Abstract
Background
Gradient echo T2*-W sequences are more sensitive than T2-W spin-echo sequences for detecting hemorrhages in the brain.
Objective
The aim of this study is to correlate presence of hemosiderin deposits in the brain of very preterm infants (gestational age <32 weeks) detected by T2*-W gradient echo MRI to white matter injury and neurodevelopmental outcome at 2 years.
Materials and methods
In 101 preterm infants, presence and location of hemosiderin were assessed on T2*-W gradient echo MRI performed around term-equivalent age (range: 40–60 weeks). White matter injury was defined as the presence of >6 non-hemorrhagic punctate white matter lesions (PWML), cysts and/or ventricular dilatation. Six infants with post-hemorrhagic ventricular dilatation detected by US in the neonatal period were excluded. Infants were seen for follow-up at 2 years. Univariate and regression analysis assessed the relation between presence and location of hemosiderin, white matter injury and neurodevelopmental outcome.
Results
In 38/95 (40%) of the infants, hemosiderin was detected. Twenty percent (19/95) of the infants were lost to follow-up. There was a correlation between hemosiderin in the ventricular wall with >6 PWML (P < 0.001) and cysts (P < 0.001) at term-equivalent age, and with a lower psychomotor development index (PDI) (P=0.02) at 2 years. After correcting for known confounders (gestational age, gender, intrauterine growth retardation and white matter injury), the correlation with PDI was no longer significant.
Conclusion
The clinical importance of detecting small hemosiderin deposits is limited as there is no independent association with neurodevelopmental outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) is a safe and valuable tool to assess development and pathology of the preterm infant’s brain [1, 2, 3, 4]. In infants born very prematurely, germinal matrix and intraventricular hemorrhage (GMH/IVH) and white matter injury are frequently encountered [5, 6, 7], while cerebellar hemorrhage is increasingly recognized [8, 9, 10, 11]. All are associated with later cognitive and motor impairment [5, 12, 13, 14, 15].
Elevation of free radicals and iron in cerebrospinal fluid is associated with both hemorrhages and white matter injury in very preterm and low birth weight infants [16, 17]. Therefore an independent influence of hemorrhages on neurodevelopmental outcome cannot be excluded.
Although the T2*-W gradient echo technique has a much higher sensitivity for detection of (small) hemorrhages, in particular hemosiderin deposits, than T1-W and T2-W MR techniques [18, 19, 10], its added clinical value for brain imaging in preterm infants has not been determined.
In the present study, we used a T2*-W gradient echo sequence for detection and location of small hemosiderin deposits in an unselected cohort of very preterm infants who underwent MRI within 3 months after term-equivalent age. We investigated the clinical significance of these hemosiderin deposits by evaluating the association with white matter injury and neurodevelopmental outcome around the corrected age of 2 years.
Materials and methods
Very preterm infants
As part of a prospective neuroimaging study performed in an unselected group of very preterm infants (gestational age <32 weeks) admitted to the tertiary neonatal unit of our hospital, 113 infants underwent MRI, preferably around term at a postmenstrual age of 40–44 weeks. For infants who were unstable around that age, MRI was postponed, resulting in an age range of 40–60 postmenstrual weeks at imaging. Thirty-four infants were imaged beyond 44 weeks. Ethical approval for the study was given by the institutional review board and informed parental consent was obtained for each infant.
In three infants, congenital malformations of the central nervous system were found on MRI and they were excluded from the study. In nine infants, a T2*-W gradient echo sequence was not performed. Six infants diagnosed with a grade III-IV intraventricular hemorrhage and post-hemorrhagic ventricular dilatation (PHVD) on brain US in the neonatal period were excluded from further analysis to ascertain that ventricular dilatation on the term MRI’s resulted ex vacuo from white matter injury.
Therefore, for this part of the study, data of 95 infants were included. Clinical parameters were collected from the patients’ files.
Image and data acquisition
All MRI examinations were performed on a 3-T MRI system (Achieva, Philips Medical Systems, Best, the Netherlands) according to a standard protocol for imaging the newborn infant’s brain [4]. The infants were sedated using chloral hydrate (55 mg/kg), were supine and were swaddled during the scanning procedure. Ear protection consisted of neonatal earmuffs (Natus Mini Muffs; Natus Medical Inc., San Carlos, CA, USA) covered by a headphone. All MRI examinations included a 3-D T1 turbo field echo sequence (TR: 9.7 ms, TE: 4.6 ms, FOV: 180 mm, matrix size: 192 × 152, flip angle: 8º, TFE factor: 128, slice thickness: 1 mm), a T2 turbo spin-echo sequence (TR: 6,269 ms, TE: 120 ms, FOV: 180 mm, matrix size: 336 × 234, TSE factor: 18, slice thickness: 2 mm), and a T2* fast field echo sequence (TR: 735 ms, TE: 16 ms, FOV: 230 mm, matrix size: 256 × 163, flip angle: 18º, slice thickness: 4 mm).
Hemosiderin deposits
To assess the presence of hemosiderin, two investigators (F. T. d. B. and S. J. S.) examined the T2*-W gradient echo images together by consensus.
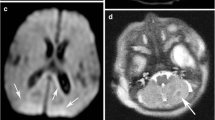
Hemosiderin deposits, originating from hemorrhages occurring in the perinatal period, were defined as hypointense signal intensity lesions or blooming effects (Fig. 1). The location of hemosiderin was noted in anatomical regions as shown in Table 2.
Cerebellar hemorrhages were identified as hypointense or blooming artifacts on T2*-W gradient echo sequences. We distinguished primary lobar or folial cerebellar hemorrhages located within the confines of the cerebellum from posterior fossa subarachnoid hemorrhage as hemorrhagic lesions outside and surrounding the cerebellum (Fig. 2).
White matter injury and ventricular size
Two investigators (F. T. d. B and L. M. L.) analyzed all T1-W and T2-W sequences together by consensus for presence of white matter injury and ventricular dilatation. White matter injury was defined as >6 non-hemorrhagic punctate white matter lesions (PWML) and/or cystic white matter lesions and/or ventricular dilatation with the exclusion of PHVD. Non-hemorrhagic PWML on MRI were defined as bilateral high signal lesions on T1-W sequences, indistinct or with a low signal intensity on T2-W sequences and not visible on T2*-W gradient echo sequences. They were discriminated from small venous infarcts, which are usually located unilaterally, mostly contain hemosiderin and are thus visible on the T2*-W gradient echo sequences. On coronal reconstructions from the 3-D T1-W images, the ventricular index was measured as the total width of the lateral ventricles, divided by 2, in analogy to ventricular index measurements on US [20]. A ventricular index between 12 and 16 mm was considered moderate dilatation and a ventricular index of more than 16 mm was considered severe dilatation [21]. Diffuse excessive high signal intensity (DEHSI) was not considered part of the spectrum of white matter injury [22].
Follow-up
Around 2 years of age, the infants were seen by an experienced neonatologist, who was unaware of the neuroimaging findings. Each child underwent a standardized neurological examination to assess the presence of cerebral palsy or abnormal muscular tone. A Gross Motor Function Classification System (GMFCS) level was assigned [23]. A GMFCS level of 2 or more was considered cerebral palsy.
The cognitive and psychomotor development was assessed by a psychology assistant, who was unaware of the neuroimaging findings, using the Dutch version of the Bayley Scales of Infant Development (BSID-III). A mental developmental index score (MDI) and a psychomotor developmental index score (PDI) were calculated for the corrected age of the child. Five infants diagnosed with cerebral palsy, in whom testing the gross and fine motor function with the BSID-III was not feasible, were assigned a PDI score of 50.
To evaluate child behavior, the Dutch version of the Child Behavior Checklist 1½ to 5 (CBCL) [24] was sent to the child’s home address prior to the follow-up visit, to be completed by either parent or caretaker. The questionnaire consists of 99 items rated on a 3-point scale. By summing the scores, an internalizing, externalizing, total and other problem score can be computed. These raw scores were transformed into normalized T-scores and used as secondary outcome parameters in the analyses, where a higher score represented more severe behavioral problems.
Statistical analyses
Data were analyzed using SPSS 17.0.1 IBM, Armonk, New York, USA. Frequency counts and percentages were used to summarize categorical parameters. For continuous parameters, mean and standard deviations are reported.
The clinical parameters of the infants with and without 2-year follow-up were compared with chi-square, Fisher exact and Mann–Whitney U tests where appropriate.
The association between presence of hemosiderin per location and white matter injury (>6 PWML, cystic lesions and ventricular dilatation) and outcome parameters was evaluated using chi-square, Fisher exact and Mann–Whitney U tests. For the relation with outcome, backward linear and logistic regression were subsequently used to adjust for known potentially confounding neonatal characteristics (gestational age, gender, intrauterine growth retardation) and white matter injury on MRI. Statistical significance was defined by two-tailed p-value <0.05.
Results
Clinical parameters
The clinical parameters of infants (n=95) are shown in Table 1. The numbers of infants with white matter injury (>6 PWML, cystic white matter lesions, ventricular dilatation) are mentioned separately.
Hemosiderin deposits
Table 2 shows the number of infants with hemosiderin deposits and the location as seen on T2*-W gradient echo. Figure 3 illustrates the different supratentorial hemosiderin locations. Hemosiderin deposits were detected in 38/95 (40%) of the infants. These were supratentorially located in 25/95 (26.3%) and infratentorially located in 23/95 (24.2%) of the infants. In 10/23 of the infants with infratentorial hemosiderin deposits, supratentorial hemosiderin deposits coexisted. Of the 22 infants with a GMH/IVH on perinatal US, 18 (81.8%) were also diagnosed as GMH/IVH on MRI. Of the 38 infants with supra- and infratentorial hemosiderin on MRI, 18 (47.4%) were diagnosed with hemorrhages on US (P < 0.0001).
Figure set illustrating Table 2 supratentorial hemosiderin deposits. a T2*- W gradient echo MRI in a 31-week very preterm infant, imaged at 43 weeks shows hemosiderin at the site of the germinal matrix on the right, and bilateral hemosiderin deposits in the occipital horns after intraventricualar hemorrhage. b Same patient on the T2-W sequence cystic periventricular leucomalacia along the occipital horns and abnormally shaped severely dilated lateral ventricles. c MRI in a 30-week very preterm infant, imaged at 44 weeks, on the T2-W sequence, on the right a germinal matrix hemorrhage and bilateral in the periventricular white matter hemosiderin deposits around the occipital horns. d MRI in a 31 + 6 weeks very preterm infant imaged at 40 weeks, on the T2-W sequence a large porencephalic hemosiderin lined cyst on the left, extending to the deep white matter
Of the 75 infants with flaring on US, 31 (41.3%) had hemosiderin deposits on MRI. Thirty-one out of 38 (81.6%) infants with hemosiderin on MRI had flares on US (P=0.61).
Association between hemosiderin deposits per location and white matter injury
Table 3 shows the associations per location between the presence of hemosiderin deposits on T2*-W gradient echo sequences and white matter injury on MRI (>6 PWML, ex-vacuo ventricular dilatation and cysts). Non-hemorrhagic PWML were mainly located above the level of the caudate head and above the level of the basal ganglia, bilaterally in a linear distribution, parallel to the atrium (Fig. 4) and sometimes at the level of the trigones distributed along the optic radiation. Sixteen infants had >6 PWML, 49 infants had moderate ventricular dilatation, 17 severe ventricular dilatation and 6 infants had cysts. Three of the infants with cysts had a unilateral cyst, after periventricular hemorrhagic infarct (PVHI), and the remaining three had bilateral cysts within the cystic PVL spectrum.
MRI in a female infant (GA 26, PMA at MRI 42 weeks). a The T1-W image shows bilaterally in the centrum semiovale linearly parallel to the atrium located high signal intense non-hemorrhagic PWML are shown, indistinct on T2-W (b) sequence and not visible on the T2*-W gradient echo sequence (c). GA gestational age, PMA postmenstrual age
Nineteen infants had one white matter injury item, seven infants had two and only two infants had all three items.
The presence of hemosiderin in the wall of the lateral ventricles was strongly associated with > 6 PWML (P < 0.001). 10 (62.5%; P=0.04) out of these 16 infants with non-hemorrhagic PWML had hemosiderin deposits either supra- and/or infratentorially.
Furthermore, there were associations between hemosiderin deposits in and around the ventricle and >6 PWML (P=0.03 and P=0.04) and between intraventricular hemosiderin deposits and severe ventricular dilatation (P=0.02). There were significant associations between hemosiderin deposits at most locations and cystic white matter lesions (P=0.003). There were no associations between infratentorial cerebellar hemosiderin deposits and supratentorial white matter injury.
Follow-up
Follow-up was available for 76 (80%) infants. Infants were lost to follow-up due to miscellaneous reasons such as rejection of participation or practical problems, including travel distance to the hospital. Mean corrected age at follow-up was 29.7 months (range: 20.1–42.1 months, SD 4.5). No difference in baseline clinical parameters or presence of hemosiderin deposits was found between infants with and without follow-up.
There were five infants with cerebral palsy. Three infants showed >6 PWML, four infants had moderate/severe dilation of the ventricles, two infants had cysts and three infants had hemosiderin deposits. There was only one infant who had all white matter injury imaging abnormalities.
Table 4 shows the unadjusted and adjusted associations between hemosiderin deposits per location and the PDI at 2 years. In infants with hemosiderin deposits supratentorially and/or in the ventricular wall, we found a lower PDI (respectively, P=0.04 and P=0.02). However, after correcting for clinical parameters (gestational age, gender and intrauterine growth retardation) and white matter injury, the association was no longer significant. Backward linear regression analyses showed that the presence of >6 PWML (P=0.002) and cystic white matter lesions (P=0.02) were independent predictors of a lower PDI. After linear regression (correction for GA, gender and intrauterine growth retardation), >6 PWML showed a significant correlation with a lower MDI score (P = 0.047). The correlation between severe ventricular dilatation and a lower MDI was not significant (P=0.700). No differences in cognitive or motor delay, cerebral palsy or CBCL scores were found between infants with (n=32) and without (n=44) hemosiderin deposits.
Discussion
In this study, we assessed presence of hemosiderin deposits, as detected by T2*-W gradient echo MRI, in an unselected cohort of very preterm infants and correlated presence and location of hemosiderin deposits with white matter injury and neurodevelopmental outcome at 2 years of corrected age. Our most important finding is that especially hemosiderin deposits located in the ventricular wall are correlated with white matter injury and a less favorable neurodevelopmental outcome. However, after adjusting for clinical parameters and white matter injury, the association between these hemosiderin deposits and a less favorable neurodevelopmental outcome was no longer significant. The presence of more than six T2*-W negative PWML and cystic white matter lesions were independent predictors of psychomotor delay.
The clinical use of gradient echo techniques, including susceptibility-weighted imaging (SWI), to detect microbleeds or hemosiderin deposits in the elderly is widely accepted [25, 19, 26]. Yet reports on the use of gradient echo techniques in infants are scarce [27, 28]. In a recent study, the use of SWI to distinguish hemorrhagic from non-hemorrhagic punctate white matter lesions in neonates was reported [29]. However, in this paper, the clinical relevance of detecting hemorrhagic lesions in relation to neurodevelopmental follow-up was not discussed.
From large cohort studies [30] it is known that amongst other pathologies, intraventricular hemorrhage is predictive of a less favorable neurodevelopmental outcome in very preterm neonates. This is especially true for grade III–IV intraventricular hemorrhages detected by US in the neonatal period [31, 32]. Ultrasound studies have also reported poorer neurodevelopmental outcomes at 20 months in extremely low birth weight infants with grade I-II intraventricular hemorrhages. However, US could have missed additional injury associated with intraventricular hemorrhages explaining the poorer outcomes [33]. As we excluded infants with PHVD diagnosed by US, the ventricular dilatation in our cohort most likely resulted from white matter injury. We found that hemosiderin deposits in and around the ventricular walls, without parenchymal involvement, are especially associated with PWML and cystic white matter lesions, and that these hemosiderin deposits are correlated with a less favorable neurodevelopmental outcome. In a recent study, it was found that GMH/IVH was associated with hemorrhagic punctate white matter lesions, possibly as a result of impaired venous drainage and increased pressure in the medullary veins resulting in periventricular white matter damage [29]. Our data showed that after correction for clinical parameters and white matter injury, the association between hemosiderin deposits in the ventricular wall and a less favorable neurodevelopmental outcome was no longer significant, while the presence of >6 non-hemorrhagic PWML and cystic white matter lesions were independent predictors of a lower PDI. Therefore, our data show that the presence of white matter injury is a better predictor of a less favorable neurodevelopmental outcome than small ventricular wall hemorrhages.
A limitation of our study is that we used a T2*-W gradient echo technique, while SWI is increasingly being used in clinical practice [29, 28]. However, the clinical significance of detecting more and/or even smaller hemosiderin deposits with SWI as compared to T2*-W gradient echo MRI [25] seems to be limited.
In this cohort of preterm infants, we defined ventricular dilatation as ex vacuo ventricular dilatation from white matter injury resulting in volume loss [34, 35]. By excluding infants with PHVD diagnosed by US during the neonatal period, we intended to differentiate between ex vacuo ventricular dilatation and PHVD. However, both forms of ventricular dilatation may co exist and are interrelated. Moreover, we may have missed hemosiderin deposits, as the age range (40–60 weeks postmenstrual age) at which the infants were imaged may be of influence on the detectability of hemosiderin originating from hemorrhages occurring in the perinatal period. Also non-hemorrhagic PWML may resolve after a period of time.
Another limitation of our study is that a substantial number of infants (20%) were lost to follow-up. However, at baseline, there were no differences in clinical parameters or presence of hemosiderin deposits between infants with or without clinical follow-up.
As cognitive delay or handicap is especially difficult to test at this young age, long-term follow-up at school age may still demonstrate differences in cognitive and behavioral performance.
Conclusion
In conclusion, we demonstrated that T2*-W gradient echo sequences detect and localize small hemosiderin deposits in very preterm infants, especially in and around the ventricular system. The clinical importance of detecting these small hemosiderin deposits is, however, limited as there is no independent association with neurodevelopmental outcome. We believe that on conventional MRI sequences white matter injury characterized as >6 non-hemorrhagic PWML, cystic lesions and/or severe ex vacuo ventricular dilatation are the more important predictors for adverse outcome.
References
Barkovich AJ, Maroldo TV (1993) Magnetic resonance imaging of normal and abnormal brain development. Top Magn Reson Imaging 5:96–122
Counsell SJ, Rutherford MA, Cowan FM et al (2003) Magnetic resonance imaging of preterm brain injury. Arch Dis Child Fetal Neonatal Ed 88:F269–F274
Rutherford M, Biarge MM, Allsop J et al (2010) MRI of perinatal brain injury. Pediatr Radiol 40:819–833
van Wezel-Meijler G, Leijser LM, de Bruïne FT et al (2009) Magnetic resonance imaging of the brain in newborn infants: practical aspects. Early Hum Dev 85:85–92
Dyet LE, Kennea N, Counsell SJ et al (2006) Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118:536–548
Leijser LM, de Bruïne FT, Steggerda SJ et al (2009) Brain imaging findings in very preterm infants throughout the neonatal period: part I. Incidences and evolution of lesions, comparison between ultrasound and MRI. Early Hum Dev 85:101–109
Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8:110–124
Grunnet ML, Shields WD (1976) Cerebellar hemorrhage in the premature infant. J Pediatr 88:605–608
Limperopoulos C, Benson CB, Bassan H et al (2005) Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics 116:717–724
Steggerda SJ, Leijser LM, Wiggers-de Bruïne FT (2009) Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology 252:190–199
Tam EW, Rosenbluth G, Rogers EE (2011) Cerebellar hemorrhage on magnetic resonance imaging in preterm newborns associated with abnormal neurologic outcome. J Pediatr 158:245–250
Limperopoulos C, Bassan H, Gauvreau K et al (2007) Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 120:584–593
Miller SP, Ferriero DM (2009) From selective vulnerability to connectivity: insights from newborn brain imaging. Trends Neurosci 32:496–505
Tam EW, Miller SP, Studholme C et al (2011) Differential effects of intraventricular hemorrhage and white matter injury on preterm cerebellar growth. J Pediatr 158:366–371
Woodward LJ, Clark CA, Pritchard VE et al (2011) Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev Neuropsychol 36:22–41
Inder T, Mocatta T, Darlow B et al (2002) Elevated free radical products in the cerebrospinal fluid of VLBW infants with cerebral white matter injury. Pediatr Res 52:213–218
Savman K, Nilsson UA, Blennow M (2001) Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilatation. Pediatr Res 49:208–212
Atlas SW, Mark AS, Grossman RI et al (1988) Intracranial hemorrhage: gradient-echo MR imaging at 1.5 T. Comparison with spin-echo imaging and clinical applications. Radiology 168:803–807
Greenberg SM, Vernooij MW, Cordonnier C (2009) Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 8:165–174
Levene MI (1981) Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child 56:900–904
Leijser LM, de Bruïne FT, Steggerda SJ et al (2009) Brain imaging findings in very preterm infants throughout the neonatal period: part I. Incidences and evolution of lesions, comparison between ultrasound and MRI. Early Hum Dev 85:101–109
de Bruïne FT, van den Berg-Huysmans AA, Leijser LM et al (2011) Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology 261:899–906
Palisano RJ, Hanna SE, Rosenbaum PL et al (2000) Validation of a model of gross motor function for children with cerebral palsy. Phys Ther 80:974–985
Achenbach TM, Ruffle TM (2000) The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 21:265–271
Goos JD, van der Flier WM, Knol DL et al (2011) Clinical relevance of improved microbleed detection by susceptibility-weighted magnetic resonance imaging. Stroke 42:1894–1900
Haacke EM, Xu Y, Cheng YC et al (2004) Susceptibility weighted imaging (SWI). Magn Reson Med 52:612–618
Niwa T, Aida N, Takahara T et al (2010) Imaging and clinical characteristics of children with multiple foci of microsusceptibility changes in the brain on susceptibility-weighted MRI. Pediatr Radiol 40:1657–1662
Tong KA, Ashwal S, Obenaus A (2008) Susceptibility-weighted MR imaging: a review of clinical applications in children. AJNR Am J Neuroradiol 29:9–17
Niwa T, de Vries LS, Benders MJ et al (2011) Punctate white matter lesions in infants: new insights using susceptibility-weighted imaging. Neuroradiology 53:669–679
Beaino G, Khoshnood B, Kaminski M et al (2010) Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Dev Med Child Neurol 52:e119–e125
Brouwer A, Groenendaal F, Van Haastert I et al (2008) Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr 152:648–654
de Vries LS, Van Haastert I, Rademaker KJ et al (2004) Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr 144:815–820
Patra K, Wilson-Costello D, Taylor HG et al (2006) Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr 149:169–173
Inder TE, Warfield SK, Wang H et al (2005) Abnormal cerebral structure is present at term in premature infants. Pediatrics 115:286–294
Ment LR, Hirtz D, Huppi PS (2009) Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol 8:1042–1055
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Bruïne, F.T., Steggerda, S.J., van den Berg-Huysmans, A.A. et al. Prognostic value of gradient echo T2* sequences for brain MR imaging in preterm infants. Pediatr Radiol 44, 305–312 (2014). https://doi.org/10.1007/s00247-013-2803-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-013-2803-1