Abstract
Most pediatric malignancies require some form of cross-sectional imaging, either for staging or response assessment. The majority of these are solid tumors and this review addresses the role of MRI, as well as other cross-sectional and functional imaging techniques, for evaluating the most common pediatric solid tumors. The primary emphasis is on neuroblastoma, hepatoblastoma and Wilms tumor, three of the most common non-central-nervous-system (CNS) pediatric solid tumors encountered in young children. The initial focus will be a review of the imaging techniques and approaches used for diagnosis, staging and early post-treatment response assessment, followed by a discussion of the role surveillance imaging plays in pediatric oncology and a brief review of other emerging imaging techniques. The lessons learned here can be applied to most other pediatric tumors, including rhabdomyosarcoma, Ewing sarcoma and osteosarcoma, as well as germ cell tumors, neurofibromatosis and other rare tumors. Although lymphoma, in particular Hodgkin lymphoma, represents one of the more common pediatric malignancies, this is not discussed in detail here. Rather, many of the lessons that we have learned from lymphoma, specifically with regard to how we integrate both anatomical imaging and functional imaging techniques, is applied to the discussion of the other pediatric solid tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Neuroblastoma
Neuroblastoma is the most common non-central nervous system (CNS) pediatric solid tumor, making up approximately 7% of all pediatric neoplasms. The adrenal gland is the most common site of origin, seen in approximately 50% of the patients, with thoracic and extra-adrenal retroperitoneal neoplasms seen in approximately 25% of the patients [1].
Neuroblastoma staging
As with most pediatric solid tumors, imaging initially involves radiographs and ultrasound, usually as a first step in the evaluation of nonspecific symptoms. Subsequent staging requires a determination of overall disease burden. The International Neuroblastoma Staging System (INSS) was established in the late 1980s and modified in 1993, and can still be found in use in many neuroblastoma treatment protocols (Table 1) [2]. This staging system focuses on characterization of the primary tumor, locoregional lymph node involvement and invasion of adjacent structures, and presence or absence of metastatic disease, and was based both on initial imaging and extent of tumor remaining after surgical resection. This system was limited in that assessment of lymph node involvement was often subjective when based on imaging, and when established at the time of surgery could not be used for up-front risk stratification. Indeed a tumor could be classified as INSS stage 1 based on imaging and stage 3 based on surgical staging or incomplete tumor excision, making it challenging to apply this staging system uniformly across different clinical trials [3].
Subsequently, it became clear that additional factors were important in establishing overall prognosis. Major prognostic factors include the INSS stage of disease, age, histology and molecular pathology. Poor prognostic factors include age greater than 18 months, MYCN (the proto-oncogene encoding oncoprotein N-myc) gene amplification, diploid deoxyribonucleic acid (DNA) content, and poorly differentiated or undifferentiated histology. Based on the imaging findings and these other prognostic factors, patients historically were classified into low, intermediate or high risk categories [1]. Although the INSS staging system remained in place for more than two decades, it largely relied on surgical criteria established at the time of tumor resection, making it difficult to risk-stratify children prior to initiating therapy. In response, the International Neuroblastoma Risk Group (INRG) developed the INRG staging system for neuroblastoma (Table 2) [4]. The INRG staging system focuses on establishing tumor stage prior to surgery or chemotherapy and is based on the presence of one or more of 20 agreed-upon “image-defined risk factors” (IDRFs), which allow children to be assigned to specific risk groups at the time of diagnosis, prior to initiating treatment. These IDRFs are surgical risk factors, based on imaging, that could influence the surgical resectability of a tumor and the potential to achieve gross total resection [3]. These IDRFs include, but are not limited to, vascular invasion, invasion of adjacent solid organs and soft-tissue structures, and extension into the spinal canal (Table 3), and have been incorporated into the comprehensive pretreatment INRG classification system, which includes age, histological classification and tumor grade, MYCN amplification status, and chromosomal aberrations, in addition to the new IDRFs [5].
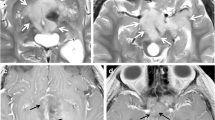
For the purposes of staging, most children are imaged using a combination of cross-sectional and functional nuclear medicine imaging techniques. Both CT and MRI are used. CT has the advantage of near-universal availability, high-quality lesion detection, and in some instances with newer, ultrafast multi-detector row scanners, the ability to perform examinations without sedation in young children [6]. MRI is more sensitive for characterizing bone marrow involvement, central nervous system (CNS) disease, and extension into the spinal canal. With the advent of hepatocellular-based contrast agents, MRI can also be used to establish the presence of focal hepatic lesions. MRI does not involve ionizing radiation, and the use of diffusion-weighted sequences, combined with the superior soft-tissue discrimination afforded by MRI as compared to CT, has led many to favor use of MRI over CT for staging neuroblastoma and identifying image-defined risk factors [7]. An example of this is shown in Fig. 1.
Neuroblastoma staging. Coronal (a) and axial (b, d) fat-suppressed T2-weighted MRI, and axial diffusion-weighted image (c) in a 3-year-old girl with suspected abdominal mass show a large midline retroperitoneal mass with encasement of the mesenteric vessels, extension into the porta hepatis, and encasement of the renal vessels, indicating multiple IDRFs. MRI also shows multiple skeletal lesions (d, arrows) that were not seen by CT (e, arrows), with additional lesions revealed by 123I-MIBG scintigraphy (f, arrows). IDRF image-defined risk factor, MIBG metaiodobenzylguanidine
Scintigraphic imaging by 123I-metaiodobenzylguanidine (MIBG) is considered standard of care for staging children with neuroblastoma. More than 90% of these children have MIBG-avid disease and MIBG uptake is identified both in primary tumors and at multiple sites of metastatic disease, including the bone and bone marrow [8]. Establishing the burden of MIBG-avid disease at baseline and after induction therapy correlates with outcome. Interestingly, children who have MIBG-non-avid disease have been found to have lower rates of adrenal primary tumors and metastatic disease and, despite having other risk factors such as MYCN amplification, have superior outcomes as compared to children with MIBG-avid disease [9]. 123I-MIBG scintigraphy should include planar imaging of the entire body, providing a comprehensive evaluation for sites of skeletal disease, as well as SPECT (single-photon emission computed tomography) imaging of the torso, typically extending from the vertex of the skull through the thighs. The use of SPECT/CT, in which either a low-dose CT or dose-optimized diagnostic CT provides for accurate anatomical localization of MIBG abnormalities, increases the specificity of the MIBG examination and is now routinely used in most centers [8, 10]. Historically, neuroblastoma staging also utilized 99mTc-methylene diphosphonate (MDP) bone scanning. This has been shown to be unnecessary, with few if any instances where conventional bone scanning led to either a change in diagnosis or a change in disease stage [11].
The role of other functional imaging agents such as 18F-FDG positron emission tomography (PET)/CT in staging neuroblastoma has been investigated. While most studies have confirmed the feasibility of FDG PET for imaging neuroblastoma, a significant number of children with neuroblastoma have FDG-negative tumors despite those tumors being intensely MIBG-avid [12, 13]. In addition, FDG is less specific than MIBG and shows uptake in inflammatory lesions as well as in normal and cytokine-stimulated marrow. As a result, the use of FDG PET imaging is not routine in neuroblastoma, and is reserved for children who are MIBG-negative (~10%), or for characterizing cases in which MIBG and conventional cross-sectional imaging modalities are discrepant [14].
Neuroblastoma response assessment
Most children being treated for neuroblastoma have re-evaluation of their primary disease after induction chemotherapy and prior to planned surgical resection. Induction chemotherapy is important for reducing tumor size and aiding in surgical resection. Children who do not respond satisfactorily to induction chemotherapy are often switched to an alternative treatment regimen. Showing a reduction in tumor size implies a response to induction chemotherapy, and Trout et al. [15] have reported that 3-D measurements most accurately quantify changes in tumor size in advanced-stage neuroblastoma, as compared to 1-D and 2-D measurements, which may underrepresent tumor response. Nonetheless, in a recent large international multicenter study aimed at determining the best method for primary tumor response assessment, comparing uni-dimensional RECIST (Response Evaluation Criteria in Solid Tumors) measurements with comprehensive 3-D volumetric assessments, none of the methods of primary site disease assessment were found to be predictive of outcome [16]. The 123I-MIBG response of the primary tumor site also had no bearing on outcome [17]. Rather, the best overall response-based predictor of outcome is the ability to obtain a reduction in overall tumor burden, both by achieving complete surgical resection and by reducing the burden of metastatic disease as determined by MIBG scan. The best measure of determining overall tumor burden is the semi-quantitative Curie score. The Curie score is based on the degree of MIBG uptake identified at 10 anatomical sites, each of which is scored 0–3 based on the degree of MIBG avidity. An example of MIBG scoring, applied at baseline and after induction chemotherapy, is shown in Fig. 2.
Curie scoring in neuroblastoma. a Anatomical regions for Curie scoring in neuroblastoma. Skeletal sites (1–9) and soft-tissue lesions are scored 0 to 3: 1 = one distinct MIBG-avid lesion; 2 = more than one MIBG-avid lesion; 3 = MIBG-avid lesions in ≥50% of a site. Max = 30. b, c Imaging in a 23-month-old boy with newly diagnosed neuroblastoma. Planar images from 123I-MIBG scintigraphy obtained at baseline (b), and following six cycles of induction chemotherapy and gross total surgical resection (c). The Curie scores for each time point are shown below each image, with the extensive uptake in the primary right adrenal tumor and throughout the skeleton seen at baseline having resolved following chemotherapy and resection. MIBG metaiodobenzylguanidine
Several studies, both from the United States and European consortia, have shown that MIBG scores predict outcome for children with MIBG-avid advanced-stage disease [18,19,20]. There is no correlation between the Curie score at diagnosis and subsequent treatment outcome. Rather, children who achieve a post-induction chemotherapy Curie score of less than 2 have been shown to have an overall better prognosis, whereas those with Curie scores greater than 2 have a poor prognosis and might benefit from alternative treatment options [20].
In summary, neuroblastoma requires a multitude of imaging studies for both staging and response assessment, including cross-sectional imaging using CT, MRI and MIBG scintigraphy. Predictors of outcome include high disease stage at diagnosis (stage that was initially determined using the INSS staging system but more recently is based on image-defined risk factors). Children with more than two IDRFs have locally advanced disease, which in turn impacts surgical resectability and outcome. The presence of MIBG-avid disease at baseline is an independent predictor of outcome, with MIBG-negative patients having fewer sites of metastatic disease and overall improved event-free survival. In keeping with the IDRF model, the extent of surgical resection has also been shown to be a predictor of outcome, with children achieving gross total resections of greater than 90% having overall better survival [21, 22]. Finally, the presence of MIBG-avid residual disease after induction chemotherapy and surgical resection of the primary tumor is associated with an overall poor outcome and warrants alternative treatment options such as 131I- MIBG therapy, antibody therapy or stem cell transplantation.
Hepatoblastoma
Hepatoblastoma is the most common primary liver tumor of childhood, occurring primarily in infants and children younger than 3 years. It is associated with syndromes such as Beckwith–Wiedemann syndrome and has a slight male predominance. Common symptoms at the time of diagnosis include weight loss, a painless palpable abdominal mass, and in some cases jaundice, symptoms that in turn prompt the initial imaging evaluation. At the time of diagnosis, serum alpha-fetoprotein levels (AFP) are almost always elevated.
Hepatoblastoma staging
The goals of hepatoblastoma staging, as with neuroblastoma, are to determine the extent of disease, which includes both local extent of disease as well as regional spread into lymph nodes and adjacent structures, vascular involvement and metastatic disease. An important advance in hepatoblastoma therapy has been adoption of the PRETEXT (pre-treatment extent of disease) staging system [23]. The PRETEXT score represents the number of contiguous liver sections that must be resected to completely excise the tumor, and is based on the Couinaud’s segmentation of the liver [24, 25]. PRETEXT has been essential in determining surgical resectability and the overall extent of tumor before any therapy commences, and now serves as the basis for preoperative risk stratification in both U.S. and European trials [26].
PRETEXT has good inter-observer reproducibility, and in addition to its prognostic value, it plays a critical role in identifying contraindications to surgery or to liver transplant.
Figure 3 shows a diagram of the PRETEXT system and Table 4 outlines the criteria used to establish PRETEXT stage. In its simplest form, PRETEXT 1 tumors are those involving one section of the liver, PRETEXT 2 tumors involve two sections, but with two adjoining sections free, while PRETEXT 3 and 4 tumors are generally unresectable, with no two adjoining sections free, although some PRETEXT 3 patients, particularly those with tumor sparing the left lateral segment, could undergo resection [26]. Additional considerations are the presence of metastatic disease, vascular invasion, and tumor rupture outside of the liver (Table 4).
Diagram of PRETEXT (pre-treatment extent of disease) staging considerations shows the dependence of PRETEXT stage on the contiguous sections of liver involvement. PRETEXT 1 and PRETEXT 2 tumors are usually resectable and involve either one or two sections of the liver, respectively, with at least two adjoining sections free. PRETEXT 3 and 4 tumors are generally unresectable, with no two adjoining sections free (some PRETEXT 3 patients, e.g., those with tumor sparing the left lateral segment might be eligible for resection). Adapted from Emre et al. [25] with permission
The ability to establish PRETEXT stage is predicated on use of sensitive and specific imaging techniques. Most children with hepatoblastoma undergo either a CT or MRI scan at diagnosis. Many surgeons prefer an angiographic or biphasic CT imaging technique that includes optimal delineation of the hepatic arterial, portal venous and hepatic venous structures, although concerns about radiation dose have led some radiologists to eschew this modality in favor of MRI [24]. MRI, while a much more lengthy examination than CT, has the advantage of multiplanar soft-tissue characterization, and when diffusion-weighted imaging techniques are used, MRI is exquisitely sensitive for detecting tiny liver lesions. Nearly all abdominal MRI examinations performed in oncology patients require the use of intravenous contrast agents. Whereas routine abdominal MRI exams most often use standard extracellular gadolinium-based agents, in children suspected of having hepatoblastoma the use of hepatocyte-specific MRI contrast agents such as gadoxetate disodium (gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid [Gd-EOB-DTPA]; Eovist®/Primovist®; Bayer Healthcare, Whippany, NJ) has been shown to be useful both in characterizing indeterminate lesions and in excluding subtle lesions in the liver that would increase the PRETEXT stage of the tumor [24, 27] (Fig. 4). Both CT and MRI are effective at showing vascular involvement, although delineation of the vasculature by CT is dependent on phase of contrast and often necessitates multi-phase scanning.
Staging and response assessment of hepatoblastoma in a 20-month-old boy. The boy was initially imaged by CT and subsequently by MRI, including coronal fat-suppressed T2-weighted (a, c) and axial post-contrast fat-suppressed VIBE imaging with Eovist (b, d). Baseline imaging (a–c): Hepatocyte-phase imaging confirms the absence of disease in the left lateral segment and shows the relationship of the mass to the bile ducts and vessels (b), confirming PRETEXT (pre-treatment extent of disease) 3 tumor. Post-induction chemotherapy (d, f): Following four cycles of chemotherapy the mass had decreased in size and pulmonary nodules (arrows) detected by CT at diagnosis (e) had either resolved or significantly decreased in size (f). The boy underwent hepatic trisegmentectomy, sparing the left lateral segment (segments 2 and 3), and continues to do well. VIBE volumetric interpolated breath-hold examination
When evaluating children for pulmonary metastatic disease, CT remains the standard of care (Fig. 4). MRI of the lungs is not yet considered sufficiently sensitive to identify small pulmonary nodules, the detection of which would impact outcome. For routine staging, there is currently no role for 18F-FDG PET/CT in hepatoblastoma staging, although it might be useful in select cases of suspected relapse where elevated AFP levels are identified but where no site of disease is revealed by conventional imaging [28].
These diagnostic imaging approaches are important because surgical resection remains the mainstay of curative therapy for hepatoblastoma. Historically, cooperative groups in the USA favored an attempt at surgical resection at the time of diagnosis whenever feasible, whereas the European consortia advocated for preoperative imaging and chemotherapy; it was out of the European approach that the current PRETEXT system arose [29]. Only 1/3 to 1/2 of the patients have resectable disease at diagnosis. When feasible, complete resection of the primary tumor results in ~90% event-free survival. For unresectable primary tumors, neoadjuvant chemotherapy has transformed the management of hepatoblastoma. Unresectable tumors include large tumors that involve multiple adjacent segments of the liver (PRETEXT 3 or 4), tumors with portal or hepatic vein involvement, and tumors with lung metastasis at diagnosis. For those children unresponsive to chemotherapy and for whom initially unresectable disease cannot be converted to resectable disease, orthotopic liver transplant remains an important option [25]. In this subpopulation of patients, however, the best survival is seen in those who have shown a response to chemotherapy, both in terms of a decrease in AFP levels and a decrease in tumor size [26]. For children who have metastases at diagnosis, all sites of extra-hepatic metastatic disease must have cleared, either as a result of neoadjuvant chemotherapy or by surgical resection, in order to be eligible for liver transplantation. According the current Children’s Oncology Group Hepatoblastoma protocol AHEP 0731, “no patient with Stage IV disease will be offered a liver transplant unless all sites of metastatic disease have either been surgically resected or documented radiographically to have disappeared as a result of neoadjuvant chemotherapy.” Small residual pulmonary nodules must be resected in order to have histological proof of metastatic disease clearance prior to transplant. Although no large studies show the importance of viable tumor in residual pulmonary nodules, in a recent analysis of 29 children with metastatic hepatoblastoma studied through the COG, 5 of 8 patients who had pulmonary nodule resections after initial chemotherapy had viable tumor still present, with 2 of the 5 patients ultimately dying of disease [30].
Hepatoblastoma response assessment
The imaging technique used to assess response in hepatoblastoma should favor the modality that best demonstrates the sites of disease established at baseline, although in most instances CT and MRI have comparable sensitivity and specificity for detecting disease. Because children often present semi-acutely, it is common for a CT to be obtained initially, followed by a transition to MRI for further imaging and response assessment. Many clinical protocols are now reporting a POSTTEXT (post treatment extent of disease) stage following initial chemotherapy, using the same grading scheme as with PRETEXT. This is particularly important for children who have PRETEXT 4 tumors at diagnosis, but after chemotherapy have a reduction in tumor size or extent and are judged to have PRETEXT 3 disease or less, increasing the likelihood of surgical success [26]. As at diagnosis, MRI with hepatocellular contrast agents is particularly effective in characterizing hepatic lesions and determining eligibility for surgical resection (Fig. 4). MRI with hepatocellular agents is also effective at characterizing the biliary tree relative to the liver disease, which might be particularly important in children with large central lesions. Some surgeons prefer a multi-phase contrast-enhanced CT as part of the post-chemotherapy preoperative assessment to establish the relationship of intrahepatic tumor to the hepatic vasculature. For children who experience a fall in serum AFP following induction chemotherapy and a significant decrease in tumor size and burden of metastatic disease, the primary role of imaging is to guide surgical management. No studies have shown the predictive value of change in tumor size and outcome.
Imaging predictors of outcome that are important in hepatoblastoma include high disease stage at diagnosis (PRETEXT 3–4) and the presence of residual disease after induction chemotherapy. The ability to achieve complete surgical resection is an independent predictor of outcome, as is the presence of metastatic disease at diagnosis. The response of metastatic disease to induction chemotherapy has also been shown to correlate with outcome, and any imaging assessment of response must include evaluation of suspected or confirmed sites of metastatic disease (Fig. 4).
In summary, the evaluation of hepatoblastoma is focused largely on establishing up-front surgical resectability, as well as guiding surgical management following induction chemotherapy and in children who do not have suitable initial responses, identifying the subset who are eligible for orthotopic liver transplant.
Wilms tumor
Wilms tumor is the most common primary pediatric renal tumor, making up approximately 80% of pediatric renal masses. As with the other tumors discussed in this review, Wilms tumor occurs mainly in young children, with the peak age at diagnosis of 3–4 years. Bilateral disease is seen in approximately 5% of patients and Wilms tumor is associated with genitourinary abnormalities and other syndromes such as Beckwith–Wiedemann syndrome and Denys–Drash syndrome [31]. The presenting symptom is typically a painless, palpable abdominal mass, and this is seen in approximately 80% of the patients.
Wilms tumor staging
The primary aim of Wilms tumor staging is directed at establishing the local stage of tumor, the presence of locoregional tumor extension, either secondary to local tumor rupture or regional lymph node involvement, the presence of bilateral disease, and the presence of metastatic disease (Fig. 5). The most common sites of metastatic disease are to the liver and lung, and children with hematogenously spread metastases are considered to have stage IV disease, independent of local tumor stage. Calcifications of either the primary tumor or lung metastases are unusual. It is important to establish the presence of vascular invasion into the renal vein or inferior vena cava (IVC), both of which can impact surgical management (Fig. 5). In a study evaluating the utility of either CT or MRI as compared to ultrasound for staging Wilms tumors, in more the half of the patients CT or MRI contributed additional information that impacted local staging, indicating that reliance solely on ultrasound for the initial characterization of Wilms tumor results in the under-staging of a significant number of children [32]. This is particularly important in the USA, where most children with suspected Wilms tumor undergo immediate nephrectomy without any preoperative chemotherapy [33], risking possible under-staging when evaluated by ultrasound alone. Both MRI and CT have similar diagnostic performance for detecting regional lymph node involvement and capsular penetration [34], whereas CT, despite having moderate specificity, has been found to have relatively low sensitivity (54–77%) in detecting preoperative tumor rupture [35], with the presence of extracapsular fluid/ascites beyond the cul-de-sac being most predictive of rupture [35]. In the study by Servaes et al. [34] examining the diagnostic performance of CT and MRI for renal tumor staging, only 2/82 patients in their cohort had confirmed preoperative tumor rupture, limiting the comparison of modalities for detecting rupture. A larger formal comparison of CT and MRI in this setting has not been performed. MRI has been shown to be superior for detecting bilateral disease, although newer-generation CT scanners with multiple detector rows, ultrafast acquisition parameters and iterative reconstruction techniques have dramatically improved image quality and high sensitivities for lesion detection.
MRI in Wilms tumor. The patient is a 4-year-old boy with left renal mass imaged by CT (a, b) and MRI (c–f), including axial fast spin-echo T2-weighted (c, e) and axial post-contrast VIBE (d, f) MR imaging. Both CT and MRI show an exophytic left renal mass with a thin rim of surrounding renal cortex. MRI (d) better shows the disruption of the renal capsule by the mass (*) and better delineates thrombus in the left renal vein (e, f; arrows), which was difficult to distinguish from contrast mixing artifact by CT (b, arrow). Lung nodules are better characterized by CT (a), although they are also evident on MRI (c). The patient underwent preoperative chemotherapy, followed by left nephrectomy, with subsequent whole-lung and left nephrectomy bed radiation. VIBE volumetric interpolated breath-hold examination
The use of CT to identify pulmonary metastases is now considered standard of care, although a rigorous comparison of the relative sensitivities of MRI versus CT in detecting small pulmonary nodules in children with Wilms tumor has not been performed. The importance of pulmonary metastases identified only by CT and not detectable by radiographs (“CT-only nodules”) has been controversial, particularly in children with favorable-histology tumors who then receive augmented chemotherapy and whole-lung radiation. In a study of 417 people with favorable-histology Wilms tumor and isolated lung metastases, Grundy et al. [36] reported that people with these so-called CT-only lung lesions have improved event-free survival, but no change in overall survival, despite additional chemotherapy and whole-lung radiation. Both European and U.S. cooperative groups, in an effort to reduce lung radiation in select children with stage IV disease, now incorporate a rigorous radiology central review of chest CT scans obtained at diagnosis [37]. While CT remains imperfect, with many small nodules detected by CT showing no evidence of malignancy upon biopsy/resection, the central review provides an opportunity to standardize the definitions of metastatic disease and improve management of pulmonary nodules.
Wilms tumor response assessment
In Wilms tumor characterizations of the primary tumor in response to chemotherapy have largely been based on European studies. In the USA, most children with Wilms tumor undergo up-front surgical resection of the primary tumor. Certain children undergo preoperative chemotherapy, including those with extension of tumor thrombus into the IVC above the level of the hepatic veins, tumor involving adjacent organs for which up-front nephrectomy would also necessitate resection of the involved adjacent organ, tumors for which complete resection is considered unlikely or for which surgical resection would pose significant morbidity, and children with bilateral disease. In contrast, European studies employ a regimen of induction chemotherapy to reduce tumor size and establish response, followed by surgery.
Based mainly on the European experience, we have learned that MRI is more sensitive for establishing the presence of bilateral disease and is the preferred methodology for response assessment [38]. This is a result of both the concerns about radiation and the improved sensitivity of MRI for detecting small bilateral renal lesions. In response to treatment, MRI has the potential to differentiate active nephrogenic rests and Wilms tumor, both of which often enhance with contrast agents and remain bright on T2-weighted and short tau inversion recovery (STIR) sequences, from inactive nephrogenic rests and treated tumor, which correspondingly show decreased enhancement and signal intensity on fluid-sensitive sequences [39]. MRI also allows for the use of diffusion-weighted imaging, which has improved sensitivity for lesion detection and provides additional specificity for lesion characterization [40]. Changes in diffusivity have also been shown to correlate with histopathology following chemotherapy, although these methodologies have not been incorporated into routine practice or used in multi-center trials to make response-based treatment decisions [41]. After primary tumor resection, follow-up imaging is also directed at ongoing assessment of the contralateral kidney because the presence of nephrogenic rests predisposes the remaining kidney to the development of Wilms tumor.
Pulmonary nodules identified at diagnosis are re-evaluated following either surgery or chemotherapy, and depending on other risk factors and the particular study protocol, patients might undergo surgical biopsy for confirmation of metastatic disease if residual nodules are present, or be randomized to respective treatment arms based on central review of the imaging data. The use of functional imaging techniques such as 18F-FDG PET/CT in the management of Wilms tumor is limited by renal uptake and excretion of the 18F-FDG radiotracer into the renal collecting system. In one small study of 12 children treated with neoadjuvant chemotherapy and surgery, changes in maximum standardized uptake value in the primary tumor correlated with histological response established at the time of surgery [42]. Other studies have suggested a role in managing relapsed patients [43], but the more widespread use of FDG PET/CT in the evaluation of Wilms tumor has not been adopted into practice.
In summary, both CT and MRI are essential in the initial staging of patients with Wilms tumor. MRI is the preferred method for identifying small contralateral lesions, assessing response to therapy, and post-surgical follow-up imaging. Current risk stratification schemes are based on stage at diagnosis and histological features such as loss of chromosomal heterozygosity, although response-based treatment algorithms that incorporate imaging criteria are being studied, particularly in children with favorable histological features in whom reduction in treatment intensity is being considered.
Surveillance imaging
Following completion of up-front chemotherapy, surgical resection and postsurgical radiation/chemotherapy, routine imaging surveillance becomes an important consideration. By definition, imaging surveillance is performed after all therapy is completed, at which time the child is considered to be free of disease or has stable residual abnormalities, some of which could be treatment-related. Many studies have pointed to concerns related to routine surveillance imaging, including time, cost, repeat exposure to radiation and contrast agents, as well as cumulative anesthesia risks [44]. These studies have also pointed to data showing little improvement in overall survival rates between children whose relapse is detected by imaging and those whose relapse is detected by clinical suspicion.
In one study of 186 pediatric solid tumor patients with 1 year and 5 years of follow-up, 1,727 imaging tests were required to detect 11 recurrences [45]. While the majority of these were plain radiographs, a significant number of ultrasounds, CTs, MRIs and nuclear medicine examinations were performed. A separate study of pediatric Hodgkin lymphoma showed that after 8 years of follow-up, the primary predictor of outcome in those patients who relapsed was the time to relapse, not how the relapse was detected [46]. Children whose relapses occurred within the first 12 months, presumably reflecting more aggressive disease, had worse outcome as compared to those whose relapses were detected more than 12 months after completion of chemotherapy, independent of whether the relapse was detected by imaging, symptoms or a combination of findings.
Interestingly, for children with hepatoblastoma in whom AFP levels can be monitored, routine imaging surveillance plays little, if any, role in detecting disease recurrence. In a study of 26 children, 5 relapses were identified [47]. AFP elevations occurred in all five children and no recurrence was identified by imaging prior to an elevation in AFP, indicating that serologic markers, when present, might be the most effective means of monitoring children after they complete therapy.
Children with neuroblastoma represent a population of patients who undergo extensive imaging both prior to and during therapy, as well as following completion of therapy. Two recent studies have shown that most relapses are detected by symptoms, physical exam or MIBG scanning [48], and that children who present with non-thoracic neuroblastoma do not benefit from any routine follow-up chest surveillance [49]. Recently completed neuroblastoma protocols from the Children’s Oncology Group (ANBL0532, ANBL1232 and ANBL15131) specify off-therapy monitoring recommendations that include a significant number of CT or MRI scans, as well as MIBG scans, for up to 5 years after therapy completion, although there are no data to indicate that this aggressive neuroblastoma surveillance imaging regimen is associated with improved outcome. Indeed, one might consider reducing surveillance imaging in non-high-risk neuroblastoma patients for whom outcome is generally good. It is not clear whether risk of repeat MRI with anesthesia and gadolinium-based contrast agents is greater than the risk of CT in the surveillance protocols, and an additional consideration would be to utilize MIBG scintigraphy alone for surveillance imaging, reserving CT and MRI for abnormalities detected by MIBG. All of these represent fruitful areas for future investigation in an effort to reduce unnecessary surveillance imaging in neuroblastoma.
Similarly, for Wilms tumor a fairly intensive off-therapy monitoring regimen is included in most clinical protocols. Despite this, no distinction has been made between low-risk, low-stage patients, standard-risk patients, and relapsed low-risk stage 1 patients, all of whom have off-therapy monitoring recommendations of up to 7 MRIs and chest CTs for the 5-year period of off-treatment surveillance. A recently submitted retrospective study from the Children’s Oncology Group Wilms tumor committee that is currently under review (E. Mullen, personal communication) recommends a reduction in the surveillance imaging approach; however, this has not been adopted in current treatment protocols.
Functional imaging techniques
As noted, functional imaging by MIBG is an essential component of neuroblastoma staging and response assessment. 18F-FDG PET/CT is reserved for those few children who are MIBG-negative. There is little role for functional imaging by FDG PET in the routine staging or response assessment of children with either hepatoblastoma or Wilms tumor. With Wilms tumor in particular, the excretion of the FDG tracer into the urinary collecting system limits primary renal tumor assessment, although functional imaging might be considered for sites of extrarenal spread or metastatic disease.
Neuroblastoma in particular has also received attention using both targeted therapeutic agents and newer functional imaging agents. A small subset of neuroblastomas is positive for the anaplastic large cell lymphoma kinase (ALK). These children have been shown to respond to the targeted agent crizotinib, and an important aspect of their response assessment has been use of functional imaging, with functional imaging by 123I-MIBG showing a dramatic anti-tumor response to the targeted therapeutic agent; however measurable decreases in tumor size did not occur [50]. Other new receptor-targeted PET agents in neuroblastoma include agents directed at the type 2 somatostatin receptors (SSTR) and the norepinephrine receptor transport (NET) protein. These new PET imaging agents are in early phases of clinical testing and could improve sensitivity for lesion detection and offer quantitative measures of tumor response, and include 124I-MIBG, 18F-meta-fluorobenzylguanidine (MFBG) [51], 68Ga-dotatate [52], 18F-fluorodopa [53] and 64Cu-sartate. In addition to providing PET-based radiopharmaceuticals for improved lesion detection, characterization and response assessment, some of these diagnostic agents also have corresponding therapeutic radionuclide constructs. These so-called theranostic agents include 64Cu/67Cu, 123I/131I, and 68Ga/177Lu. In each case, the diagnostic radiopharmaceutical is used to characterize the tumor and establish binding avidity, followed by targeted radiotherapy with the similarly constructed therapeutic radionuclide.
Other pediatric malignancies
The principles outlined here present a systematic approach for staging, response assessment and disease surveillance in the most common abdominal malignancies. The choice of imaging modality is largely dependent on the type of disease being surveyed; however the data show an increasing role for MRI in characterizing and assessing response in the most common tumors. For other tumors such as the malignant sarcomas and lymphomas similar principles apply. In many instances the use of functional imaging techniques has been incorporated into ongoing clinical trials, although there are few instances where functional imaging alone is being used to dictate treatment decisions. Increasingly, imaging at the time of diagnosis is being incorporated into risk stratification algorithms in an effort to direct the most aggressive therapies to children at greatest risk of a poor outcome, while reducing treatment intensity for those predicted to have a more favorable course of disease.
Summary
Pediatric solid tumors require a comprehensive imaging approach at the time of staging and for response assessment and disease surveillance. The choice of imaging approach initially is aimed at lesion characterization and staging. The use of functional imaging techniques is largely reserved for tumors such as neuroblastoma, where a combination of anatomical and functional imaging is essential for staging and risk stratification. Although clinicians rely mainly on anatomical imaging techniques for staging and assessing response in hepatoblastoma and Wilms tumor, they are beginning to incorporate functional imaging such as FDG PET into routine staging and response assessment for many other pediatric tumors — in particular the sarcomas. Multiple newer agents and techniques are on the horizon, but for the most common pediatric solid tumors, the assessment is still focused largely on establishing the extent of the child’s disease, determining the likelihood of achieving a complete surgical resection, identifying the presence of metastatic disease, and determining the risk factors that are most predictive of outcome.
References
Irwin MS, Park JR (2015) Neuroblastoma: paradigm for precision medicine. Pediatr Clin N Am 62:225–256
Brodeur GM, Pritchard J, Berthold F et al (1993) Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 11:1466–1477
Brisse HJ, McCarville MB, Granata C et al (2011) Guidelines for imaging and staging of neuroblastic tumors: consensus report from the international neuroblastoma risk group project. Radiology 261:243–257
Monclair T, Brodeur GM, Ambros PF et al (2009) The international neuroblastoma risk group (INRG) staging system: an INRG task force report. J Clin Oncol 27:298–303
Cohn SL, Pearson AD, London WB et al (2009) The international neuroblastoma risk group (INRG) classification system: an INRG task force report. J Clin Oncol 27:289–297
Callahan MJ, MacDougall RD, Bixby SD et al (2018) Ionizing radiation from computed tomography versus anesthesia for magnetic resonance imaging in infants and children: patient safety considerations. Pediatr Radiol 48:21–30
Dumba M, Jawad N, McHugh K (2015) Neuroblastoma and nephroblastoma: a radiological review. Cancer Imaging 15:5
Sharp SE, Trout AT, Weiss BD et al (2016) MIBG in neuroblastoma diagnostic imaging and therapy. Radiographics 36:258–278
DuBois SG, Mody R, Naranjo A et al (2017) MIBG avidity correlates with clinical features, tumor biology, and outcomes in neuroblastoma: a report from the Children's Oncology Group. Pediatr Blood Cancer 64. https://doi.org/10.1002/pbc.26545
Nadel HR (2014) SPECT/CT in pediatric patient management. Eur J Nucl Med Molec Imaging 41:S104–S114
Gauguet JM, Pace-Emerson T, Grant FD et al (2017) Evaluation of the utility of (99m) Tc-MDP bone scintigraphy versus MIBG scintigraphy and cross-sectional imaging for staging patients with neuroblastoma. Pediatr Blood Cancer 64. https://doi.org/10.1002/pbc.26601
Papathanasiou ND, Gaze MN, Sullivan K et al (2011) 18F-FDG PET/CT and 123I-metaiodobenzylguanidine imaging in high-risk neuroblastoma: diagnostic comparison and survival analysis. J Nucl Med 52:519–525
Sharp SE, Shulkin BL, Gelfand MJ et al (2009) 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J Nucl Med 50:1237–1243
Park JR, Bagatell R, Cohn SL et al (2017) Revisions to the international neuroblastoma response criteria: a consensus statement from the National Cancer Institute clinical trials planning meeting. J Clin Oncol 35:2580–2587
Trout AT, Towbin AJ, Klingbeil L et al (2017) Single and multidimensional measurements underestimate neuroblastoma response to therapy. Pediatr Blood Cancer 64:18–24
Bagatell R, McHugh K, Naranjo A et al (2016) Assessment of primary site response in children with high-risk neuroblastoma: an international multicenter study. J Clin Oncol 34:740–746
Schmidt M, Simon T, Hero B et al (2008) The prognostic impact of functional imaging with (123)I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: results of the German neuroblastoma trial NB97. Eur J Cancer 44:1552–1558
Decarolis B, Schneider C, Hero B et al (2013) Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: results of the cologne interscore comparison study. J Clin Oncol 31:944–951
Ladenstein R, Lambert B, Potschger U et al (2018) Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: the SIOPEN/HR-NBL1 and COG-A3973 trials. Eur J Nucl Med Molec Imaging 45:292–305
Yanik GA, Parisi MT, Shulkin BL et al (2013) Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children's Oncology Group. J Nucl Med 54:541–548
Fischer J, Pohl A, Volland R et al (2017) Complete surgical resection improves outcome in INRG high-risk patients with localized neuroblastoma older than 18 months. BMC Cancer 17:520.
von Allmen D, Davidoff AM, London WB et al (2017) Impact of extent of resection on local control and survival in patients from the COG A3973 study with high-risk neuroblastoma. J Clin Oncol 35:208–216
Roebuck DJ, Aronson D, Clapuyt P et al (2007) 2005 PRETEXT: a revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol 37:123–132
Shelmerdine SC, Roebuck DJ, Towbin AJ et al (2016) MRI of paediatric liver tumours: how we review and report. Cancer Imaging 16:21
Emre S, Umman V, Rodriguez-Davalos M (2012) Current concepts in pediatric liver tumors. Pediatr Transplant 16:549–563
Aronson DC, Meyers RL (2016) Malignant tumors of the liver in children. Semin Pediatr Surg 25:265–275
Meyers AB, Towbin AJ, Geller JI et al (2012) Hepatoblastoma imaging with gadoxetate disodium-enhanced MRI — typical, atypical, pre- and post-treatment evaluation. Pediatr Radiol 42:859–866
Cistaro A, Treglia G, Pagano M et al (2013) A comparison between (18F)F-FDG PET/CT imaging and biological and radiological findings in restaging of hepatoblastoma patients. Biomed Res Int 2013:709037. https://doi.org/10.1155/2013/709037
Meyers RL, Tiao G, de Ville de Goyet J et al (2014) Hepatoblastoma state of the art: pre-treatment extent of disease, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr 26:29–36
O'Neill AF, Towbin AJ, Krailo MD et al (2017) Characterization of pulmonary metastases in children with hepatoblastoma treated on children's oncology group protocol AHEP0731 (the treatment of children with all stages of hepatoblastoma): a report from the Children's Oncology Group. J Clin Oncol 35:3465–3473
Millar AJW, Cox S, Davidson A (2017) Management of bilateral Wilms tumours. Pediatr Surg Int 33:737–745
McDonald K, Duffy P, Chowdhury T et al (2013) Added value of abdominalcross-sectional imaging (CT or MRI) in staging of Wilms' tumours. Clin Radiol 68:16–20
Fernandez CV, Mullen EA, Chi YY et al (2018) Outcome and prognostic factors in stage III favorable-histology Wilms tumor: a report from the Children's Oncology Group study AREN0532. J Clin Oncol 36:254–261
Servaes S, Khanna G, Naranjo A et al (2015) Comparison of diagnostic performance of CT and MRI for abdominal staging of pediatric renal tumors: a report from the Children's Oncology Group. Pediatr Radiol 45:166–172
Khanna G, Naranjo A, Hoffer F et al (2013) Detection of preoperative Wilms tumor rupture with CT: a report from the Children's Oncology Group. Radiology 266:610–617
Grundy PE, Green DM, Dirks AC et al (2012) Clinical significance of pulmonary nodules detected by CT and not CXR in patients treated for favorable histology Wilms tumor on national Wilms tumor studies-4 and -5: a report from the Children's Oncology Group. Pediatr Blood Cancer 59:631–635
Dome JS, Graf N, Geller JI et al (2015) Advances in Wilms tumor treatment and biology: progress through international collaboration. J Clin Oncol 33:2999–3007
McHugh K (2007) Renal and adrenal tumours in children. Cancer Imaging 7:41–51
Irtan S, Ehrlich PF, Pritchard-Jones K (2016) Wilms tumor: "state-of-the-art" update, 2016. Semin Pediatr Surg 25:250–256
Littooij AS, Humphries PD, Olsen OE (2015) Intra- and interobserver variability of whole-tumour apparent diffusion coefficient measurements in nephroblastoma: a pilot study. Pediatr Radiol 45:1651–1660
Littooij AS, Nikkels PG, Hulsbergen-van de Kaa CA et al (2017) Apparent diffusion coefficient as it relates to histopathology findings in post-chemotherapy nephroblastoma: a feasibility study. Pediatr Radiol 47:1608–1614
Qin Z, Tang Y, Wang H et al (2015) Use of 18F-FDG-PET-CT for assessment of response to neoadjuvant chemotherapy in children with Wilms tumor. J Pediatr Hematol Oncol 37:396–401
Moinul Hossain AK, Shulkin BL, Gelfand MJ et al (2010) FDG positron emission tomography/computed tomography studies of Wilms' tumor. Eur J Nucl Med Molec Imaging 37:1300–1308
Kaste SC (2011) Oncological imaging: tumor surveillance in children. Pediatr Radiol 41:505–508
Howell L, Mensah A, Brennan B et al (2005) Detection of recurrence in childhood solid tumors. Cancer 103:1274–1279
Voss SD, Chen L, Constine LS et al (2012) Surveillance computed tomography imaging and detection of relapse in intermediate- and advanced-stage pediatric Hodgkin's lymphoma: a report from the Children's Oncology Group. J Clin Oncol 30:2635–2640
Rojas Y, Guillerman RP, Zhang W et al (2014) Relapse surveillance in AFP-positive hepatoblastoma: re-evaluating the role of imaging. Pediatr Radiol 44:1275–1280
Owens C, Li BK, Thomas KE et al (2016) Surveillance imaging and radiation exposure in the detection of relapsed neuroblastoma. Pediatr Blood Cancer 63:1786–1793
Federico SM, Brady SL, Pappo A et al (2015) The role of chest computed tomography (CT) as a surveillance tool in children with high-risk neuroblastoma. Pediatr Blood Cancer 62:976–981
Mosse YP, Voss SD, Lim MS et al (2017) Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a Children's Oncology Group study. J Clin Oncol 35:3215–3221
Pandit-Taskar N, Zanzonico P, Staton KD et al (2018) Biodistribution and dosimetry of (18)F-meta-fluorobenzylguanidine: a first-in-human PET/CT imaging study of patients with neuroendocrine malignancies. J Nucl Med 59:147–153
Kong G, Hofman MS, Murray WK et al (2016) Initial experience with gallium-68 DOTA-octreotate PET/CT and peptide receptor radionuclide therapy for pediatric patients with refractory metastatic neuroblastoma. J Pediatr Hematol Oncol 38:87–96
Piccardo A, Lopci E, Conte M et al (2012) Comparison of 18F-dopa PET/CT and 123I-MIBG scintigraphy in stage 3 and 4 neuroblastoma: a pilot study. Eur J Nucl Med Molec Imaging 39:57–71
Acknowledgments
The author is grateful to Annika T. Voss for the artwork in Fig. 3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Voss, S.D. Staging and following common pediatric malignancies: MRI versus CT versus functional imaging. Pediatr Radiol 48, 1324–1336 (2018). https://doi.org/10.1007/s00247-018-4162-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4162-4