crohn disease without using contrast media [31, 39, 40]. ADC-measurements facilitate quantitative assessment of disease activity in patients with CD and could be helpful in assessing therapeutic response in patients with subsequent follow up examinations.
MR enterography is preferred to MR enteroclysis at many institutions, mainly with regard to practicability and patient comfort. However, it was demonstrated that MR enteroclysis using a nasojejunal tube allows for a significantly better small bowel loop distension compared to MR enterography, leading to an optimized depiction of bowel wall pathologies, and consequently a higher diagnostic accuracy for the imaging of morphological wall changes in inflammatory bowel disease [41]. It is therefore suggested to perform MR enteroclysis for the initial workup of patients with suspected CD, while MR enterography may be employed for follow-up examinations in patients with confirmed disease [19]. Moreover, DW-MRI is known to be limited by low spatial resolution and decreased signal-to-noise ratio compared to morphological MRI sequences. Therefore, an optimized small bowel distension may additionally improve the diagnostic accuracy of DW-MRI for the differentiation of acute and chronic inflammatory small bowel changes.
This study is limited by its retrospective design. It was also conducted at a single center and using single scanner with a relatively small sample size. Prospective studies with larger number of patients are needed to clarify the efficacy of DWI in differentiating between acute and chronic inflammatory bowel disease in patients with CD. In addition, the study is limited by the lack of histopathological validation for the jejunum and proximal/mid ileum. For those small bowel segments, the comprehensive MRI protocol for the present and the follow-up examinations including unenhanced and contrast-enhanced morphology-based and DW-MRI sequences served as reference standard. Another limitation of the present investigation is the combined analysis of small bowel and colon segments. Differences in wall thickness and lumen diameter between colon and small bowel loops may have an influence on the semiquantitative ADC maps and may therefore limit the applicability of DWI-MRI for the comprehensive evaluation of the gastrointestinal tract.
In conclusion, DWI improves detection and enables qualitative and quantitative differentiations of acute from chronic bowel inflammatory changes in patients with CD.
References
Munkholm P, Langholz E, Davidsen M, et al. (1995) Disease activity courses in a regional cohort of Crohn’s disease patients. Scand J Gastroenterol 30:699–706
Maccioni F, Viola F, Carozzo F, et al. (2012) Differences in the location and activity of intestinal Crohn’s disease lesions between adult and paediatric patients detected with MRI. Eur Radiol 22(11):2465–2477
Prassopoulos P, Papanikolaou N, Grammatikakis J, et al. (2001) MR enteroclysis imaging of Crohn’s disease. Radiographics 21 Spec No:S161–S172
Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, et al. (2002) MR enteroclysis: technical considerations and clinical applications. Eur Radiol 12:2651–2658
Gourtsoyiannis NC, Papanikolaou N, Karantanas A (2006) Magnetic resonance imaging evaluation of small intestinal Crohn’s disease. Best Pract Res Clin Gastroenterol 20:137–156
Maccioni F, Viscido A, Broglia L, et al. (2000) Evaluation of Crohn disease activity with magnetic resonance imaging. Abdom Imaging 25(3):219–228
Al-Hawary M, Zimmermann EM (2012) A new look at Crohn’s disease: novel imaging techniques. Curr Opin Gastroenterol 28(4):330–340
Zappa M, Stefanescu C, Cazals-Hatem D, et al. (2011) Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn’s disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis 17:984–993
Adler J, Punglia D, Dillman JR, et al. (2012) CT enterography findings correlate with tissue inflammation in resected small bowel Crohn’s disease. Inflamm Bowel Dis 18(5):849–856
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. Am J Roentgenol 188:1622–1635
Taouli B, Koh DM (2010) Diffusion-weighted imaging of the liver. Radiology 254:47–66
Oto A, Zhu F, Kulkarni K, et al. (2009) Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol 16(5):597–603
Oto A, Kahyan A, Williams JTB, et al. (2011) Active Crohn´s disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging 33:615–624
Tolan DJ, Greenhalgh R, Zealley IA, et al. (2010) MR enterographic manifestations of small bowel Crohn disease. Radiographics 30(2):367–384
Sandborn WJ, Loftus EV (2004) Balancing the risks and benefits of infliximab in the treatment of inflammatory bowel disease. Gut 53:780–782
Fidler JF, Guimaraes L, Einstein DM (2009) MR imaging of the small bowel. Radiographics 29:1811–1825
Negaard A, Paulsen V, Sandvik L, et al. (2007) A prospective randomized comparison between two MRI studies of the small bowel in Crohn’s disease, the oral contrast method and MR enteroclysis. Eur Radiol 17:2294–2301
Schreyer AG, Geissler A, Albrich H, et al. (2004) Abdominal MRI after enteroclysis or with oral contrast in patients with suspected or proven Crohn’s disease. Clin Gastroenterol Hepatol 2:491–497
Masselli G, Gualdi G (2012) MR imaging of the small bowel. Radiology 264(2):333–348
Wesbey GE, Moseley ME, Ehman RL (1984) Translational molecular self-diffusion in magnetic resonance imaging. II. Measurement of the self-diffusion coefficient. Invest Radiol 19:491–498
Maccioni F, Patak MA, Signore A, et al. (2012) New frontiers of MRI in Crohn’s disease: motility imaging, diffusion-weighted imaging, perfusion MRI, MR spectroscopy, molecular imaging, and hybrid imaging (PET/CT). Abdom Imaging 37(6):974–982
Guzman R, Barth A, Lövblad KO, et al. (2002) Use of diffusion-weighted magnetic resonance imaging in differentiating purulent brain processes from cystic brain tumors. J Neurosurg 97:1101–1107
Reddy JS, Mishra AM, Behari S, et al. (2006) The role of diffusion-weighted imaging in the differential diagnosis of intracranial cystic mass lesions: a report of 147 lesions. Surg Neurol 66:246–250
Leuthardt EC, Wippold FJ 2nd, Oswood MC, et al. (2002) Diffusion-weighted MR imaging in the preoperative assessment of brain abscesses. Surg Neurol 58:395–402
Wong AM, Zimmerman RA, Simon EM, et al. (2004) Diffusion-weighted MR imaging of subdural empyemas in children. Am J Neuroradiol 25:1016–1021
Kiroğlu Y, Calli C, Yunten N, et al. (2006) Diffusion-weighted MR imaging of viral encephalitis. Neuroradiology 48:875–880
Han KT, Choi DS, Ryoo JW, et al. (2007) Diffusion-weighted MR imaging of pyogenic intraventricular empyema. Neuroradiology 49:813–818
Kiryu S, Dodanuki K, Takao H (2009) Free-breathing diffusion weighted imaging for the assessment of inflammatory activity in Crohn’s disease. J Magn Reson Imaging 29:880–886
Kilickesmez O, Atilla S, Soylu A, et al. (2009) Diffusion-weighted imaging of the rectosigmoid colon: preliminary findings. J Comput Assist Tomogr 33(6):863–866
Oussalah A, Laurent V, Bruot O, et al. (2010) Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut 59:1056–1065
Buisson A, Joubert A, Montoriol PF, et al. (2013) Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther 37(5):537–545
Taouli B, Aj Tolia, Losada M, et al. (2007) Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. Abdom Imaging 189:799–806
Namimoto T, Yamashita Y, Sumi S, et al. (1997) Focal liver masses: characterization with diffusion-weighted echo-planar MR imaging. Radiology 204:739–744
Ichikawa T, Haradome H, Hachiya J, et al. (1998) Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. Am J Roentgenol 170:397–402
Amano Y, Kumazaki T, Ishihara M (1998) Single-shot diffusion-weighted echo-planar imaging of normal and cirrhotic livers using a phased-array multicoil. Acta Radiol 39:440–442
Kim T, Murakami T, Takahashi S, et al. (1999) Diffusion-weighted single-shot echoplanar MR imaging for liver disease. Am J Roentgenol 173:393–398
Taouli B, Vilgrain V, Dumont E, et al. (2003) Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology 226:71–78
Annet L, Peeters F, Abarca-Quinones J, et al. (2007) Assessment of diffusion weighted MR imaging in liver fibrosis. J Magn Reson Imaging 25:122–128
Hordonneau C, Buisson A, Scanzi J, et al. (2014) Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol 109(1):89–98
Neubauer H, Pabst T, Dick A, et al. (2013) Small-bowel MRI in children and young adults with Crohn disease: retrospective head-to-head comparison of contrast-enhanced and diffusion-weighted MRI. Pediatr Radiol 43(1):103–114
Masselli G, Casciani E, Polettini E, et al. (2008) Comparison of MR enteroclysis with MR enterography and conventional enteroclysis in patients with Crohn’s disease. Eur Radiol 18(3):438–447
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Schmid-Tannwald, C., Schmid-Tannwald, C.M., Morelli, J.N. et al. The role of diffusion-weighted MRI in assessment of inflammatory bowel disease. Abdom Radiol 41, 1484–1494 (2016). https://doi.org/10.1007/s00261-016-0727-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0727-6