Abstract
Sonography can be used in the management of pediatric soft-tissue vascular anomalies for diagnosing, for assessing lesion extent and for evaluating complications and response to therapy. The sonographic technique includes a combination of gray-scale imaging with color and spectral Doppler techniques. However the interpretation of the sonographic findings requires correlation with the clinical findings, some of which can be easily obtained at the time of scanning. This has to be combined with the use of appropriate nomenclature and the most updated classification in order to categorize these children into the appropriate management pathway. In this article, which is part 1 of a two-part series, the authors review the current classification of vascular anomalies, provide a clinical and a sonographic approach to these lesions, and review the most relevant clinical and sonographic features of vascular tumors including infantile and congenital hemangiomas, tufted angioma, kaposiform hemangioendothelioma, pyogenic granuloma, intramuscular capillary-type hemangioma and angiosarcoma.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vascular anomalies are a heterogeneous group of vascular tumors and vascular malformations for which there is a rapidly growing field of knowledge. Much has been learned in the last two decades with the growth of multidisciplinary teams, treatment options and outcomes research and the growing understanding of the genetic basis of vascular anomalies. As this knowledge has grown, technology has also allowed for the knowledge to be shared and compared much quicker, frequently in real time. There has been an evolution from a basic diagnostic checklist that might have included a few diagnoses such as hemangioma, arteriovenous malformation and “lymphangioma” to a new list that has not only modified the concepts of these diagnoses but has also added nearly a hundred diagnostic possibilities including complex combined vascular anomalies, among others.
Epidemiologically, with the overall rarity of many of these vascular anomalies there is a lack of accurate incidence and prevalence data for most. All of the current epidemiologic data available are challenged by historically inconsistent diagnostic criteria being used along with inaccurate nomenclature. The importance of knowing the most common of these entities is essential to radiologists and other clinicians who see these patients. The proper classification and diagnosis of these entities directly leads the patients to specific expanding treatment pathways. The options of conservative management and surgery as the only choices have long been replaced at specialized vascular anomaly centers with medical therapies including sirolimus and propranolol as well as image-guided approaches including cryotherapy, endovenous laser ablation, sclerotherapy and embolization, with surgical resection usually reserved as a second-line therapy today.
Sonography is often the ideal initial imaging evaluation, and in skilled hands it provides a great amount of helpful information that aids in the correct diagnosis when combined with the clinical history and physical exam. Sonography also screens as to who needs further advanced imaging or histologic correlation. The inherent clinical interaction with a patient by the sonographer and radiologist provides a key clinical opportunity to note valuable visual features and clinical history in a minute or less, which can aid in the most helpful sonographic impression possible and is the purpose of this manuscript.
In part 1 of this series, we review the classification proposed by the International Society for the Study of Vascular Anomalies (ISSVA), focusing on entities that are more pertinent to pediatric radiologists and more frequently evaluated with ultrasound. These are listed in Table 1. We also propose a clinical and sonographic approach to these lesions and highlight the most relevant clinical and sonographic features of vascular tumors, which are summarized in Table 2. In part 2 of this series we review the most relevant clinical and sonographic features of vascular malformations, including complex combined vascular malformations that are often associated with syndromes.
International Society for the Study of Vascular Anomalies classification
The ISSVA classification system has helped bridge the nomenclature divide and has helped different specialties and specialists worldwide to speak the same language when discussing vascular tumors and malformations. The implementation of this classification set the groundwork for accurate diagnosis and improved treatment that has evolved into the development of a subspecialty specifically dedicated to the management of patients with vascular anomalies and in the creation of specialized centers. All of this together has allowed the start of outcome-based clinical research in this field. The ISSVA classification system was last revised in April 2014, expanding upon the growing body of knowledge of these rare disorders and also incorporating the increasing knowledge of the genetic basis for these diseases, including the genetic mutations associated with many of them [1]. The complete classification can be accessed at www.issva.org. While the basic division of vascular tumors and vascular malformations remains unchanged, both lists have significantly grown in size as the understanding of these entities has grown and specific subgroups with distinct phenotypes and genotypes have been discovered and further characterized. The focus still holds true, however, that the accurate diagnostic evaluation that leads a patient down the current tumor or malformation pathway toward a given diagnosis still characterizes its underlying cellular origin, protein expression and clinical course, just as Mulliken and Glowacki [2] first proposed 35 years ago.
Clinical approach
Understanding vascular anomalies and the current nomenclature, classification and treatment options allows radiologists to give the most clinically relevant information in their readings to help the referring physician or the referring vascular anomaly team to decide the best diagnosis and treatment plan. Additionally, arming oneself with a few clinical “Aunt Minnie” appearances of these lesions is also helpful to gain the most information during the short interaction between the radiologist and the patient at the time of the ultrasound examination.
A few quick questions that can be very helpful for a sonographer or radiologist to ask include the following: When was the lesion first seen or noticed? Was the lesion present at birth? Is the lesion growing and, if it is, is it in proportion to the patient or very quickly and out of proportion? Is there a family history of similar lesions? When did the lesion start to hurt? While there are many more possible questions, these are very helpful in problem-solving for both the simple more common lesions and for complex rarer lesions and should be part of the diagnostic toolkit of general and pediatric radiologists and of dedicated vascular anomaly specialists alike.
The observation of the skin before turning down the lights and applying the ultrasound gel on the skin allows for an invaluable clinical glance at the lesion before the ultrasound machine and transducer take over. Illustrative examples of the clinical presentation of some vascular anomalies are included in both parts of this series.
The radiologist should attempt to obtain information by using sonography that is helpful in the clinical decision-making process. This includes the following: the anatomical spaces involved by the lesion, to the extent possible; the type of vessels affected; the presence or absence of intralesional clotting and age of clot; presence of a fatty hamartomatous component to the lesion; and whether there is a single well-defined lesion or there are multifocal lesions throughout an anatomical region.
Although there is much more to the clinical practice of vascular anomalies, capturing the important decision-making points for these children allows radiologists to accurately intervene in those points and to understand what portions of the sonographic examination are key aspects of the decision-making process. Additionally, having the clinical knowledge base of some of the classic appearances helps for a more accurate sonographic impression and also improves efficiency in that a skilled provider will know exactly what needs to be characterized on ultrasound and what to look for before ever laying down the probe.
Sonographic approach
Sonography is generally the first imaging modality used in the evaluation of soft-tissue masses and suspected soft-tissue vascular anomalies. In addition to the fact that sonography is easily accessible, costs little relative to other modalities, does not require sedation and lacks radiation, sonography can be used to easily assess these lesions because they are often small and relatively superficial. However in some cases the sonographic findings might not be specific and differentiation between types of vascular anomalies might not be possible or the findings might overlap with those of other benign or malignant soft-tissue masses [3, 4]. Furthermore, in some instances the extent of the lesions might not be accurately assessed with ultrasound because of their large size or extension into areas that are difficult to penetrate with the US beam, such as the cranial cavity or the joint space. In these situations, MR imaging might be indicated. In some cases, particularly when dealing with small superficial lesions, excisional biopsy might follow an inconclusive sonogram.
High-frequency linear-array transducers are generally used because they are well suited to the size and superficial location of most vascular anomalies. These transducers provide high spatial resolution that allows for the identification of the different soft-tissue planes, which can be helpful in the diagnosis and management of these lesions. Use of stand-off pads and even submersion of hands and feet in water baths can facilitate visualization of very superficial lesions [5]. For larger and deeper lesions, the use of low-frequency curved-array transducers might be necessary.
The sonographic diagnosis relies on a combination of gray-scale, spectral Doppler and color Doppler sonography. Gray-scale features to be assessed in vascular anomalies include echogenicity and internal architecture (including recognition of solid and cystic components), margins, visible vessels, clots and intralesional calcifications. Echogenicity should be compared to adjacent subcutaneous fat or muscle. Spectral Doppler analysis includes type of waveform (arterial or venous), magnitude of velocities, resistive index and phasicity. Color Doppler is used to determine presence or absence of vascularity. When flow is present, the degree of vascularity can be estimated by counting the number of vessels (color pixels) per square centimeter in a region of interest within the area of greatest intralesional vascularity. Dubois et al. [6] categorized lesions as high vascular density if there were more than five vessels per square centimeter, moderate vascular density if there were two to four vessels per square centimeter, and low vascular density if there were fewer than two vessels per square centimeter. This approach, however, requires the routine use of standardized color Doppler settings to make all exams comparable. In practice, one does not have to meticulously count the vessels within a lesion, but rather it is often sufficient to do a visual estimation of the vascular density and also to do a comparison with the vascular density of the adjacent soft tissues, always paying attention to the Doppler parameters used.
Compressibility of the lesion with the transducer is another feature that can be evaluated with sonography. It has been reported that neoplastic lesions are not compressible and therefore compressibility can be a helpful finding in the differentiation of vascular anomalies, particularly venous malformations, from other causes of soft-tissue masses [7].
Vascular tumors
Infantile hemangioma
Infantile hemangioma is the most common vascular tumor, with a reported prevalence of 5–10% in Caucasian infants by the age of 1 year [8]. Most are small and do not need to be imaged. However when these lesions are deeper or there is uncertain degree of anatomical involvement, sonography is commonly the first modality requested. It is important to note that these masses are not present at birth. However, there might be a flat, pink, prodromal lesion present, which turns red and grows into a mass during the proliferative phase of the hemangioma. This bright strawberry red color of the skin is quite characteristic of infantile hemangiomas (Fig. 1). The proliferation usually starts in the first weeks to months after birth, reaching its maximal growth at about a year and followed by a slow involution [9]. Cutaneous bleeding and ulceration are the most common complications that require treatment, with reported occurrence in 15% of focal lesions and in up to 38% of diffuse head and neck lesions [10]. The opportunity in the interaction with the child during the ultrasound examination comes from identifying lesions that have associations with underlying developmental disorders or deep hemangioma involvement given their anatomical location (Fig. 2). These syndromes are referred to as PHACE, which is the association of posterior fossa brain malformations, large infantile hemangiomas of the face, neck or scalp, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities [11], and LUMBAR, which is the association of lower body infantile hemangiomas with other regional anomalies including urogenital anomalies, ulceration, myelopathy, bony deformities, anorectal malformations, arterial anomalies and renal anomalies [12]. The recognition of these characteristic associations dictates the need for further imaging.
Association between infantile hemangioma and structural malformations. a Clinical image in a 3-month-old boy who presented with a facial hemangioma for evaluation with sonography prior to starting propranolol therapy. He was found to have a frontonasal segmental hemangioma. Subsequent MR brain imaging findings were consistent with PHACE syndrome. Parental consent was obtained for publication of this photograph. b Clinical image of a 5-month-old girl who presented with a large hemangioma overlying the lumbosacral spine. Subsequent MR imaging findings of the lumbar spine were consistent with LUMBAR syndrome. Neither lesion in these two children was present at birth, but both had pink flat prodromal lesions. LUMBAR lower body infantile hemangiomas, urogenital anomalies, ulceration, myelopathy, bony deformities, anorectal malformations, arterial anomalies and renal anomalies, PHACE posterior fossa brain malformations, hemangiomas of the face, neck or scalp, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities
The sonographic appearance of infantile hemangioma depends as to whether it is examined during the proliferative phase or the involutive phase. In the proliferative phase, infantile hemangioma is a well circumscribed mass of variable echogenicity, often confined to the subcutaneous soft tissues. In a series by Paltiel et al. [3], 65% of infantile hemangiomas were hypoechoic in relation to adjacent normal soft tissues (Fig. 3), 19% were hyperechoic and 16% were of heterogeneous echogenicity. In our experience a higher proportion of lesions are hyperechoic in relation to adjacent subcutaneous fat (Fig. 4) or are of heterogeneous echogenicity (Fig. 5), which is in agreement with a more recent series describing a heterogeneous echogenicity in 42% of infantile hemangiomas [13]. Intralesional vessels are only occasionally visible on gray-scale imaging and calcifications are rare. In general, the gray-scale sonographic appearance is nonspecific [6]. The use of color Doppler substantially facilitates the diagnosis of infantile hemangioma by showing a characteristic pattern of high vascular density, and this prominent vascularity might also be noted in the adjacent soft tissues (Figs. 3, 4 and 5). Spectral Doppler analysis reveals the presence of both arterial and venous flow (Fig. 5). The arterial flow is typically of low resistance with relatively high velocities and usually without associated arteriovenous shunting [14]. Rogers et al. [15] described as a typical finding of infantile hemangioma the presence of well-defined lobules with large vessels in the septa separating the lobules rather than presence of vessels throughout the lesion (Fig. 6). In our experience, this appearance is rather uncommon, with increased vascularity more frequently noted diffusely throughout the lesion.
Infantile hemangioma in an 8-month-old girl who presented with a small red nodule in the chest wall that had been growing for the last 5–6 months. a Transverse sonogram shows a well-defined nodule (arrows) of irregular margins involving the dermis and the superficial aspect of the subcutaneous tissues. The nodule is hypoechoic compared with the adjacent soft tissues. b Longitudinal color Doppler sonogram shows high vascular density, characteristic of this type of lesion, with associated increased vascularity of the adjacent soft tissues
Infantile hemangioma in a 12-month-old boy who presented with a small nodule in the right shoulder noticed a few months prior. a Transverse sonogram shows a relatively well-defined hyperechoic nodule (arrows) confined to the subcutaneous soft tissues. b Transverse color Doppler sonogram shows high vascular density, characteristic of infantile hemangiomas
Infantile hemangioma in an 8-month-old girl who presented with an enlarging nodule in the interscapular region. a Transverse sonogram shows a well-defined subcutaneous nodule (arrows) with heterogeneous echogenicity containing hyperechoic and hypoechoic areas, and a central hyperechoic band (arrowheads) that likely represents fibro-fatty tissue. b Longitudinal color Doppler sonogram shows high vascular density, characteristic of infantile hemangiomas. c Spectral Doppler analysis shows the presence of low-resistance arterial flow within the hemangioma
Infantile hemangioma in a 4-month-old girl who presented with a mobile soft lump in the posterior aspect of the neck that was first noticed at 2–3 weeks of age. a Longitudinal sonogram shows a heterogeneous subcutaneous mass (arrows) with a multilobulated appearance owing to the presence of multiple hypoechoic nodules and hyperechoic tissue between the lobules and in the periphery of the lesion. b Longitudinal Color Doppler sonogram shows high vascular density, although the prominent vascularity is mainly confined to the hyperechoic tissue with less or absent flow noted in the hypoechoic areas. This girl underwent MR imaging (not shown), which confirmed the diagnosis of infantile hemangioma (courtesy Dr. Shema Hameed, Evelina London Children’s Hospital, London, UK)
Usually imaging of infantile hemangioma is not required in the involutive phase. During this stage, sonography shows progressive decrease in size as well as increase in echogenicity and decrease of vascular density, both as a result of fibro-fatty replacement [16]. Occasionally sonography is required if the lesion appears to stop involuting or shows increase in size. This can be caused by excessive fatty infiltration within the hemangioma. Sonography, in this case, shows the excess fatty tissue with similar echogenicity to adjacent subcutaneous fat and lack of significant vascularity (Fig. 7).
Involuted infantile hemangioma in a 10-year-old girl who had a history of typical infantile hemangioma but whose involution appeared to have stopped, with persistent and slowly enlarging palpable nodule in the left upper arm. Transverse color Doppler sonogram shows an ill-defined, raised nodule (box) of similar echogenicity to adjacent subcutaneous fat (F) and with only minimal vascularity. This represents exuberant fatty proliferation, which can happen occasionally during the involutive phase of infantile hemangiomas and might be confused with development of a new tumor
In hemangiomas that require medical treatment with propranolol, sonography has proved to be useful in assessing response by showing decrease in tumor volume and vascular density [17]. This is particularly useful in the management of lesions with deeper soft-tissue involvement that can be difficult to assess clinically.
Congenital hemangioma
Congenital hemangiomas are present at birth by definition. If not provided by the referring physician, this information should be obtained at the time of the initial questioning before performing the US examination because it facilitates differentiation from other causes of soft-tissue mass, especially from the more common infantile hemangioma. These lesions are biologically distinct in that they undergo their rapid growth phase in utero and, based on their behavior after birth, are defined as rapidly involuting congenital hemangioma (RICH) or non-involuting congenital hemangioma (NICH) [18, 19]. RICH shows regression before 14 months of age, in contrast to the slower involution seen in infantile hemangioma, which often takes years to be completed [18]. NICH does not show involution and grows commensurately with the child, resembling the natural history of vascular malformations [19], which is discussed in part 2 of this series. More recently, a third category called partially involuting congenital hemangioma (PICH) has been proposed [20]. PICH initially behaves as a RICH but the involution is aborted and the residual lesion then behaves as a NICH. When it is not possible to differentiate a congenital hemangioma from an infantile hemangioma by imaging or history, the two hemangioma types can be histologically distinguished by the presence or absence of a specific immunohistochemical stain, glucose transporter 1 (GLUT-1). Infantile hemangiomas stain positively for GLUT-1, whereas congenital hemangiomas stain negative for GLUT-1 [21].
Congenital hemangiomas show sonographic features that resemble those of infantile hemangioma, although some particular differences have been described [13, 15, 20]. Most congenital hemangiomas are of heterogeneous echogenicity, which is largely a result of visualization of intralesional vessels on gray-scale imaging [13] (Fig. 8). Intralesional calcifications are not common but are seen with a higher frequency as compared to infantile hemangiomas [13]. Color Doppler interrogation shows a high vascular density with both arterial and venous flow present [13, 20]. Interestingly, venous flow tends to be prominent and in fact many of the visible vessels on gray-scale imaging correspond to dysplastic veins that traverse the lesion and are larger centrally [13, 15] (Fig. 9). Arteriovenous microshunting can be detected, particularly in NICH. Venous thrombi, which are never seen in infantile hemangiomas, have also been described in congenital hemangiomas [22].
Rapidly involuting congenital hemangioma (RICH) in a 6-week-old boy who had antenatal diagnosis of a left arm mass. a Longitudinal sonogram shows a large heterogeneous subcutaneous mass (arrows) that compresses the muscles (M) in the posterior aspect of the upper arm. The lesion contains large visible vessels (arrowheads), a finding more commonly described in congenital hemangiomas and rarely seen in infantile hemangiomas. b Longitudinal color Doppler sonogram confirms the presence of multiple large vessels, showing the typical high vascular density of hemangiomas. The final diagnosis of RICH was only made months later on the basis of the clinical evolution and imaging findings
Non-involuting congenital hemangioma (NICH) in a 3-year-old boy with a right upper arm mass that had been growing slowly since birth. a Transverse sonogram shows a heterogeneous subcutaneous mass (arrows) with a large intralesional vessel (V). b Transverse color Doppler sonogram shows that the large vessel is a vein with slightly pulsatile flow, likely reflecting some arteriovenous microshunting. There are also multiple smaller vessels denoting the characteristic high vascular density of hemangiomas. The diagnosis of NICH was made on the basis of the clinical evolution and sonographic findings
Pyogenic granuloma (lobular capillary hemangioma)
The history of a quickly growing vascular skin lesion with bleeding in a patient older than 6 months of age is common for this lesion, which is also known as a lobular capillary hemangioma. Common locations include the head, neck and upper extremities [23]. Many times this lesion occurs at a site of previous trauma and is composed of friable lobulated tan to red tissue (Fig. 10) that is easily traumatized and bleeds. Most pyogenic granulomas are not referred for imaging but as with other fast-flow lesions, it is recommended that an ultrasound department have a bleeding plan in place with hemostasis pads if they decide to image these lesions. The treatment for pyogenic granuloma is usually cauterization or surgery, depending on size.
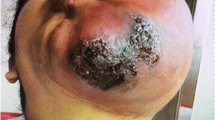
Clinical image of pyogenic granuloma in a 12-year-old boy who presented with a nodular lesion in the lower lip that quickly grew over the course of a month after questionable minor trauma. On exam, he was found to have a friable polypoid vascular lesion (arrow), which bled after only minimal manipulation. This was treated with a single carbon dioxide laser photoevaporation treatment
There is little information on the sonographic appearance of pyogenic granuloma because most lesions are diagnosed clinically. The more polypoid superficial lesions tend to be well-circumscribed, but those that are confined to the subcutaneous tissues often have less defined margins [23, 24]. Independent of their location, they tend to be small in size, hypoechoic compared to subcutaneous fat and with high vascular density on color Doppler interrogation [23, 24] (Fig. 11). Occasionally pyogenic granulomas show patchy hyperechoic areas and a prominent feeder vessel on color Doppler sonography [25].
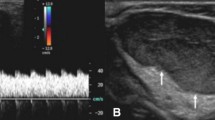
Pyogenic granuloma in a 9-year-old boy who presented with a slowly growing nodule in the index finger. a Longitudinal sonogram shows a well-defined, slightly heterogeneous nodule (arrows) in the subcutaneous tissues in the anterior aspect of the index finger in the region of the proximal interphalangeal joint, superficial to the flexor tendon. The mass is predominantly hypoechoic to the adjacent soft tissues. b Longitudinal color Doppler sonogram shows high vascular density within the nodule. Although the sonographic appearance can be compatible with a hemangioma, the clinical history does not support this diagnosis. Excisional biopsy was required to make the diagnosis of pyogenic granuloma. P phalanx, PP proximal phalanx
Tufted angioma and kaposiform hemangioendothelioma
Tufted angioma and kaposiform hemangioendothelioma are two closely related tumors that can be locally aggressive and can be associated with systemic complications, particularly the Kasabach–Merritt phenomenon, which is characterized by profound thrombocytopenia, sometimes with microangiopathic hemolytic anemia and secondary consumption of coagulation factors [26, 27]. The Kasabach–Merritt phenomenon is not seen with other entities and therefore its presence is indicative of one of these two tumors.
A growing red to purple plaque, sometimes tender, is the most common presentation of kaposiform hemangioendothelioma, usually in the first years of age and often at birth. Helpful observations can be made in the ultrasound suite that are suggestive of this diagnosis, including the associated hypertrichosis that is sometimes seen in this entity (Fig. 12) as well as serpiginous thin red surface lesions next to the mass, which have been found to be associated with extension of tumor through the associated dilated lymphatics [28, 29]. The treatment of these disorders now includes medical management and sometimes surgical resection [29].
Clinical image of kaposiform hemangioendothelioma in a 1-year-old boy who presented with a painful growing purple plaque and associated hypertrichosis (arrow) that were not present at birth. The diagnosis of kaposiform hemangioendothelioma was confirmed on histology. The boy had marked improvement with sirolimus therapy
On sonography, these two tumors tend to have heterogeneous echogenicity with ill-defined margins, making them difficult to differentiate from adjacent soft tissues [4, 22, 30, 31]. Tufted angiomas tend to be more superficial and thinner, not exceeding 1 cm in thickness and surrounded by hyperechoic subcutaneous fat [4]. Kaposiform hemangioendothelioma can be focal or diffuse and might be confined to subcutaneous fat, confined to muscle or involve all soft-tissue planes and even underlying bone [28] (Fig. 13). Kaposiform hemangioendothelioma tends to be larger, with more prominent vascularity than tufted angioma, and has also been reported to have frequent calcifications, which are not seen with tufted angioma. Dubois et al. [4] reported that most kaposiform hemangioendotheliomas show moderate vascular density on color Doppler interrogation, less than is seen with infantile hemangiomas. However, in our experience these tumors usually show high vascular density with visible vessels on gray-scale imaging (Fig. 13), which is also shown in some cases illustrated in the literature [30].
Kaposiform hemangioendothelioma in a 2-day-old boy who had antenatal diagnosis of a left thigh mass and presented at birth with a large red-purple tumor that extended from the groin to the knee. There were no features of Kasabach–Merritt phenomenon. a Transverse sonogram shows a large mass (cursors) that is predominantly subcutaneous but shows extension into the underlying musculature (arrows). The mass is heterogeneous, more hypoechoic in its superficial component and more hyperechoic in its deeper aspect. Some vessels are seen on this gray-scale image within the mass (arrowheads). F femur. b Transverse color Doppler sonogram confirms the high vascular density of the lesion. The diagnosis of kaposiform hemangioendothelioma was based on the combination of clinical and imaging findings including subsequent MR imaging. The lesion continued to grow after birth, requiring treatment with vincristine and sirolimus
Intramuscular capillary-type hemangioma
This is a rare tumor that should not be confused with the more common intramuscular venous malformation. Unfortunately, many cases described in the literature as intramuscular hemangiomas are indeed venous malformations. However, intramuscular capillary-type hemangioma is a well-defined entity that can present at any age, does not show involution and often presents clinically as a non-tender soft-tissue mass [32].
On sonography, as implied by its name, this type of hemangioma is confined to skeletal muscle. It is of heterogeneous echogenicity, although mainly isoechoic to the adjacent muscular tissue [32] (Fig. 14). It shows areas of increased echogenicity that correspond with fat and are more frequently peripheral, creating a hyperechoic rim (Fig. 14), but can also be seen centrally within the mass. Sometimes the mass is diffusely hyperechoic compared to spared muscle [33], probably reflecting a more diffuse fatty component. Another described sonographic pattern is of diffuse involvement of one or two adjacent muscles, causing expansion, but no discrete mass and with relative preservation of the fibrillar pattern of the muscle [32, 34, 35]. In this pattern, the capillary and adipocytic components of the hemangioma are intermixed between the fibers of the affected muscle [34]. Doppler sonography reveals high vascular density with high-resistance arterial flow [32]. These vessels can be visible on gray-scale imaging [34].
Intramuscular capillary-type hemangioma in a 17-year-old boy who recently noticed a lump in the right paraspinal region. a Longitudinal sonogram using extended field-of-view technique shows a large intramuscular mass. The mass has two components. The larger component is more centrally located within the lesion and appears as a hypoechoic solid mass (arrows) containing some prominent vessels. The other component appears as a hyperechoic rim, thicker inferiorly, where it is seen interposed between normal muscle fibers (arrowheads). This hyperechoic component represents fatty tissue. b Longitudinal power Doppler sonogram shows high vascular density. The final diagnosis was made at histology following surgical excision, which was indicated because the clinical and imaging findings could not exclude malignancy
The clinical and imaging findings in intramuscular capillary-type hemangioma do not allow differentiation from malignant neoplasms and therefore biopsy is recommended in all of these cases [32].
Angiosarcoma
This is an extremely rare tumor in children that can present at any age and often shows rapid growth [36]. Deyrup et al. [36] reported soft-tissue angiosarcoma to be more common in the lower extremities, whereas Ferrari et al. [37] described it as more common in the head and neck, presenting as a purple lesion of the scalp. None of the authors of the present article has seen a case, and there are no reports of the sonographic findings in pediatric cases of cutaneous angiosarcoma.
Differentiation of vascular tumors from other neoplastic lesions
One of the concerns at the time of imaging a vascular tumor is its differentiation from other non-vascular soft-tissue masses, particularly malignant neoplasms. While a solid knowledge base in vascular tumors along with clinical history and sonographic findings many times can lead the astute radiologist to a confident diagnosis or a narrow differential diagnosis, numerous scenarios do not fit into one of the classic clinical and imaging presentations. From a clinical point of view, red flags include a painful mass (especially in an older child), rapid growth after 12 months of age, enlarged lymph nodes adjacent to the lesion and any associated necrotic tissue in a mass not present at birth. Imaging red flags for vascular tumors include lymphadenopathy, adjacent soft-tissue or bone destruction, calcifications in a non-congenital lesion, internal hemorrhage or necrosis in a non-congenital lesion and any history of concomitant lung, bone or liver lesions. Any of these findings are suggestive of a more aggressive lesion including myofibromatosis, sarcomas and the rarer malignant hemangioendotheliomas as well as various types of metastatic disease. Therefore any of these findings warrants further evaluation with MR imaging, usually followed with histologic correlation.
Conclusion
Sonography is a useful tool in the evaluation of pediatric soft-tissue vascular anomalies, providing valuable information for diagnosis and in some cases for monitoring treatment and complications. This is usually achieved without the need of sedation, which in association with the lack of radiation makes it ideal for the pediatric age group. However sonography might be limited in some cases by lesion extent or location or the inherent characteristics of the lesion, in which case MR imaging might be indicated. The effect of using sonography in the evaluation of vascular anomalies is maximized when correlation is made with the clinical findings and when proper terminology and the most updated ISSVA classification is used, especially during the interaction with the multidisciplinary team that is often involved in the care of these children.
References
International Society for the Study of Vascular Anomalies (2014) ISSVA classification of vascular anomalies. http://wwwissvaorg/UserFiles/file/Classifications-2014-Finalpdf Accessed 12 Apr 2017
Mulliken J, Glowacki J (1982) Classification of pediatric vascular lesions. Plast Reconstr Surg 70:120–121
Paltiel HJ, Burrows PE, Kozakewich HP et al (2000) Soft-tissue vascular anomalies: utility of US for diagnosis. Radiology 214:747–754
Dubois J, Garel L, David M et al (2002) Vascular soft-tissue tumors in infancy: distinguishing features on Doppler sonography. AJR Am J Roentgenol 178:1541–1545
Shah SH, Callahan MJ (2013) Ultrasound evaluation of superficial lumps and bumps of the extremities in children: a 5-year retrospective review. Pediatr Radiol 43:S23–S40
Dubois J, Patriquin HB, Garel L et al (1998) Soft-tissue hemangiomas in infants and children: diagnosis using Doppler sonography. AJR Am J Roentgenol 171:247–252
Trop I, Dubois J, Guibaud L et al (1999) Soft-tissue venous malformations in pediatric and young adult patients: diagnosis with Doppler US. Radiology 212:841–845
Frieden I, Enjolras O, Esterly N (2003) Vascular birthmarks and other abnormalities of blood vessels and lymphatics. In: Schachner LA, Hansen RC (eds) Pediatric dermatology, 3rd edn. Mosby, New York, pp 833–862
Enjolras O, Wassef M, Chapot R (2007) Color atlas of vascular tumors and vascular malformations, 1st edn. Cambridge University Press, New York
Waner M, North PE, Scherer KA et al (2003) The nonrandom distribution of facial hemangiomas. Arch Dermatol 139:869–875
Frieden I, Reese V, Cohen D (1996) PHACE syndrome: the association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol 132:307–311
Iacobas I, Burrows PE, Frieden IJ (2010) LUMBAR: association between cutaneous infantile hemangiomas of the lower body and regional congenital anomalies. J Pediatr 157:795–801
Gorincour G, Kokta V, Rypens F et al (2005) Imaging characteristics of two subtypes of congenital hemangiomas: rapidly involuting congenital hemangiomas and non-involuting congenital hemangiomas. Pediatr Radiol 35:1178–1185
Dubois J, Garel L (1999) Imaging and therapeutic approach of hemangiomas and vascular malformations in the pediatric age group. Pediatr Radiol 29:879–893
Rogers M, Lam A, Fischer G (2002) Sonographic findings in a series of rapidly involuting congenital hemangiomas (RICH). Pediatr Dermatol 19:5–11
Sheybani EF, Eutsler EP, Navarro OM (2016) Fat-containing soft-tissue masses in children. Pediatr Radiol 46:1760–1773
Bingham MM, Saltzman B, Vo NJ et al (2012) Propranolol reduces infantile hemangioma volume and vessel density. Otolaryngol Head Neck Surg 147:338–344
Boon LM, Enjolras O, Mulliken JB (1996) Congenital hemangioma: evidence of accelerated involution. J Pediatr 128:329–335
Enjolras O, Mulliken JB, Boon LM et al (2001) Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg 107:1647–1654
Mulliken JB, Enjolras O (2004) Congenital hemangiomas and infantile hemangioma: missing links. J Am Acad Dermatol 50:875–882
Nasseri E, Piram M, McCuaig CC (2014) Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol 70:75–79
Dubois J, Alison M (2010) Vascular anomalies: what a radiologist needs to know. Pediatr Radiol 40:895–905
Lee GK, Suh KJ, Lee JH et al (2010) Lobular capillary hemangioma in the soft tissue of the finger: sonographic findings. Skeletal Radiol 39:1097–1102
Cantisani V, Del Vecchio A, Fioravanti E et al (2016) Color-Doppler US features of a pyogenic granuloma of the upper dorsum tongue. J Ultrasound 19:67–70
Kikusawa A, Oka M, Shimizu H et al (2012) Subcutaneous lobular capillary hemangioma with sonography and computed tomography findings. Eur J Dermatol 22:276–277
Colmenero I, Hoeger PH (2014) Vascular tumours in infants. Part II: vascular tumours of intermediate dignity and malignant tumours. Br J Dermatol 171:474–484
Kasabach H, Merritt K (1940) Capillary hemangioma with extensive purpura: report of a case. Am J Dis Child 59:1063–1070
Drolet BA, Trenor CC 3rd, Brandão LR (2013) Consensus-derived practice standards plan for complicated kaposiform hemangioendothelioma. J Pediatr 163:285–291
Ballah D, Cahill AM, Fontalvo L et al (2011) Vascular anomalies: what they are, how to diagnose them, and how to treat them. Curr Probl Diagn Radiol 40:233–247
Kollipara R, Dinneen L, Rentas KE et al (2013) Current classification and terminology of pediatric vascular anomalies. AJR Am J Roentgenol 201:1124–1135
Gruman A, Liang MG, Mulliken JB et al (2005) Kaposiform hemangioendothelioma without Kasabach–Merritt phenomenon. J Am Acad Dermatol 52:616–622
Yilmaz S, Kozakewich HP, Alomari AI et al (2014) Intramuscular capillary-type hemangioma: radiologic–pathologic correlation. Pediatr Radiol 44:558–565
Fernandez-Pineda I, Jenkins JJ, Santiago TC et al (2016) Awareness of intramuscular capillary type hemangioma in the differential diagnosis of soft-tissue tumors in children. Pediatr Blood Cancer 63:2252–2253
Merrow AC, Gupta A, Adams DM (2014) Additional imaging features of intramuscular capillary-type hemangioma: the importance of ultrasound. Pediatr Radiol 44:1472–1474
Chaudry G, Yilmaz S, Alomari AI (2014) Reply to Merrow et al regarding additional imaging features of intramuscular capillary-type hemangioma and the importance of ultrasound. Pediatr Radiol 44:1475
Deyrup AT, Miettinen M, North PE et al (2011) Pediatric cutaneous angiosarcomas: a clinicopathologic study of 10 cases. Am J Surg Pathol 35:70–75
Ferrari A, Casanova M, Bisogno G et al (2002) Malignant vascular tumors in children and adolescents: a report from the Italian and German soft tissue sarcoma cooperative group. Med Pediatr Oncol 39:109–114
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no financial interests, investigational or off-label uses to disclose.
Rights and permissions
About this article
Cite this article
Johnson, C.M., Navarro, O.M. Clinical and sonographic features of pediatric soft-tissue vascular anomalies part 1: classification, sonographic approach and vascular tumors. Pediatr Radiol 47, 1184–1195 (2017). https://doi.org/10.1007/s00247-017-3885-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-3885-y