Abstract
CT is considered the first-line study for acute intracranial injury in children because of its availability, detection of acute hemorrhage, and lack of sedation. An MRI study with rapidly acquired sequences can obviate the need for sedation and radiation. We compared the detection rate of rapid non-sedated brain MRI to CT for traumatic head injury in young children. We reviewed a series of children 6 years of age or less who presented to our ED during a 5-year period with head trauma and received a non-sedated brain MRI and CT within 24 h of injury. Most MRI studies were limited to triplane T2 and susceptibility sequences. Two neuroradiologists reviewed the MRIs and CTs and assessed the following findings: fracture, epidural hematoma (EDH)/subdural hematoma (SDH), subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH), and parenchymal injury. Thirty of 33 patients had radiologically identified traumatic injuries. There was an overall agreement of 82 % between the two modalities. Skull fracture was the only injury subtype which had a statistically significant difference in detection between CT and MRI (p = 0.0001), with MRI missing 14 of 21 fractures detected on CT. While not statistically significant, MRI had a higher detection rate of EDH/SDH (p = 0.34), SAH (p = 0.07), and parenchymal injuries (p = 0.50). Non-sedated MRI has similar detection rates to CT for intracranial injury in young children presenting with acute head trauma and may be an alternative to CT in select patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a prevalent condition in the USA that accounts for nearly half a million visits to the emergency department and approximately two thousand deaths in children aged 0 to 14 years per year [1].
Computed tomography (CT) is currently recommended by the American College of Radiology as the first-line imaging modality for the evaluation of TBI in children because it takes minutes to perform, is widely available, and does not require sedation [2]. CT, however, uses ionizing radiation. Recent large cohort studies have shown a positive correlation between lifetime risk of developing cancer and CT-associated radiation dose and have shown that the lifetime risk of cancer is higher in young children [3, 4]. Such data have prompted a search for alternative imaging modalities.
Magnetic resonance imaging (MRI) is an alternative modality that avoids ionizing radiation while offering greater anatomic detail of the brain. While there is limited research comparing the performance of MRI to CT in pediatric TBI, prior studies have shown that MRI typically has similar detection rates compared to CT for extra-axial hemorrhage and often higher detection rates of intraparenchymal injury [5–10]. Young children, especially in the 2-month to 6-year age group, often cannot tolerate the enclosure of the MRI unit and may move during the MRI exam, which can produce motion artifact compromising diagnostic quality, and thus, MRI often requires anesthesia in this age group. In addition to the risks at the time of sedation, the general anesthetics used for MRI sedation have also been potentially associated with developmental delay and behavioral problems in children [11–13]. Therefore, rapid MRI sequences have been developed to avoid this risk.
Rapid or “quick-brain” MRI studies utilize modified MR imaging protocols with shorter acquisition times. These tailored protocols have become an accepted technique to evaluate and follow patients with hydrocephalus [14, 15] with expanding use to other indications such as macrocephaly [16]. A recently published study by Mehta et al. showed that rapid MRI performed favorably compared to CT in children from 0–19 years of age with trauma, with a similar detection rate for extra-axial hemorrhage and higher detection rates for contusions and diffuse axonal injury (DAI) [17]. To our knowledge, this study is the only publication that compares the detection rate of rapid MRI without sedation to CT for traumatic intracranial injury in pediatric patients. In our experience, children 2 months to 6 years of age are the most likely to require sedation for MRI. Therefore, we focused our study on comparing the detection rate of rapid, non-sedated MRI to CT for the assessment of acute traumatic injury in patients 6 years of age or less.
Methods
Patient cohort
Institutional review board approval was obtained for the study. We conducted a retrospective review for MRIs on pediatric head trauma patients performed at the emergency department of our institution, an American College of Surgeons’ certified level 1 pediatric trauma center, between January 2010 and May 2015. We searched the imaging PACS (Picture Archiving and Communication System) and the admission records from the pediatric ED using the key words “trauma, pediatric, head, and MRI.” Inclusion criteria included patients 6 years of age or less who presented with mild traumatic head injury (Glasgow Coma Scale >13) and who received a rapid non-sedated MRI and CT within 24 h of injury. We reviewed the clinical and imaging records for patient age, gender, and mechanism of injury.
Imaging protocol
At our institution, rapid MRI with structural and blood-sensitive sequences has become the preferred initial modality for the evaluation of pediatric head trauma in order to reduce radiation exposure. In children in whom CT is performed first (typically at another institution), indications for additional rapid MRI include an inconclusive or technically limited CT, a finding that requires close interval follow-up, a negative CT in a child with persistent symptoms, or a negative CT in a child with a high risk for hemorrhage and in whom the presence of hemorrhage might alter management. We only perform head CT as the initial study if the wait for MRI is considered unacceptably long for appropriate patient care. We do not routinely perform both a head CT and MRI unless there is a clinical indication for which the modalities would provide complementary information.
CTs from both our institution and outside institutions (that often accompanied the children from the referring outside hospital) were included in the study. Head CT studies performed at our institution were acquired helically on a 64 detector row GE LightSpeed scanner (GE Healthcare, Little Chalfont, UK) with the following typical parameters: 150 mA, 100 kV, pitch 0.9, rotation time 1 s. All CTs included soft tissue algorithm (slice thickness range 1.25 to 5 mm) and bone algorithm (slice thickness range 0.625 to 2.5 mm). Additional coronal and sagittal reformatted images with soft tissue algorithm were reconstructed routinely (slice thickness range 1.25 to 2.5 mm).
All rapid MRI studies at our institution were performed on either a Siemens 3T Tim Trio (Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil or a GE 1.5T scanner (GE Healthcare, Little Chalfont, UK) using an eight-channel head coil. All MRIs in the study included three-plane half Fourier acquisition (HASTE Siemens) or single-shot fast spin echo (SSFSE GE) sequences in the axial, coronal, and sagittal planes, and echo planar imaging (EPI) susceptibility images with the following typical parameters: axial HASTE or SSFSE (TR 1037 ms, TE 120 ms, 3-mm slice thickness, 30–40 s), coronal HASTE or SSFSE (TR743 ms, TE 120 ms, 3-mm slice thickness, 30–40 s), and sagittal HASTE or SSFSE (TR 1383 ms, TE 120 ms, 3-mm slice thickness, 30–40 s) and axial EPI susceptibility (TR 12,000 ms, TE 25 ms, 3-mm slice thickness, 12–15 s) sequences. Total scan time for the four core sequence protocol (triplane HASTE/SSFSE and axial EPI susceptibility) was 1.5 to 2 min. Additional sequences including SWAN, T2/FLAIR, DWI, and T1 were obtained if requested by the radiologist or treating physician and if tolerated by the child.
To perform a pediatric MRI without sedation, a parent or other caregiver accompanies the child into the MRI room and then lies with the child on the scanner table so that the caregiver can provide reassurance to the child throughout the scan. The caregiver then holds the child’s head between her or his hands for additional tactile reassurance and to aid in control of head motion, and the technologist places the coil over the child’s head. The child and parent/caregiver are then moved into the scanner bore together and the scan is performed.
Imaging interpretation
The CT and rapid MRI studies were de-identified and reviewed concurrently by two board-certified neuroradiologists, (JY) and (SR). All CT and rapid MRI studies were reviewed independently by the two readers and with at least a 24-h interval between the review of the MRI and CT on the same patient, to prevent recall bias.
The CTs and MRIs were reviewed for the following findings: skull fracture, epidural hematoma (EDH)/subdural hematoma (SDH) (combined into the same category because the majority of the extra-axial collections were less than 4 mm in thickness and were often too small to place definitively into the subdural or epidural compartment), subarachnoid hemorrhage (SAH), intraventricular hemorrhage (IVH), diffuse axonal injury (DAI), and contusions.
Statistical analysis
McNemar’s test (p value) for correlated proportions was used to test the hypothesis that there was no difference between CT and MRI in the detection of each type of injury. Cohen kappa statistics (k) were performed to assess the agreement between the two imaging modalities in injury detection. Overall percentage agreement of the CT and MRI findings was also analyzed; for skull fractures, for example, the percentage agreement is defined as the total number of cases where a fracture is seen on both CT and MRI + the number of cases where neither CT nor MRI show a fracture, divided by the number of patients (33).
Results
A total of 33 patients met inclusion criteria. The mean age was 23 months with a range of 3 days to 6 years. 67 % were male and 33 % were female. The mechanisms of injury included fall (79 %) and blunt trauma (21 %).
All patients received a CT, 18 of which were acquired at an outside institution, and a rapid non-sedated MRI, all of which were performed at our institution. Both the CT and MRI were performed within 24 h of injury with an average time between imaging studies of 7 h (range of 1 to 23 h). Typically, CT was acquired first, but in four cases, MRI was acquired before CT.
Of the 33 patients, 30 had radiologically identified traumatic injuries (Table 1). Twenty-five patients had intracranial injuries, 16 of which had accompanying skull fractures, and five patients had isolated skull fractures. Overall, the correlation of traumatic findings between CT and rapid MRI was moderate to good (k = 0.59), with no statistically significant difference in injury detection (p = 0.69). The overall agreement was 82 %.
When subdivided by injury type, the degree of correlation between CT and MRI was not as strong. In our study, 21 skull fractures were found on CT, of which only seven were seen on MRI (k = 0.27 and an overall agreement of 58 %). This injury subtype was the only one which had a statistically significant difference in detection between CT and MRI (p = 0.0001).
There was no statistically significant difference in the detection of EDH/SDH between CT and MRI (p = 0.34) with k = 0.39 and an overall agreement of 70 %. Between the two modalities, there were a total of 24 extra-axial collections, six of which were greater than 4 mm in maximal thickness (four SDH and two EDH). MRI had a higher detection rate (e.g., number of instances in which the MRI or CT is interpreted as positive for a particular finding) for EDH/SDH, identifying 21 extra-axial collections, seven of which were not seen on CT. CT detected 17 extra-axial collections, three of which were not seen on MRI. The missed hemorrhages by either modality were small and all less than 4 mm in thickness.
There was no statistically significant difference in the detection of SAH between CT and MRI (p = 0.07) with k = 0.41 and an overall agreement of 76 %. There were a total of 13 cases of SAH, 12 of which were detected by MRI and only six by CT. The SAH missed by either modality was subtle.
There were no cases of intraventricular hemorrhage (IVH).
There was no statistically significant difference in the detection of parenchymal injuries (contusions and DAI) between CT and MRI (p = 0.50) with k = 0.48 and an overall agreement of 91 %. However, MRI had a higher detection rate of parenchymal injuries, identifying one case of DAI and contusion in the same patient and one case of contusion in a different patient, all of which were not seen on CT. There was one case of contusion seen on CT which was also identified by MRI.
Discussion
The results of this study suggest that MRI has similar detection rates as CT for intracranial injury in young patients presenting with head trauma, with many cases demonstrating concordant results (Fig. 1). Furthermore, although there was no statistically significant difference in detection, due to the relatively small number of patients with each injury type, MRI had higher detection rates for EDH/SDH, SAH, and parenchymal injuries. However, MRI was inferior to CT in the detection of skull fractures, missing 14 of 21 skull fractures. This injury subtype was the only one which had a statistically significant difference in detection between CT and MRI (p = 0.0001). While both imaging modalities missed findings, they were small and/or subtle and did not require neurosurgical intervention.
MRI had a higher detection rate of EDH/SDH, with seven positive cases in which CT was negative. Most of these hemorrhages were overlying the cerebral convexities, suggesting that the increased contrast resolution of MRI compared to CT may be useful in identifying these small extra-axial hemorrhages (Fig. 2). However, MRI missed three cases of EDH/SDH, two of which were located along the tentorial leaflets. A possible explanation why MRI may not detect hemorrhages as well in this location could be due to the combination of the small size (<4 mm) and orientation of the hematomas, which allowed the increased spatial resolution of CT and contrast between the relatively high density of the hemorrhage/tentorium compared to adjacent brain parenchyma to have better detection in this location.
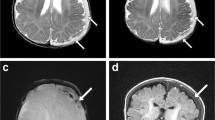
MRI also had a higher detection rate of SAH. There were seven cases in which MRI identified SAH and CT did not (Fig. 3). In these instances, the subarachnoid hemorrhages were small in volume and identified best on the susceptibility sequences.
a A focus of susceptibility is seen in a right superior parietal sulcus on an axial susceptibility image consistent with subarachnoid hemorrhage; no corresponding abnormality is seen on the axial T2 (b) or axial CT (c) images; another case shows similar findings in which several foci of subarachnoid hemorrhage are seen on the susceptibility sequence (d), but not on the T2 (e) or CT (f) images
While there were only three cases of parenchymal injury, MRI had a higher detection rate for these injuries (Fig. 4). This result was consistent with prior studies and not unexpected given the higher tissue contrast resolution of MRI compared to CT [18, 19]. While small contusions and DAI do not necessitate neurosurgical intervention, the presence of these injuries has been shown to be associated with worse outcomes following minor head trauma [20]. Additional studies are ongoing to assess how findings from early MRI in pediatric trauma influence management (e.g., admission decisions, anticonvulsant prophylaxis, use of adjuvant treatments such as fluid management, escalation or de-escalation of care, follow-up imaging, and discharge/follow-up care).
Axial susceptibility image (a) demonstrates a hemorrhagic contusion seen in the left inferior frontal lobe which was not seen on the corresponding axial CT image (b); axial susceptibility (c) and axial DWI (d) images of the same patient show foci of traumatic axonal injury which were also not seen on CT
MRI was significantly worse compared to CT in the detection of skull fractures, missing 14 of 21 skull fractures, which is consistent with prior studies [5, 17]. However, in our study, all of the missed fractures on MRI were linear and non-displaced and did not require neurosurgical or other specific intervention (Fig. 5). Arrey et al. performed a retrospective review of 326 pediatric patients with linear nondisplaced skull fractures and found that none of them had neurological deficits on exam and none required surgical intervention [21]. Nonetheless, if skull fracture diagnosis is important, for example, in the evaluation of possible non-accidental injury or high-energy mechanisms with a high likelihood of fracture, our protocol is to obtain a low-dose 3D skull CT following the rapid MRI. This protocol has less radiation compared to routine head CT and also is very helpful in detecting subtle fractures.
Axial CT image on bone windows (a) shows a linear, non-displaced right occipital skull fracture that was not identified on the corresponding axial T2 MR image (b); another case shows a left parietal skull fracture seen on a coronal CT image (c) that was not seen on the corresponding coronal T2 MR image (d); of note, on the axial T2 MR image (b), the hands of the parent or technologist can be seen holding the patient’s head during image acquisition
One additional advantage to early MRI imaging of pediatric patients with head injury is additional information about the cervical spine in the acute phase if clinically indicated. While most awake children can be cleared by clinical examination, inclusion of a sagittal image in the rapid MRI protocol which covers the cervicomedullary junction and upper cervical spine can be a helpful adjunct in the early evaluation of many pediatric trauma patients who otherwise may be difficult to assess.
One of the limitations of our study was the time interval separating the CT and MRI scans, which on average was 7 h and may have allowed for evolution and potentially increased conspicuity of injuries. Conversely, some lesions have been known to dissipate rapidly (such as some epidurals associated with skull fractures or subdural/subarachnoid hemorrhage which appear to “wash out” quickly). However, this time frame is significantly shorter compared to previously published studies in which MRI was often performed days to weeks after the initial CT. Two of the more recently published studies which had shorter durations between the acquisition of CT and MRI still had an average of 19 and 24 h of separation [5, 17].
While there are obstacles to completion of MRI studies in a timely manner, a published study from our institution demonstrated the feasibility of rapid MRI in the evaluation of pediatric acute head injury. Cohen et al. retrospectively compared a cohort of 45 pediatric patients who received a rapid brain MRI in the ED with an age-matched cohort of 45 patients who received a non-contrast head CT [22]. The difference in the length of stay was only 41 min, which improved over time during the study as the staff became more familiar with the protocol.
The stability of patients and the safety of scanning them is another consideration when implementing rapid MRI in the imaging workup of trauma. In our study, all patients had sustained head trauma at the milder end of the clinical spectrum (GCS > 13). Ongoing studies include children who present with more severe head trauma and undergo MRI within the first 1–2 days of injury. The use of MRI in the management of pediatric head trauma is feasible and shows higher detection rates of certain parenchymal and extra-axial lesion types. Continued study to investigate the clinical utility and cost-effectiveness of rapid MRI in the acute evaluation of pediatric head injury appears warranted from the current results.
Conclusion
Non-sedated rapid MRI has similar detection rates as CT for intracranial injury in young children presenting with acute head trauma. However, MRI is inferior to CT for the detection of linear non-displaced skull fracture. If the diagnosis of a skull fracture is important for clinical assessment, then a reduced-dose 3D skull CT can be obtained for further evaluation. The clinical utility, cost-effectiveness, and effect on outcomes of rapid MRI for acute pediatric head trauma evaluation warrant further study.
References
Faul M, Xu L, Wald MM, Coronado VG (2010) Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. Centers for Disease Control and Prevention, Atlanta
Ryan ME, Palasis S, Saigal G et al (2014) ACR appropriateness criteria head trauma—child. J Am Coll Radiol 11:939–947
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
Mathews JD, Forsythe AV, Brady Z et al (2013) Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 346:f2360
Roguski M, Morel B, Sweeney M et al (2015) Magnetic resonance imaging as an alternative to computed tomography in select patients with traumatic brain injury: a retrospective comparison. J Neurosurg Pediatr 15:529–34
Buttram SD, Garcia-Filion P, Miller J, Youssif M, Brown SD, Dalton HJ, Adelson PD (2015) Computed tomography vs magnetic resonance imaging for identifying acute lesions in pediatric traumatic brain injury. Hosp Pediatr 5:79–84
Kemp AM, Rajaram S, Mann M et al (2009) What neuroimaging should be performed in children in whom inflicted brain injury (iBI) is suspected? A systematic review. Clin Radiol 64:473–483
Foerster BR, Petrou M, Lin D, Thurnher MM, Carlson MD, Strouse PH, Sundgren PC (2009) Neuroimaging evaluation of non-accidental head trauma with correlation to clinical outcomes: a review of 57 cases. J Pediatr 154:573–577
Sigmund GA, Tong KA, Nickerson JP, Wall CJ, Oyoyo U, Ashwal S (2007) Multimodality comparison of neuroimaging in pediatric traumatic brain injury. Pediatr Neurol 36:217–226
Sato Y, Yuh WT, Smith WL, Alexander RC, Kao SC, Ellerbroek CJ (1989) Head injury in child abuse: evaluation with MR imaging. Radiology 173:653–657
Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell DT, Wong JH, Tait AR (2000) Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth 84:743–748
Cote CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C (2000) Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics 106:633–644
Wang X, Xu Z, Miao CH (2014) Current clinical evidence on the effect of general anesthesia on neurodevelopment in children: an updated systematic review with meta-regression. PLoS One 9:e85760
O’Neill BR, Pruthi S, Bains H et al (2013) Rapid sequence magnetic resonance imaging in the assessment of children with hydrocephalus. World Neurosurg 80:e307–312
Ashley WW Jr, McKinstry RC, Leonard JR, Smyth MD, Lee BC, Park TS (2005) Use of rapid-sequence magnetic resonance imaging for evaluation of hydrocephalus in children. J Neurosurg 103:124–130
Missios S, Quebada PB, Forero JA, Durham SR, Pekala JS, Eskey CJ, Duhaime AC (2008) Quick-brain magnetic resonance imaging for nonhydrocephalus indications. J Neurosurg Pediatr 2:438–444
Mehta H, Acharya J, Mohan AL, Tobias ME, LeCompte L, Jeevan D (2015) Minimizing radiation exposure in evaluation of pediatric head trauma: use of rapid MR imaging. AJNR Am J Neuroradiol 37:11–18
Lee H, Wintermark M, Gean AD, Ghajar J, Manley GT, Mukherjee P (2008) Focal lesions in acute mild traumatic brain injury and neurocognitive outcome: CT versus 3T MRI. J Neurotrauma 25:1049–1056
Mittl RL, Grossman RI, Hiehle JF, Hurst RW, Kauder DR, Gennarelli TA, Alburger GW (1994) Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol 15:1583–1589
Yuh EL, Mukherjee P, Lingsma HF et al (2013) Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 73:224–235
Arrey EN, Kerr ML, Fletcher S, Cox CS Jr, Sandberg DI (2015) Linear nondisplaced skull fractures in children: who should be observed or admitted? J Neurosurg Pediatr 16:703–708
Cohen AR, Caruso P, Duhaime AC, Klig JE (2015) Feasibility of “rapid” magnetic resonance imaging in pediatric acute head injury. Am J Emerg Med 33:887–890
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Young, J.Y., Duhaime, AC., Caruso, P.A. et al. Comparison of non-sedated brain MRI and CT for the detection of acute traumatic injury in children 6 years of age or less. Emerg Radiol 23, 325–331 (2016). https://doi.org/10.1007/s10140-016-1392-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-016-1392-3