Abstract
Background
Abusive head trauma (AHT) is an important cause of morbidity in infants. Identifying which well-appearing infants are at risk for AHT and need neuroimaging is challenging, and concern about radiation exposure limits the use of head CT. Availability of an MRI protocol that is highly sensitive for intracranial hemorrhage would allow for AHT screening of well-appearing infants without exposing them to radiation.
Objective
To develop a screening MRI protocol to identify intracranial hemorrhage in well-appearing infants at risk for AHT.
Materials and methods
Infants enrolled in a parent study of well-appearing infants at increased risk for AHT were eligible for the current study if they underwent both head CT and conventional brain MRI. A derivation cohort of nine infants with AHT was used to identify sequences that provided the highest sensitivity for intracranial hemorrhage. A validation cohort of 78 infants including both controls with normal neuroimaging and cases with AHT was used to evaluate the accuracy of the selected sequences.
Results
Three pulse sequences — axial T2, axial gradient recalled echo (GRE) and coronal T1-W inversion recovery — were 100% sensitive for intracranial hemorrhage in the derivation cohort. The same sequences were 100% sensitive (25/25) and 83% specific (44/53) for intracranial hemorrhage in the validation cohort.
Conclusion
A screening MRI protocol including axial T2, axial GRE and coronal T1-W inversion recovery sequences is highly sensitive for intracranial hemorrhage and may be useful as a screening tool to differentiate well-appearing infants at risk for AHT who should undergo head CT from those who can safely be discharged without head CT. Additional research is needed to evaluate the feasibility of this approach in clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abusive head trauma (AHT) is the leading cause of morbidity and mortality from traumatic brain injury in infants. Multiple studies have demonstrated that physicians often fail to make the proper diagnosis of AHT in infants who present with nonspecific symptoms such as vomiting, lethargy, bruising and fussiness [1–5]. In a landmark study published in 1999 [5], Jenny and colleagues demonstrated that 31% (54/173) of children diagnosed with AHT had been evaluated by a physician after the injury occurred but the diagnosis of AHT was missed. In this study, the most common incorrect diagnoses were viral gastroenteritis and viral syndrome. Of the five deaths in the study, four were thought to have been preventable if the diagnosis of abuse had been made at the initial encounter. Unfortunately, despite continued education within the medical community, there are still missed opportunities to identify AHT. In a recent multi-center study conducted 15 years after the study by Jenny and colleagues, more than one-quarter of children with AHT had a prior missed opportunity to diagnose abuse (Megan Letson, MD, personal communication).
Head CT is considered the gold-standard modality for evaluating infants and young children with possible AHT [6] because it can be completed within a minute without the need for sedation, is available in virtually all hospitals in the United States, 24 h a day and 7 days a week [7], and is highly sensitive for subdural hemorrhage. Although many types of brain injury are seen in AHT, subdural hemorrhage is the most common and is present in more than 90% of cases [8, 9]. Although CT is an effective imaging modality in these cases, it necessitates use of radiation and there has been increasing concern about radiation risks, particularly cancer, in young children [10, 11]. The lack of improvement in diagnosis of AHT could be related to physician concern about radiation risk, combined with recent recommendations to greatly limit head CT use in well-appearing young children after closed head injury [12, 13].
Conventional brain MRI has been used primarily as an adjunct to CT in cases of suspected AHT to better delineate ischemia, diffuse axonal injury, cerebellar injury and other subtle parenchymal abnormalities [6, 14]. The use of a conventional brain MRI for the initial evaluation of infants with possible AHT is not feasible for several reasons, including the difficulty of adding an MRI with sedation or general anesthesia to an already crowded MRI schedule. The fact that infants and young children cannot eat or drink for several hours prior to sedation/anesthesia makes conventional MRI impractical in an emergent setting. Concerns about the effect of anesthesia on the developing brain [15] have also limited the routine use of MRI as a screening tool. Although head ultrasonography eliminates the radiation exposure and does not require sedation, it is neither sensitive nor specific enough to be used as a diagnostic tool in suspected AHT [6, 16]. Lack of 24/7 access to an experienced head sonographer in some hospitals also limits sonography’s usefulness in emergency departments.
Rapid-sequence MRI has been used for almost 10 years for evaluation of shunt malfunction in children with hydrocephalus [17, 18]. Rapid-sequence MRI, a fast T2-weighted sequence, eliminates the radiation risk associated with CT as well as the cost and risks associated with the sedation needed for routine MRI. In a recently published study by Rozovsky and colleagues [19], however, six of the seven abnormalities missed by rapid-sequence MRI were intracranial hemorrhages. The lack of blood-sensitive sequences, such as gradient-recalled echo (GRE), susceptibility-weighted imaging (SWI) and T1-weighted imaging might have contributed to the poor detection of intracranial hemorrhage in this study, suggesting that single-shot fast spin echo sequences alone are not adequate to screen for AHT.
The objective of this study was to derive and validate a screening MRI protocol, obtained from conventional MRI sequences, specifically designed to identify intracranial hemorrhage, the intracranial abnormality most frequently associated with AHT.
Materials and methods
Children in the current study were selected from a larger prospective study funded by the National Institutes of Health titled “Novel Approaches to Screening for Inflicted Childhood Neurotrauma” (R01HD055986). Children were eligible for enrollment in the parent study if they were age 30–364 days, were well-appearing (defined as a Glasgow coma scale score of 13–15), did not have a temperature greater than 38.3°C/101°F within the prior 24 h, and presented to one of three participating emergency departments for evaluation of a nonspecific symptom. The nonspecific symptoms included fussiness/irritability, vomiting, poor feeding, seizure/abnormal movements, apparent life-threatening event, swelling on the head, macrocephaly or bruise. These symptoms are often presenting symptoms in infants with missed AHT. The only exclusion criterion was having had prior abnormal neuroimaging (e.g., an infant with known hydrocephalus) or a chief complaint of trauma/closed head injury. Infants were eligible for enrollment in the current neuroimaging sub-study if they were enrolled at our institution, had undergone both head CT and conventional brain MRI as part of the parent study, and were either diagnosed with AHT (cases) or had normal neuroimaging (controls). The diagnosis of AHT in each case was made by the hospital-based Child Protection Team, a multi-disciplinary team that includes child abuse pediatricians. Defining AHT based on the decision of the Child Protection Team is a standard approach in research [5, 20, 21]. The institutional review board of our institution approved the study. Informed consent was obtained from the parents of all control subjects. A waiver of informed consent was approved for all children with AHT. The children in the current study were retrospectively selected from the larger cohort of children enrolled in the prospective parent study.
Eligible children were divided into two groups: (1) a derivation cohort of 9 children with AHT and (2) a validation cohort of 78 children that included children with AHT and control children with normal brain imaging (Fig. 1). For children in the derivation cohort, seven pulse sequences (sagittal T1, axial T1, axial T2, coronal T2, coronal T1 inversion recovery, axial diffusion-weighted imaging [DWI], axial proton density and axial GRE) were reviewed independently by a pediatric neuroradiologist (L.F., 15 years of experience) and a pediatric neurosurgeon (E.T.K., 18 years of experience) who designated each sequence as normal, abnormal or equivocal. Because there were 9 children in the derivation cohort and 7 pulse sequences for each MRI, 63 sequences were evaluated by each reader for a total of 126 sequences.
The validation cohort was used to validate the subset of sequences identified in the derivation cohort as providing the highest sensitivity for intracranial hemorrhage and to evaluate the specificity of the sequences. The validation cohort included a different set of children with AHT (cases) as well as children with normal neuroimaging (controls). There was no overlap in the cases between the derivation and validation cohorts. For the validation cohort, the same pediatric neuroradiologist and neurosurgeon evaluated only the three sequences that were the most sensitive in the derivation cohort and classified each as normal (no intracranial hemorrhage), abnormal (intracranial hemorrhage) or equivocal (possible intracranial hemorrhage). If one or more of these three sequences were interpreted as equivocal or abnormal, the screening MRI was assessed as being abnormal. The pediatric neuroradiologist and neurosurgeon were blinded to the results of the head CT. The reference standard for detection of hemorrhage was considered to be the head CT because that is the reference standard to which the screening MRI was being compared.
Data collection
The following data were collected for each subject: age (in months), gender, race (white or not white), and reason for presentation. There were seven possible reasons for presentation: fussiness and/or vomiting, fussiness and poor feeding, possible seizure activity, apparent life-threatening event, head swelling, macrocephaly or bruising. The time between the head CT and brain MRI was also calculated.
Imaging protocol
The imaging protocol used was the standard brain MRI used at Children’s Hospital of Pittsburgh of UPMC: (1) coronal T1-W inversion recovery (repetition time/echo time [TR/TE] 2,500/minimum ms, field of view [FOV] 20 cm, slice thickness 5 mm); (2) axial DWI (TR/TE 1,000/minimum ms, FOV 26 cm, slice thickness 4 mm); (3) axial T2-W fast spin echo (FSE) (TR/TE 4,000/70 ms, FOV 20 cm, slice thickness 5 mm); (4) sagittal T1-W (TR/TE 2,500/minimum ms, FOV 20 cm, slice thickness 5 mm); (5) axial proton density (PD) (TR/TE 2,200/20 ms, FOV 20 cm, slice thickness 5 mm), and (6) axial GRE (TR/TE 700/14.5 ms, FOV 22 cm, slice thickness 4 mm) or susceptibility-weighted angiography (SWAN TR/TE minimum/26 ms, FOV 20 cm, slice thickness 3 mm). All scans were performed on a 1.5-T GE MR scanner (HD 16.0 platform, GE Healthcare, Waukesha, WI). All MRIs performed prior to February 2011 used GRE as the blood-sensitive sequence. After February 2011, SWAN was used as the standard blood-sensitive sequence.
Statistical analysis
We used descriptive statistics to describe the derivation and validation cohorts.
Results
All children were enrolled between December 2006 and January 2013.
Derivation cohort
Nine children were included in the derivation cohort, with a mean (SD) age of 3.9 (2.5) months. All were white and 67% (6/9) were male. The infants presented for evaluation of fussiness (n = 3), possible seizure activity (n = 3), vomiting (n = 1), macrocephaly (n = 1) or bruising (n = 1). The brain MRI was performed a median (25–75%) of 40 h (range 14–71 h) after the head CT.
The three sequences with the highest sensitivity for identification of intracranial hemorrhage were axial T2, axial GRE and coronal T1 inversion recovery. Concordance between the readers was 100%: both agreed that axial T2 was abnormal in all nine of these children; GRE (n = 6)/SWAN (n = 3) was abnormal in 8 of 9 cases, and coronal T1 inversion recovery was abnormal in all 8 children in whom it was performed. Based on these data from the derivation cohort, the three most sensitive sequences — axial T2, axial GRE and coronal T1 inversion recovery — were evaluated in the validation cohort. A comparison of the sequences in the conventional MRI and the proposed screening MRI are found in Table 1.
Validation cohort
Seventy-eight children were included in the validation cohort with a mean (SD) age of 5.2 (3.5) months; 77% (60/78) were white and 44% (34/78) were male. Thirty-two percent (25/78) had AHT and the remaining 68% (53/78) were controls. The children presented for evaluation of fussiness and/or vomiting (n = 18), possible seizure activity (n = 34), apparent life-threatening event (n = 16), scalp swelling (n = 4), macrocephaly (n = 4) or bruising (n = 2). The brain MRI was performed a median (25–75%) of 37 h (range 23–53 h) after the head CT. There was no difference in the demographics or the time between CT and MRI in the derivation and validation cohorts.
Of the 78 children in the validation cohort, 35 had a GRE sequence, 30 had a SWAN sequence and 13 did not have a blood-sensitive sequence completed. All 13 children without a blood-sensitive sequence were controls. In one control subject, the coronal T1 inversion recovery sequence was not completed.
Controls
In 83% (44/53) of controls, both readers interpreted all three sequences in the screening MRI as normal (Table 2). In 17% of controls (9/53) at least one reader thought at least one of the three sequences was abnormal; there was no overlap between the five subjects the neuroradiologist interpreted as having an abnormal sequence and the four subjects the neurosurgeon interpreted as having an abnormal sequence.
Cases
Both readers interpreted the screening MRI as abnormal in all 25 children with AHT (Table 2). Concordance between readers was 100%. Both readers agreed that axial T2 and GRE/SWAN were abnormal in all 25 cases and that coronal T1 inversion recovery was abnormal in 23 of 25 cases. The sensitivity and specificity of the screening MRI for intracranial hemorrhage were, therefore 100% and 83%, respectively. Figures 2 and 3 demonstrate abnormal axial T2, axial GRE and coronal T1 inversion recovery sequences in children with AHT.
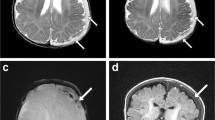
Abnormal screening head MRI sequences in 2.5-month-old boy with abusive head trauma and Glasgow coma scale score of 15. a Axial T2-W sequence demonstrates a subdural hemorrhage (arrow) along the left convexity. This sequence was assessed as abnormal. b Axial gradient recalled echo sequence demonstrates interhemispheric and left convexity subdural hemorrhage (arrow). This sequence was assessed as abnormal. c Coronal T1-W inversion recovery sequence demonstrates subdural hemorrhage along left convexity (arrow). This sequence was assessed as abnormal
Abnormal screening head MRI sequences in a 2-month-old boy with abusive head trauma and Glasgow coma scale score of 15. a Axial T2-W sequence demonstrates bilateral subdural convexity hemorrhages (arrows). This sequence was assessed as abnormal. b Axial gradient recalled echo sequence demonstrates bilateral subdural convexity hemorrhages (arrows). This sequence was assessed as abnormal. c Coronal T1-W inversion recovery sequence demonstrates bilateral mixed-signal-convexity subdural hemorrhages (arrows)
Discussion
In this study we evaluated whether a screening MRI that can be done quickly and potentially without sedation could be used to screen for intracranial hemorrhage in well-appearing infants who are at high risk for abusive head trauma. Our data demonstrated that a screening MRI protocol that includes axial T2, axial GRE and a coronal T1 inversion recovery was 100% sensitive for identification of intracranial hemorrhage. It is important to recognize that the screening MRI is appropriate only in well-appearing infants in whom neuroimaging is not emergent but is being done to rule out a brain injury. This is the group in whom AHT is most likely to be missed because head CTs are not routinely performed in well-appearing children. The goal of the screening MRI protocol is not to replace the diagnostic head CT or brain MRI, but rather to discriminate the subset of infants who require a head CT from those who can safely be discharged with a screening MRI only, thereby eliminating the need for any radiation exposure. Although the risk from radiation exposure from a single head CT using ALARA principles (dose as low as reasonably achievable) is low, it is not zero. Very young children — those at greatest risk of AHT — are also the ones at greatest risk of developing leukemia from radiation from head CT [22]. Because our data do not suggest any loss in accuracy using the screening MRI in place of a head CT, using MRI and not exposing infants to radiation is clearly preferable to using CT and exposing them to even a low dose of radiation. The question of whether an abnormal screening MRI could be followed by a conventional MRI rather than a head CT is one that individual institutions can decide based on local circumstances. At our institution, logistics and practicality dictate that head CT is likely the only option to follow up an abnormal screening MRI. In order to complete a full MRI in an infant, sedation or general anesthesia is almost always needed; sedation requires that infants do not eat or drink for several hours prior to the MRI. Because infants who undergo a screening MRI need to be fed and bundled, virtually all infants who have undergone a screening MRI are ineligible for immediate sedation. One of the advantages of the screening MRI is that it is relatively quick and can be fit into a busy MRI schedule between other scheduled procedures. It is unlikely that an MRI machine used for a screening MRI would be available for 45 min to 1 h for a full MRI immediately after the screening MRI.
Although the primary goal of the screening MRI is to improve identification of AHT with an initial screen that avoids radiation exposure from head CT, another important clinical use is to decrease head CT usage in infants with a normal screening MRI. Of the 53 infants with a normal CT, 44 had a normal screening MRI and therefore would not have needed to undergo a subsequent CT. This corresponds to an 83% decrease in head CT use.
We recognize that based on our data — in which axial T2 and GRE/SWAN were abnormal in all 25 cases and coronal T1 inversion recovery was abnormal in 23 of 25 cases of AHT in the validation cohort — we could have recommended a single-sequence screening MRI of an axial T2 or GRE/SWAN rather than a three-sequence MRI. Although this would have increased the specificity, we intentionally created redundancy by including multiple sequences; although the screening MRI was optimized to identify intracranial hemorrhage because this is the abnormality most associated with AHT, atraumatic abnormalities such as stroke, venous vein thrombosis and brain tumors as well as non-abusive traumatic brain injuries could present with the same clinical symptoms in the same age-group. Because non-traumatic insults, for example, are much rarer than AHT in this age group, we were not able to evaluate them as part of the current study. We plan to evaluate non-AHT abnormalities as part of a larger prospective study. We also plan to evaluate the use of DWI in this prospective study because even though DWI was not a critical sequence in identification of intracranial hemorrhage, it might be critical in pediatric stroke and can be performed in less than 1 min. A fluid-attenuated inversion recovery (FLAIR) sequence was not included in the analysis because it is not part of the routine MRI protocol for children younger than 18 months at our institution. In this age group, the brain is uniformly bright on FLAIR from lack of myelination, and intraparenchymal pathology is, therefore, hard to detect. As described in the methods section, we did evaluate proton density, which is similar to FLAIR and shows abnormal signal in the subarachnoid space in the presence of blood, but this sequence was not ultimately included in the screening MRI. Striking a balance between sensitivity and the length of time it takes to perform the selected sequences is a critical issue for a screening MRI because the screen must be done without sedation.
There are a number of limitations to the approach of using the screening MRI protocol. A shortened MRI protocol has the same shortcomings as a complete standard conventional MRI protocol in that it is not sensitive to skull fracture. If there is clinical concern for an isolated skull fracture because of scalp swelling, a head CT rather than a screening MRI would be indicated. The screening MRI is also not intended to be performed in critically ill children; although it is faster than a routine MRI, it is not nearly as rapid as a head CT. All children in this study had mild AHT and were well-appearing, and there was little clinical risk associated with the additional time needed for transport to the radiology suite and for the optimized limited MRI. The most significant limitation of this approach is a practical one regarding the accessibility to MRI. At our institution there is 24/7 access to MRI, but this is not universally true. A recent survey of 260 hospitals demonstrated that on-site MRI was available in 66% (171/260) of hospitals [7]. Thus our results have applicability only to some hospitals. It is likely, however, that over time accessibility to MRI will increase.
There are several limitations to the study design. As part of the protocol, we determined whether there was intracranial hemorrhage. We did not identify the abnormality itself (e.g., subdural hemorrhage vs. subarachnoid hemorrhage vs. parenchymal contusion) or the age of the hemorrhage (acute vs. chronic) because in clinical practice an abnormal screen would not be used to identify a specific abnormality or diagnosis but rather to identify which children need additional imaging. In clinical practice, we would expect that the screening MRI result would be reported as a dichotomous abnormal or normal. We would not want the report to identify the specific abnormality because it is important that the screening tool not replace a diagnostic test. Because of this design, however, we do not know the sensitivity of the screening MRI for specific abnormalities. Because of the high prevalence of children with AHT in the study population, the population is not representative of the children who would undergo a screening MRI protocol in clinical practice where the proportion of abnormal images is likely to be far lower. This difference in prevalence of abnormalities would likely change negative predictive value of the screening MRI in clinical practice. Another potential limitation relates to the fact that in order to be eligible for enrollment, infants had to undergo both head CT and brain MRI. It is possible that infants who undergo both types of imaging for clinical care are not representative of the infants who only undergo a head CT. However, because the goal of the study was to compare the reference standard CT to a screening MRI, it would not have been possible to perform this study without enrolling children who had both CT and MRI. In addition, because all infants had a complete conventional MRI protocol performed with sedation or general anesthesia, movement artifact was not an issue. In clinical practice, the screening MRI would be done without sedation, which might increase the possibility of movement artifact and a resulting change in sensitivity or specificity. It is important to note that while the full MRI is routinely done with a 2-NEX (number of excitations) signal average, it is likely that a 1-NEX signal average could be used for a screening MRI. Use of a 1-NEX signal average would decrease the time of the three proposed sequences from 11 min 46 s to 5 min 27 s. Finally, it was not possible to directly compare a rapid-sequence MRI to the screening MRI in this study because single-shot fast spin echo T2 (ssT2 FSE) sequences that are part of the rapid sequence MRI are not included in a standard full MRI protocol. In a future prospective study, we plan to evaluate whether ssT2 FSE can be used to replace the axial T2 motion-correction sequence in the screening MRI because this has the potential to decrease the time of the screening MRI even further than is possible by using a 1-NEX signal average alone.
Conclusion
We identified three sequences that could be used as a screening protocol that has the potential to be used in clinical practice as a first-level screening in infants in whom a head CT would otherwise be needed. In infants with a normal screening MRI, the screening tool might eliminate the radiation exposure of a head CT. For physicians the option of a screening tool that does not require radiation or sedation might lower the threshold to screen for intracranial hemorrhage in infants at increased risk of AHT. The clinical indication for this screening MRI would be the same population of children who were eligible for the current study — afebrile infants who present to the emergency department for evaluation of one of a set of clinical criteria that place them at high risk of AHT. It is important to note that infants who present for evaluation of a closed head injury and in whom the Pediatric Emergency Care Applied Research Network (PECARN) rule [12] can be used to assess the need for head CT would not have met the inclusion criteria for the current study. Future research will focus on the feasibility of incorporating the screening MRI protocol into the flow of the emergency department as well as the feasibility of obtaining high-quality images in infants without sedation.
References
Sieswerda-Hoogendoorn T, Bilo RA, van Duurling LL et al (2013) Abusive head trauma in young children in the Netherlands: evidence for multiple incidents of abuse. Acta Paediatr 102:e497–e501
Petska HW, Sheets LK, Knox BL (2013) Facial bruising as a precursor to abusive head trauma. Clin Pediatr 52:86–88
Oral R, Yagmur F, Nashelsky M et al (2008) Fatal abusive head trauma cases: consequence of medical staff missing milder forms of physical abuse. Pediatr Emerg Care 24:816–821
Ricci L, Giantris A, Merriam P et al (2003) Abusive head trauma in Maine infants: medical, child protective, and law enforcement analysis. Child Abuse Negl 27:271–283
Jenny C, Hymel KP, Ritzen A et al (1999) Analysis of missed cases of abusive head trauma. JAMA 281:621–626
Sieswerda-Hoogendoorn T, Boos S, Spivack B et al (2012) Abusive head trauma part II: radiological aspects. Eur J Pediatr 171:617–623
Ginde AA, Foianini A, Renner DM et al (2008) Availability and quality of computed tomography and magnetic resonance imaging equipment in U.S. emergency departments. Acad Emerg Med 15:780–783
Keenan HT, Runyan DK, Marshall SW et al (2004) A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics 114:633–639
Sieswerda-Hoogendoorn T, Boos S, Spivack B et al (2012) Educational paper: abusive head trauma part I. Clinical aspects. Eur J Pediatr 171:415–423
Menoch MJ, Hirsh DA, Khan NS et al (2012) Trends in computed tomography utilization in the pediatric emergency department. Pediatrics 129:e690–e697
Brenner DJ, Hall EJ (2007) Computed tomography — an increasing source of radiation exposure. N Engl J Med 357:2277–2284
Bressan S, Romanato S, Mion T et al (2012) Implementation of adapted PECARN decision rule for children with minor head injury in the pediatric emergency department. Acad Emerg Med 19:801–807
Hennelly KE, Mannix R, Nigrovic LE et al (2013) Pediatric traumatic brain injury and radiation risks: a clinical decision analysis. J Pediatr 162:392–397
Foerster BR, Petrou M, Lin D et al (2009) Neuroimaging evaluation of non-accidental head trauma with correlation to clinical outcomes: a review of 57 cases. J Pediatr 154:573–577
Yu CK, Yuen VM, Wong GT et al (2013) The effects of anaesthesia on the developing brain: a summary of the clinical evidence. F1000Res 2:166
Kemp AM, Rajaram S, Mann M et al (2009) What neuroimaging should be performed in children in whom inflicted brain injury (iBI) is suspected? A systematic review. Clin Radiol 64:473–483
Patel DM, Tubbs RS, Pate G et al (2014) Fast-sequence MRI studies for surveillance imaging in pediatric hydrocephalus. J Neurosurg Pediatr 13:440–447
Iskandar BJ, Sansone JM, Medow J et al (2004) The use of quick-brain magnetic resonance imaging in the evaluation of shunt-treated hydrocephalus. J Neurosurg 101:147–151
Rozovsky K, Ventureyra EC, Miller E (2013) Fast-brain MRI in children is quick, without sedation, and radiation-free, but beware of limitations. J Clin Neurosci 20:400–405
Keenan HT, Runyan DK, Marshall SW et al (2003) A population-based study of inflicted traumatic brain injury in young children. JAMA 290:621–626
Hymel KP, Abshire TC, Luckey DW et al (1997) Coagulopathy in pediatric abusive head trauma. Pediatrics 99:371–375
Miglioretti DL, Johnson E, Williams A et al (2013) The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr 167:700–707
Acknowledgments
Data contained in this manuscript were presented at the following conferences: Annual Meeting of the Helfer Society, Annapolis, MD, April 6, 2014; Pediatric Academic Societies & Asian Society for Pediatric Research Joint Meeting, Vancouver, Canada, May 4, 2014; Society for Pediatric Radiology (SPR) Annual Meeting, Washington D.C., May 14, 2014. Funding was provided by the National Institutes of Health (NICHD R01HD055986).
We would like to thank Emily Heineman, Pamela Rubin and Marci Provins for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Flom, L., Fromkin, J., Panigrahy, A. et al. Development of a screening MRI for infants at risk for abusive head trauma. Pediatr Radiol 46, 519–526 (2016). https://doi.org/10.1007/s00247-015-3500-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-015-3500-z