Abstract
Background
Obstructed total anomalous pulmonary venous connection (TAPVC) is frequently misdiagnosed as pulmonary disease and without operative correction early death is common. It is important to make a correct diagnosis before surgery.
Objective
The purpose of this study was to describe the chest radiographic features of obstructed TAPVC and compare CT angiography with transthoracic echocardiography in the evaluation of obstructed TAPVC.
Materials and methods
Eighteen children with obstructed TAPVC were assessed. Their clinical and imaging data were retrospectively reviewed. The characteristic radiographic findings were analyzed and compared with surgical results, and the diagnostic accuracy of CT angiography and transthoracic echocardiography was evaluated in terms of pulmonary venous drainage and obstruction detection.
Results
The common radiographic features included pulmonary venous congestion or edema or both (16 of 18 cases, 89%), and absence of cardiomegaly (12 of 18 cases, 67%). CT angiography correctly diagnosed TAPVC and clearly revealed the draining sites in all children (five with supracardiac TAPVC, three with cardiac TAPVC, eight with infracardiac TAPVC and two with mixed TAPVC). The diagnostic agreement between CT angiography and surgery was 100%. Transthoracic echocardiography only correctly revealed the draining sites in 11 children (5 with supracardiac TAPVC, 2 with cardiac TAPVC and 4 with infracardiac TAPVC). The diagnostic agreement between transthoracic echocardiography and surgery was 61%. The diagnostic accuracy of CT angiography was higher than that of transthoracic echocardiography (P = 0.0156). Thirty-four sites of obstruction were correctly detected by CT angiography (11 in the mediastinum, 1 at the diaphragmatic level, 9 below the diaphragm and 13 stenotic individual pulmonary veins in the lung). The diagnostic agreement between CT angiography and surgery was 92%. Transthoracic echocardiography only correctly detected 15 sites of obstruction (11 in the mediastinum, 1 at the diaphragmatic level and 3 below the diaphragm). The diagnostic agreement between transthoracic echocardiography and surgery was 41%. The rate of detection for sites of obstruction with transthoracic echocardiography was much lower than that of CT angiography (P = 0.0002).
Conclusion
In children with obstructed TAPVC, plain radiographs usually show a characteristic pattern of pulmonary venous congestion or edema, or both, and a normal cardiac silhouette. CT angiography is superior to transthoracic echocardiography in the evaluation of pulmonary venous drainage and obstruction, especially in children with infracardiac and mixed TAPVC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
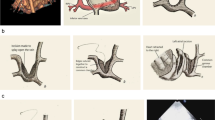
Total anomalous pulmonary venous connection (TAPVC) accounts for about 1–2% of congenital heart disease [1]. Embryologically, TAPVC results from the persistent connection of the pulmonary veins to the right side of the heart. Darling et al. [2] grouped the lesions into four types according to the site where the connection occurs: (1) Supracardiac TAPVC involves connections to the right superior vena cava, azygous vein or left innominate vein; (2) Cardiac TAPVC involves connections to the coronary sinus or right atrium; (3) Infracardiac TAPVC involves connections below the diaphragm to the portal venous system, ductus venosus or inferior vena cava; and (4) Mixed TAPVC is a combination of any of the above connections. TAPVC is further categorized as either obstructed or nonobstructed. Clinically, patient symptomatology is dependent on the size of atrial communication and the presence or absence of pulmonary venous obstruction [3]. Children with obstructed TAPVC always present with profound cyanosis, marked dyspnea and no or insignificant-sounding murmurs. They are frequently misdiagnosed as having pulmonary disease, and without operative correction early death is common [1, 3]. It is important to make a correct diagnosis before surgery.
Chest radiography and transthoracic echocardiography are usually the initial screening and diagnostic modalities in children with obstructed TAPVC, but making this diagnosis is a challenge, especially in neonates and infants. With developing technology, CT angiography has become a good diagnostic modality for use in the preoperative evaluation of obstructed TAPVC. In this retrospective study, we reviewed our experience of chest radiography, CT angiography and transthoracic echocardiography in 18 children with obstructed TAPVC, and we compared the diagnostic accuracy of CT angiography and transthoracic echocardiography in the evaluation of obstructed TAPVC. The purpose of this study was to delineate the diagnostic radiographic signs of obstructed TAPVC and evaluate the advantages of CT angiography relative to transthoracic echocardiography in the evaluation of obstructed TAPVC.
Materials and methods
Patients
We searched the electronic medical records at our hospital and found 18 children who had surgically confirmed obstructed TAPVC during the study period of January 2009 to June 2012. These 18 children (11 boys, 7 girls) were enrolled in the study. Children ranged in age from 1 day to 3 years. Ninety-four percent (17/18) were younger than 4 months, and 61% (11/18) were neonates. Fourteen had isolated TAPVC and four had complex TAPVC. All children had experienced cyanosis, respiratory distress and low blood oxygen saturation. Five children had no significant heart murmur. The remainder had a heart murmur.
This retrospective study was approved by the Institutional Review Board. Informed consent was waived.
Chest radiography
All patients underwent chest radiographs during good inspiration. Anteroposterior (AP) and lateral or only AP 40-inch supine films were obtained in 17 neonates and infants. In the 18th child, a 3-year-old boy, posteroanterior (PA) and lateral 6-foot upright films were obtained.
All images were reviewed independently by two radiologists with more than 5 years of experience in cardiac imaging who had no knowledge of the surgical or other imaging findings at the time of review. Heart size, heart and mediastinal shape, and vascularity were evaluated.
CT angiography
Sedation was obtained with oral chloral hydrate (0.5 mL/kg) administered 30 min before the anticipated CT angiography time for each child. Then CT angiography was performed with a 64-slice multidetector CT scanner (LightSpeed VCT; GE Healthcare, Waukesha, WI) using the following parameters: spiral non-ECG-gated mode, 80–120 kV, 80–140 mA, 0.4 s/r rotation time, 0.625-mm detector collimation, 1.25-mm slice thickness and 0.625-mm reconstruction interval of thin axial slices for post-processing. A 2 mL/kg bolus application of Iohexol (Omnipaque 350; GE Healthcare, Waukesha, WI) was injected by a mechanical power injector followed by a normal saline flush (1 mL/kg) at a rate of 1 mL/s via a peripheral venous cannula. We used the SmartPrep technique (GE Healthcare) and set the descending aorta at the carina level as the region of interest (ROI). Scanning automatically commenced when the CT value of the ROI rose to 100 HU. CT scan started at the level of the thoracic inlet down to the upper abdomen. Images were acquired during quiet respiration. Multiplanar reconstruction, maximum-intensity projection and volume-rendered images from various angles were produced with an Advantage Workstation 4.3 (GE Healthcare).
The recorded dose length product (DLP) was multiplied by 2 to account for the scanner use of a 32-cm phantom for reference [4]. The effective radiation dose was derived from the total DLP multiplied by the age- and gender-specific conversion factors for DLP-based CT dosimetry based on International Commission of Radiological Protection (ICRP) [5].
All images were reviewed independently by two radiologists with more than 5 years of experience in cardiac imaging who had no knowledge of the surgical or other imaging findings at the time of review. The draining sites and the sites of obstruction were recorded and correlated with the surgical findings. Pulmonary veins were considered to be obstructed if the axial area of pulmonary vein or draining vein was reduced by 50% or more from the largest measured area [6], or the draining vein had a connection to the portal vein [7].
Transthoracic echocardiography
Transthoracic echocardiography was performed with an ultrasonic cardioscope (Vivid 7 Dimension, GE Healthcare, Waukesha, WI) and reviewed by a pediatric cardiologist with more than 5 years of experience in cardiac imaging who had no knowledge of the surgical or other imaging findings at the time of review. The draining sites and the sites of obstruction were recorded and correlated with the surgical findings. Pulmonary veins were considered to be obstructed if the color Doppler flow imaging showed peak velocity of >1.3 m/s, or the draining vein had a connection to the portal vein. Trans-esophageal echocardiogram was not performed.
Statistical analysis
We used Stata 7.0 statistical software package (StataCorp, College Station, TX). The findings presented in surgical reports were used as the reference standard. McNemar’s χ 2 test was performed to compare the diagnostic accuracy of CT angiography with transthoracic echocardiography in terms of pulmonary venous drainage and obstruction detection. P < 0.05 was considered statistically significant.
Results
Chest radiography
The chest radiographic findings are summarized in Table 1. Of the 18 children, 16 (89%) had varying degrees of pulmonary venous congestion, edema, or both; 12 (67%) had no cardiomegaly (Figs. 1, 2, 3 and 4). One child with cardiac TAPVC had only vascular engorgement. One of the six children with cardiomegaly had associated dextrocardia.
Chest radiography and CT angiography in a 38-day-old boy with supracardiac total anomalous pulmonary venous connection (TAPVC). a Chest radiograph shows vascular congestion and interstitial and alveolar pulmonary edema; the cardiac configuration and size are normal. b Three-dimensional volume-rendered CT angiogram (posterior view) demonstrates four individual pulmonary veins joining in a retrocardiac venous confluence (C) and draining into the left innominate vein (LIV) via a vertical vein (VV). The stenosis (arrow) of the vertical vein is clearly depicted
Chest radiography and CT angiography in a 29-day-old girl with supracardiac total anomalous pulmonary venous connection (TAPVC). a Chest radiograph shows pulmonary venous congestion, interstitial and alveolar pulmonary edema, cardiomegaly and right superior mediastinal widening. b, c Maximum-intensity projection CT angiography demonstrates two left pulmonary veins (LPV) and one right inferior pulmonary vein (RIPV) joining and draining to the superior vena cava (SVC) via a vertical vein (VV); seven tiny right superior pulmonary veins (arrows, all not shown) drain to the vertical vein directly. Note the stenosis (*) at the connection between the vertical vein and the dilated superior vena cava
Chest radiography and CT angiography in a 7-day-old boy with infracardiac total anomalous pulmonary venous connection (TAPVC). The boy was misdiagnosed as neonatal respiratory distress syndrome on the initial chest radiograph. a Chest radiograph shows hazy-appearing lungs and a small heart. b, c Maximum-intensity projection CT angiography demonstrates two right pulmonary veins (RSPV right superior pulmonary vein, RIPV right inferior pulmonary vein) and one left superior pulmonary vein (LSPV) joining and draining to the middle hepatic vein (HV) via a vertical vein (VV); the left inferior pulmonary vein (LIPV) drains to the vertical vein directly. Note the stenosis (arrow) at the connection between the vertical vein and the middle hepatic vein. The left superior pulmonary vein and the right inferior pulmonary vein are also stenotic
Chest radiography and CT angiography in a 1-day-old girl with infracardiac total anomalous pulmonary venous connection (TAPVC). The girl was misdiagnosed with pneumonia on the initial chest radiograph. a Chest radiograph shows a normal cardiac silhouette. The lungs appear hazy and granular and the findings mimic pulmonary disease. b Maximum-intensity projection CT angiography demonstrates individual pulmonary veins (LPV left pulmonary vein, RPV right pulmonary vein) forming a venous confluence and draining to a vertical vein (VV) that passes downward through the diaphragm. The vertical vein enters the ductus venosus (*), which drains into the inferior vena cava (IVC). Note the stenosis (arrow) at the connection between the vertical vein and the ductus venosus
CT angiography
The mean volume CT dose index (CTDIvol) was 1.28 ± 0.31 mGy (range, 0.78–1.92 mGy). The mean resulting total DLP was 46.65 ± 6.58 mGy × cm (range, 38.36–58.18 mGy × cm). The mean effective radiation dose was 1.02 ± 0.28 mSv (range, 0.76–1.49 mSv).
CT angiography correctly diagnosed TAPVC in all children. Five children had supracardiac pulmonary venous connection, three children had cardiac pulmonary venous connection, eight children had infracardiac pulmonary venous connection and two children had a mixed connection.
CT angiography also clearly depicted the draining sites in all children (Table 2). Of the five children with supracardiac TAPVC, four had a connection to the left innominate vein (Figs. 1 and 5), and one had a connection to the superior vena cava (Fig. 2). In three children with cardiac TAPVC, pulmonary veins connected to the coronary sinus. Among the eight children with infracardiac TAPVC, five had connections to the portal vein, two had connections to the hepatic vein (Figs. 3 and 6), and one had a connection to the ductus venosus (Fig. 4). Of the two children with mixed TAPVC, one had a combination of supracardiac and infracardiac connections (Fig. 7), and the other had a combination of supracardiac and cardiac connections (Fig. 8). The diagnostic agreement between CT angiography and surgery was 100%.
CT angiography in a 23-day-old boy with supracardiac total anomalous pulmonary venous connection (TAPVC). A 3D volume-rendered image (a, posterior view) and maximum-intensity projection image (b, anterior view) show four pulmonary veins joining and draining to the left innominate vein (white arrow, b) via a vertical vein (VV); one left superior pulmonary vein (LSPV) drains to the vertical vein directly. Note that the two left superior pulmonary veins (black arrows, a) are tiny as well as the vertical vein; the left innominate vein had diffuse stenosis and was surrounded by abundant collateral vessels (not shown)
CT angiography in an 8-day-old boy with infracardiac total anomalous pulmonary venous connection (TAPVC). Maximum-intensity projection image demonstrates three left and two right tributary veins forming a venous confluence that connects to the hepatic vein (HV) via a vertical vein (VV); the vertical vein is compressed by the diaphragm (white arrow) and is stenotic (*) at its connection to the hepatic vein. Note that the two left superior pulmonary veins (black arrows) are small
CT angiography in a 10-day-old boy with mixed total anomalous pulmonary venous connection (TAPVC). The boy was misdiagnosed with pneumonia on the initial chest radiograph. a, b Maximum-intensity projection images demonstrate the right superior pulmonary vein (arrow) and the other three pulmonary veins (RIPV right inferior pulmonary vein, LSPV left superior pulmonary vein, LIPV left inferior pulmonary vein) connecting with a tortuous vertical vein (VV) without forming a distinct confluence. The vertical vein has two draining sites: one extends upward and drains into the superior vena cava (SVC), the other passes downward through the diaphragm and enters into the portal vein (PV)
CT angiography in a 2-month-old girl with mixed total anomalous pulmonary venous connection (TAPVC). a Maximum-intensity projection image shows the left superior pulmonary vein (LSPV) draining into the left innominate vein (LIV) via a vertical vein (VV); the stenosis (white arrow) of the vertical vein is clearly depicted. b, c Maximum-intensity projection images show the other three pulmonary veins (RSPV right superior pulmonary vein, RIPV right inferior pulmonary vein, and LIPV left inferior pulmonary vein) draining into the coronary sinus (CS); the left inferior pulmonary vein is stenotic (black arrow, c) at the orifice
Thirty-four sites of obstruction were correctly detected by CT angiography (Table 3). Among them, 11 were in the mediastinum, one was at the diaphragmatic level, nine were below the diaphragm and 13 were stenotic individual pulmonary veins in the lung. Of these sites of obstruction, six were caused by extrinsic compression (dilated pulmonary artery or diaphragm), 22 were caused by intrinsic stenosis (pulmonary vein hypoplasia) and six were infracardiac-type draining into the portal vein without pulmonary vein stenosis. Three sites of obstruction were not detected by CT angiography—one was an individual pulmonary vein stenosis at its connection to the draining vein and two resulted from stenosis at the junction between the hepatic vein and the right atrium (the hepatic veins drained directly into the right atrium in these two children). The diagnostic agreement between CT angiography and surgery was 92%.
Transthoracic echocardiography
Transthoracic echocardiography correctly diagnosed the draining sites in 11 children with TAPVC (Table 2). Transthoracic echocardiography did not identify the exact draining sites in three children (two with infracardiac TAPVC and one with mixed TAPVC), and made a misdiagnosis in four children (one with cardiac TAPVC, two with infracardiac TAPVC and one with mixed TAPVC). The diagnostic agreement between transthoracic echocardiography and surgery was 61%, significantly lower than that of CT angiography (P = 0.0156).
Transthoracic echocardiography correctly detected 15 sites of obstruction (Table 3)—11 were in the mediastinum, one was at the diaphragmatic level and three were below the diaphragm. Of these sites of obstruction, four were caused by extrinsic compression (dilated pulmonary artery or diaphragm), eight were caused by intrinsic stenosis (pulmonary vein hypoplasia) and three were infracardiac-type draining into the portal vein without pulmonary vein stenosis. The stenotic individual pulmonary veins in the lung were not detected by transthoracic echocardiography. The diagnostic agreement between transthoracic echocardiography and surgery was 41%. The rate of detection for pulmonary vein obstruction by transthoracic echocardiography was much lower than that of CT angiography (P = 0.0002).
Discussion
In children with obstructed TAPVC, the obstruction is either within the anomalous connecting vein or at its connection to the systemic circulation. Infracardiac TAPVC is the most common obstructed type. The reported incidence of pulmonary venous obstruction in children with infracardiac TAPVC is about 95–100% [8]. In our study, children with infracardiac TAPVC accounted for 44% of the obstructed veins. In one child with mixed TAPVC, the obstruction was also caused by the infracardiac connection. Generally speaking, the longer the venous channel connecting to the right atrium, the higher incidence of pulmonary venous obstruction. Infracardiac TAPVC has the longest venous channel of all types, as the draining vein travels with the esophagus through the diaphragm. Besides the intrinsic pulmonary vein stenosis, the vein could also be compressed when the diaphragm contracts. When the anomalous connection is to the portal vein, the high resistance of the hepatic parenchymal circulation creates its own barrier to blood flow for the pulmonary vein [7, 9]. Therefore, pulmonary venous obstruction is far more likely to occur in children with infracardiac TAPVC.
In our study, most children were critically ill soon after birth and because of their symptoms were easily identified by parents or a health care practitioner. In these infants with obstructed TAPVC, the impedance of pulmonary blood flow results in high pulmonary venous pressure leading to passive congestion and pulmonary edema; and moist rales can sometimes be auscultated. The presence of pulmonary venous obstruction reduces the blood flow returning into the right atrium, left-to-right shunt is almost obliterated and the right side of the heart is not dilated. Meanwhile, the obligatory right-to-left shunt is also attenuated and the left-side chambers become small. Consequently, the cardiac configuration is normal in almost all of these children. In children with pulmonary hypertension, cardiac murmurs can be insignificant because they are covered by the sonorous pulmonary second heart sound. Five children (28%) in our study had no cardiac murmurs. These children are usually misdiagnosed as pulmonary disease because of the prominent pulmonary lesions, normal cardiac configuration and insignificant murmurs [3].
Of the 18 children in our study, chest radiographs revealed varying degrees of passive vascular engorgement or pulmonary edema or both in 89%, and absence of cardiomegaly in 67%. Only two children with cardiac TAPVC, whose severity of pulmonary venous obstruction was relatively mild, had no passive congestion and pulmonary edema. Among the six children with cardiomegaly, four had complex TAPVC. One child with supracardiac TAPVC, two with infracardiac TAPVC and one with mixed TAPVC were misdiagnosed as pneumonia, and one child with infracardiac TAPVC was misdiagnosed as neonatal respiratory distress syndrome on initial chest radiographs. Radiographs did not show the figure-8 heart in four children with supracardiac TAPVC whose vertical vein connected to the left innominate vein. The fifth child with supracardiac type showed right superior mediastinal widening caused by dilatation of the superior vena cava. The figure-8 heart is regarded as a characteristic radiographic finding in the supracardiac TAPVC group, but this diagnostic cardiac configuration might not manifest during neonatal and infantile periods until there is a physiological decrease in pulmonary vascular resistance. Children with obstructed supracardiac TAPVC have clinical symptoms caused by passive congestion or pulmonary edema and they are usually admitted to the hospital during the neonatal or early infantile period. Therefore, in neonates and infants who are admitted to the hospital because of cyanosis and dyspnea, the diagnosis of obstructed TAPVC should be considered when the chest radiograph shows passive congestion or pulmonary edema with a normal cardiac configuration. If these children have cardiac murmurs, then this diagnosis is strongly suggested, and further imaging examination such as transthoracic echocardiography is necessary.
Transthoracic echocardiography is the first-line study for assessing children with suspected TAPVC. Transthoracic echocardiography is usually an adequate diagnostic tool to provide preoperative data to the surgeon. Oh et al. [10] reported that sensitivity and specificity of transthoracic echocardiography in the diagnosis of TAPVC were 97% and 99%, respectively. Diagnosis of the specific type of TAPVC was correct in 24 of 34 patients (71%) in their study [10]. Transthoracic echocardiography can also evaluate the presence or absence of pulmonary venous obstruction. Doppler echocardiography can detect the pulmonary venous obstruction and assess the severity of obstruction by measuring the blood velocity of the stenotic pulmonary vein [11]. An incorrect diagnosis by transthoracic echocardiography might occur because of a restricted acoustic window, insufficient spatial resolution or an interpretation error by the operator. We have sometimes experienced difficulty in elucidating the draining vein outside the mediastinum. Of the 18 children in our study, transthoracic echocardiography only correctly diagnosed the draining sites in 11. Transthoracic echocardiography could not clearly show the draining veins in four children with infracardiac TAPVC and two children with mixed TAPVC because of the poor acoustic windows. One child with cardiac-type was misdiagnosed as mixed TAPVC because the cardiologist mistook the left superior vena cava for an abnormal draining vein. Although transthoracic echocardiography is a useful modality to assess the velocity and the pressure gradients of blood flow, it is less useful for detecting the distal pulmonary veins. In our study, transthoracic echocardiography only detected 15 sites of obstruction with most obstructive sites being in the mediastinum.
These errors could be reduced by CT angiography. CT angiography is a good diagnostic tool for use in the preoperative evaluation of neonates and infants with TAPVC [11–14]. Oh et al. [10] compared multidetector CT (MDCT) with echocardiography in the evaluation of TAPVC. In their study, MDCT correctly depicted the drainage site of the common pulmonary vein, stenosis of the vertical vein and the course of the atypical vessel into the systemic vein (sensitivity 100%, specificity 100%). The specificity of echocardiography was 100% for the three defined findings; the sensitivity, however, was 87%, 71% and 0%, respectively. In our study, CT angiography clearly depicted the draining sites in all children and detected 34 sites of obstruction not only in the mediastinum but also below the diaphragm and in the lung. CT angiography is superior to transthoracic echocardiography in the evaluation of pulmonary venous drainage and obstruction, especially outside the mediastinum. But CT angiography could lead to a missed diagnosis of mild pulmonary vein stenosis according to our diagnostic criteria. Three sites of obstruction were not detected by CT angiography in our study because the axial area of the stenotic pulmonary vein was not reduced by 50% from the largest measured area.
We categorize pulmonary venous obstruction into three types: (1) extrinsic compression by surrounding organs, (2) intrinsic stenosis caused by pulmonary vein hypoplasia, and (3) infracardiac-type draining into the portal vein without pulmonary vein stenosis. Pulmonary venous obstruction caused by extrinsic compression is seen in infracardiac and supracardiac TAPVC. In infracardiac TAPVC, the draining vein might be obstructed, frequently at the level of the diaphragm because of extrinsic narrowing. In supracardiac TAPVC, the draining vein might be compressed between the pulmonary artery and the left mainstem bronchus, which is augmented if pulmonary arterial pressure increases [9]. Intrinsic pulmonary vein stenosis might be local or diffuse and can be seen in the all types of TAPVC. It is caused by pulmonary vein hypoplasia and is usually accompanied by thickening of the vessel walls. Patients with TAPVC have in utero pulmonary vein obstruction. If this occurs early in gestation, generalized hypoplasia of the pulmonary vascular bed can result, with arterialization of the pulmonary veins [6]. This might account for children in whom small pulmonary veins are seen on CT angiography. Children with infracardiac TAPVC whose draining vein connects to the portal vein are almost always obstructed because the blood passes through the hepatic sinusoids [15]. In our study, CT angiography clearly revealed the positional relationship between the pulmonary vein and the surrounding organs, and made it possible to accurately evaluate the cause of pulmonary venous obstruction.
One limitation of our study is the small sample size, which limited the statistical power of the evaluation. Despite this weakness, our results point out the special imaging features of obstructed TAPVC. In addition, two children underwent CT angiography with 120-kV tube voltage and 140-mA tube current, which increased the effective radiation dose. CT radiation dose should be minimized particularly in the younger population because they have unequivocally higher radiosensitivity and longer life expectancy than the older population [5]. When equal or greater diagnostic yields are expected, CT angiography should be replaced by alternative imaging modalities with no or less ionizing radiation. MRI is such a modality with no ionizing radiation and it can provide both anatomical and blood flow information. Various studies have shown that MRI might prove to be the best non-invasive technique to diagnose TAPVC. By virtue of its multi-planar capability, MRI identifies the anomalous course and connections of the pulmonary veins. Furthermore, because of ECG-gating and the inherently superb contrast resolution of MRI, it evaluates the intracardiac anatomy particularly well [16, 17]. But it is difficult to examine patients with critical general conditions using MRI because MRI scans may be long. Therefore, in spite of the ionizing radiation, CT angiography is regarded as an important complementary modality to transthoracic echocardiography for neonates and infants with obstructed TAPVC who are critically ill.
Conclusion
Most children with obstructed TAPVC present with symptoms in the neonatal or early infantile period. Chest radiographs usually show a characteristic pattern of pulmonary venous congestion or edema or both, and a normal cardiac configuration and size. CT angiography is superior to transthoracic echocardiography in the evaluation of pulmonary venous drainage and obstruction, especially in children with infracardiac and mixed TAPVC.
References
Silverman FN (1993) Caffey’s pediatric X-ray diagnosis. Mosby, St. Louis
Craig JM, Darling RC, Rothney WB (1957) Total pulmonary venous drainage into the right side of the heart: report of 17 autopsied cases not associated with other major cardiovascular anomalies. Lab Invest 6:44–64
Swischuk LE (1997) Total anomalous pulmonary venous return. In: Swischuk LE (ed) Imaging of the newborn, infant, and young child, 4th edn. Williams & Wilkins, Baltimore, pp 239–245
Paul JF, Rohnean A, Elfassy E et al (2011) Radiation dose for thoracic and coronary step-and-shoot CT using a 128-slice dual-source machine in infants and small children with congenital heart disease. Pediatr Radiol 41:244–249
Goo HW (2012) CT radiation dose optimization and estimation: an update for radiologists. Korean J Radiol 13:1–11
Seale AN, Uemura H, Webber SA et al (2010) Total anomalous pulmonary venous connection: morphology and outcome from an international population-based study. Circulation 122:2718–2726
Goo HW, Park IS, Ko JK et al (2003) CT of congenital heart disease: normal anatomy and typical pathological conditions. Radiographics 23:S147–S165
Yang S (2005) Total anomalous pulmonary venous connection, TAPVC. In: Yang S (ed) Pediatric cardiology, 3rd edn. PMPH, Beijing, p 278
Stein P (2007) Total anomalous pulmonary venous connection. AORN J 85:509–520
Oh KH, Choo KS, Lim SJ et al (2009) Multidetector CT evaluation of total anomalous pulmonary venous connections: comparison with echocardiography. Pediatr Radiol 39:950–954
Ohtsuki S, Baba K, Kataoka K et al (2005) Usefulness of helical computed tomography in diagnosing pulmonary vein stenosis in infants. Acta Med Okayama 59:93–98
Kim TH, Kim YM, Suh CH et al (2000) Helical CT angiography and three-dimensional reconstruction of total anomalous pulmonary venous connections in neonates and infants. AJR Am J Roentgenol 175:1381–1386
Kawano T, Ishii M, Takaqi J et al (2000) Three-dimensional helical computed tomographic angiography in neonates and infants with complex congenital heart disease. Am Heart J 139:654–660
Liu J, Wu Q, Xu Y et al (2012) Role of MDCT angiography in the preoperative evaluation of anomalous pulmonary venous connection associated with complex cardiac abnormality. Eur J Radiol 81:1050–1056
Livolsi A, Kastler B, Marcellin L et al (1991) MR diagnosis of subdiaphragmatic anomalous pulmonary venous drainage in a newborn. J Comput Assist Tomogr 15:1051–1053
Dillman JR, Yarram SG, Hernandez RJ (2009) Imaging of pulmonary venous developmental anomalies. AJR Am J Roentgenol 192:1272–1285
Gulati G, Sharma S (2003) A rare form of supracardiac total anomalous pulmonary venous drainage-evaluation by computed tomography and magnetic resonance imaging. Clin Radiol 58:172–175
Acknowledgements
We appreciate the assistance of Drs. Xuecun Liang and Xiaojing Ma, Department of Echocardiography, Children’s Hospital, Fudan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, Q., Pa, M., Hu, X. et al. Role of plain radiography and CT angiography in the evaluation of obstructed total anomalous pulmonary venous connection. Pediatr Radiol 43, 827–835 (2013). https://doi.org/10.1007/s00247-012-2609-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2609-6