Abstract
Background
Total anomalous pulmonary venous connection (TAPVC), with an intrapulmonary segment (IPV), a meandering abnormally located confluence and obligatory drainage of one lung into the other before entering the systemic circulation, is a rare anomaly and we term it as the meandering intrapulmonary TAPVC (MITAPVC).
Material and methods
We report five patients with an unusual variation of the TAPVC channel. A review of literature was done to identify this association of TAPVC with an intrapulmonary vein and absence of a confluence in its usual location.
Results
In our study, 4 patients with neo-confluence creation had excellent outcome while one with partial correction required catheter-based intervention, but succumbed to persistent pulmonary hypertension refractory to therapy. A literature search showed 25 additional such patients. Two groups were noted, one with isolated lesions (N = 16) and the other with heterotaxy or complex intracardiac lesions (N = 14). Of the 20 surgical interventions, only 12 survived, most of them in the isolated group (N = 10). Mortality was due to incomplete surgery (4/4), inappropriate surgery (3/3), and complete and appropriate surgery (1/11) respectively.
Conclusion
The MITAPVC is often associated with heterotaxy and complex lesions. However, the isolated version is being increasingly recognised. Non-recognition or inappropriate surgical correction of MITAPVC is associated with fatal outcomes. Evaluation by a computerised tomography (CT) scan, meticulous dissection and demonstration of the entire channel, creation of a neo-confluence and appropriate palliation for the heterotaxy is the key to ensure good outcome. This is not a new entity, but deserves a separate subclassification under TAPVC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Total anomalous pulmonary venous drainage (TAPVC) has been classified as supracardiac, infracardiac, cardiac, or mixed variety by Craig, Darling and Rothney [1]. Herlong’s classification [2] is a more recent and detailed one, sequentially analysing segments from pulmonary vein until the mitral valve. However, in both the formats, TAPVC is described to have a retrocardiac confluence into which all the four veins drain, which does not communicate directly with the left atrium (LA). The left and right veins drain independently into this confluence, which is usually located inferior to the central branch pulmonary arteries.
A condition in which there are total anomalous pulmonary venous return, absent confluence in the usual location, a dilated intrapulmonary vein (IPV) segment, and sequential emptying of the pulmonary venous return into a tortuous pulmonary venous channel (implying one lung venous return draining into the other lung before emptying into the systemic circulation) is a rare entity. It has been described in heterotaxy with right atrial isomerism with complex intracardiac lesions and carries a high mortality. The isolated variety is being increasingly recognised and carries a good prognosis with complete correction.
The chest roentgenogram often gives an impression of a scimitar vein, or is not contributory. Whenever an echocardiogram fails to demonstrate the confluence in usual location or fails to trace the entire course of the confluence or demonstrate the communication to the systemic vein, further imaging is warranted. A computerised tomography (CT) scan helps in delineating the complete pulmonary venous anatomy. This condition is often mistaken for scimitar vein (SV), or intrapulmonary vertical vein (IPVV), or meandering pulmonary vein (MPV).
On inspection during surgery, this is characterised by TAPVC with extrapericardial, intrapulmonary vein on one or both sides and a caudally displaced horizontal component, which may be single or multiple, but is capable of carrying only one lung return. Direct anastomosis of this horizontal component to the left atrium may lead to either distortion or inadequately relieved pulmonary venous obstruction. We propose to name this as “meandering intrapulmonary total anomalous pulmonary venous connection (MITAPVC)”.
In this article, we report five cases and present a review of literature. We also differentiate it from similar conditions and describe the various surgical options that may be needed to manage this complex condition.
Patients and methods
Case # 1
Two-month-old male infant, weighing 3.3 kg, presented to the outpatient with difficulty in breathing. The clinical examination was unremarkable and chest roentgenogram showed plethoric lungs. Echocardiogram revealed a supracardiac TAPVC with a restrictive patent foramen ovale (PFO) and no patent ductus arteriosus (PDA).
Case # 2
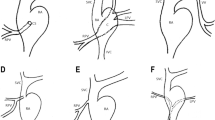
Three-week-old female neonate weighing 2.6 kg presented with chest retractions. Chest roentgenogram showed plethoric lung fields. Echocardiography showed a dilated unobstructed and abnormally located common pulmonary venous channel with all four veins draining via a left vertical vein (LVV) as a supracardiac TAPVC. CT scan (Fig. 1a) showed the abnormal course of the pulmonary vein. There was a small patent ductus arteriosus and the PFO was unrestrictive. Figure 1 depicts the schematic representation of the pulmonary venous anatomy for both these children. Echocardiogram demonstrated an abnormally located horizontal channel. Both these children underwent a sternotomy for repair of the TAPVC. During dissection of the common chamber, it was noted that the right-sided veins descended inferiorly up to the diaphragm and subsequently turned to the left along the superior surface of the diaphragm, received the left-sided veins in sequence before emptying into the innominate vein. In both instances, the pulmonary venous channel had a dilated segment which was outside the pericardium, in the pleuro pericardial space. The whole of the common vein was traced, and opened from right superior vein to the left superior vein (Fig. 1a, Video 1 and 2). The medial lips of the opened vein were directly anastomosed forming a large neo-confluence (Fig. 1b, Video 3), and the left atrium was anastomosed to this using William’s approach [3] (Fig. 1b, c). The vertical vein was ligated close to its junction with the innominate vein. The postoperative echo showed all four veins draining unobstructed into LA (Fig. 1c, Video 4).
a CT scan image of case # 2, pulmonary veins as seen from the posterior aspect, schematic representation of the pulmonary venous anatomy, incision on the pulmonary venous channel. b After incision, posterior wall anastomosed, left atriotomy. c Left atriotomy being anastomosed to the posterior lip, anterior part of the anastomosis completed, post op echo showing the pulmonary veins draining into LA
Case # 3
Forty-day-old male infant, weighing 3.5 kg, presented with breathlessness and was found to have a supracardiac TAPVC draining into the superior vena cava (SVC). 2D echocardiography failed to demonstrate a confluence posterior to the left atrium. A CT scan (Fig. 2a) demonstrated the unusual course of the common channel. At surgery, there was a common channel which originated as the left superior pulmonary vein, descending to collect the left inferior vein, crossing the midline over the diaphragm to pick up the right inferior vein and right superior vein in a sequential manner, before draining into the posterior aspect of the SVC in its extra pericardial portion (Fig. 2b, Video 5). The mouth of the drainage into the SVC was narrowed and obstructed. The vertical vein was in the right pleuropericardial space. The common channel was opened along its entire length, and using the classical Shumacker approach [4] was anastomosed to the LA (Fig. 2b, c, Videos 6, 7, 8). The anterior part of the anastomosis was augmented with a glutaraldehyde-treated autologous pericardial patch (Fig. 2c). Postoperative echocardiography showed widely open unrestricted anastomosis. The baby had an uneventful recovery (Video 9).
a CT scan image of case # 3, with the schematic representation. b Marking out the incision on the pulmonary vein, right atriotomy, atriotomy carried across the interatrial septum into the left atrium. c Posterior layer of the anastomosis between the pulmonary veins and the left atrium being completed, anterior part being closed with a pericardial patch to create a roomy anastomosis, right atriotomy closed partly onto the pericardial patch, and partly directly
Case # 4
Two-month-old male infant, weighing 3.4 kg, presented with difficulty in breathing. On echocardiographic evaluation, he was diagnosed to have an infracardiac TAPVC, or a mixed TAPVC with left inferior pulmonary vein draining into portal system. The echocardiography imaging however failed to demonstrate a good confluence, and a CT scan was not done. On inspection during surgery, the right veins joined to form a common channel with a longer course to the left (Fig. 3a). The vein could not be traced joining the vertical vein, as was demonstrated in the pre-operative imaging. The descending vein could not be identified despite extensive dissection in the posterior mediastinum. Pleural space was not entered, as it was felt that the descending vein was obstructed and so could be left alone. The horizontal portion was opened and anastomosed to the LA. The child had an uneventful recovery and was discharged. The child presented a month later with distress. Angiogram (Fig. 3a, b) and the echocardiogram (Fig. 3b) confirmed the patent descending vein, a gradient at the anastomosis and an intrapulmonary segment on the left side, which was missed at first surgery. The child had a balloon dilatation of the anastomosis and the right pulmonary vein, and was discharged home a second time. However, as the child did not report for a scheduled review at 3 months, a telephonic review revealed that the child had expired.
a Schematic representation of case # 4, with the dilated left vertical vein, and the descending vein, angiogram done after the child presented with PAH, showing the dilated intrapleural vertical vein on the left (black arrows). b Angiogram showing the obstructed anastomosis, echo showing the obstruction to the left veins
Case # 5
Ten weeks, 2.2 kg, term baby presented with respiratory distress. Echocardiogram suggested a TAPVC, but the confluence could not be demonstrated; hence, a CT angiogram was done (Fig. 4). This showed a left superior vein, joining the left inferior vein, and coursing in an abnormal location. This channel sequentially collected the right inferior and right superior vein, and drained into the SVC.
At surgery, it was noted that the left pulmonary veins formed a channel, which descended up to the diaphragm, crossed over to the right, passing behind the right main bronchus, and collected the right pulmonary veins in a sequential manner. The right part of the channel was intrapulmonary and dilated, before draining into the SVC (Fig. 4). The channel was transected before the bronchus, opened along its long axis, into both left and right veins. The neo-confluence was created in front of the bronchus, and the left atrial anastomosis was done using William’s posterior approach.
All the above children had no common confluence, as is usually seen in TAPVC. It was replaced by a common venous channel with a convoluted course in a more caudad position, into which the individual pulmonary veins were draining sequentially. In all the children, one lung drained into the other before joining the systemic. All of them had a dilated segment of the pulmonary vein, which was intrapulmonary and confined within the pleuropericardial space. All children underwent a repair using cardiopulmonary bypass, cardioplegic arrest and moderate hypothermia. The guiding principle was creation of a neo-confluence, using the dilated intrapulmonary segments, before performing a wide anastomosis with the left atrium. A classical Shumacker [4] approach was used in case # 3, while William’s approach [3] was used for the others. In the fourth child, extensive dissection of the mediastinum revealed only a single channel located caudally, into which right veins were opening and the left end was going downwards into the diaphragm. The pleura was not opened as the descending vein was shown to be obstructed at portal vein entry. The horizontal portion was anastomosed to the LA. This channel proved to be inadequate, given the adverse outcome after the discharge.
Review of literature
The earliest report was from Shone [5], but the condition was more fully described and characterised as an intrapulmonary vertical vein by Everhart [6], Sutherland [7], Delisle [8] and Brenner [9]. They also emphasised that the anomaly involves one lung draining into the other before draining into the systemic circulation. Table 1 summarises the details of all the 30 patients [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20].
They could be grouped into “Isolated” (N = 16), “Heterotaxy/Right isomerism” (N = 12) and “complex intracardiac lesions” (N = 2). Majority of them drained into the right SVC (N = 20). Left SVC (N = 2), brachiocephalic trunk (N = 5), infradiaphragmatic inferior vena cava (N = 2) and the portal vein (N = 1) formed the other drainage sites. Two had multiple channels between the left and right systems, in all others the horizontal channel was single.
Figure 5 summarises the management and outcomes in these children. Ten of these patients did not have surgical intervention. They include autopsy reports (N = 5), died before any surgical intervention could be planned (N = 3), and radiology reports, without reference to any surgery (N = 2).
Twenty patients underwent surgical intervention; however, 2 of them did not have any surgical details mentioned. Four had an “incomplete surgery”, where the TAPVC repair was attempted, but obstruction was not completely resolved, all of them expired. Three of them were categorised as “inappropriate surgery”, where the TAPVC was either not recognised or not addressed, and a palliation was done for the cyanosis. This group included Blalock-Taussig shunts (N = 2) and Potts shunt (N = 1) and all of them died. Eleven patients had “complete and appropriate surgery”, where the TAPVC was corrected completely with Warden-type repair (N = 4). Two of these had an additional bidirectional Glenn (BDG), to palliate a single ventricle situation, of which only one died. The 2 infracardiac variants, and 1 with a right vertical vein, had a classical anastomosis done. Four had reconstruction with creation of a neo-confluence.
The MITAPVC nature of the channel either was not recognised or was addressed by performing an anastomosis to the horizontal component, which led to inadequate correction of TAPVC. In the cyanotic group, performing a shunt in presence of an obstructed TAPVC physiology would have been detrimental. The obstructed nature of MITAPVC probably was responsible for the mortality just after admission, before any planned procedure. The better survival in the more recent series with isolated MITAPVC probably indicates better understanding, earlier assessment, and appropriate intervention. Formation of a neo-confluence and augmentation of the interatrial septum and LA, which ensured an unobstructed pulmonary venous drainage, were the reason for improved outcomes.
Improved pre-operative imaging and surgical planning
The transthoracic echocardiography is the commonest modality of diagnosing a TAPVC. However, reports suggest that delineation of the course of the venous channel and its termination is very operator dependent and correlation with surgical findings varies from 60 to 81% [21,22,23]. The mixed and unusual variants were more likely to be missed. Transoesophageal echocardiography is an alternative, but of limited value in neonates. Angiography has been relegated to history, except in a postoperative situation, where intervention may be needed.
The CT scan, with a 3D reconstruction, was however far more accurate in delineating the patho-morphological features of the TAPVC [24,25,26]. Magnetic resonance imaging (MRI) is another modality which is useful in defining the anatomy, but the need for anaesthesia and long data acquisition time outweigh its advantages and limit its applicability in neonates.
Excluding 10 patients, who were autopsy reports, the main diagnostic tool was 2-dimensional echocardiography. Nine of them had a CT scan done to define the TAPVC. Of the 4 angiograms done, 2 were in the postoperative period, one of whom had an angioplasty done for restenosis. One child had a MRI done for the TAPVC. In 4 children, the diagnostic modality was not clear. In 2 of the children, the diagnosis was made on table, after meticulous dissection.
Liberal use of CT scan to delineate the anatomy has made a difference to surgical planning and outcomes. All our survivors had a CT scan imaging which demonstrated the abnormal course and connection and indicated the surgical strategy. The lone mortality in the current report was where the CT scan was not done, and consequently, the MITAPVC was incompletely addressed. CT imaging is indicated whenever the confluence cannot be imaged in its usual location, the entire course of the confluence cannot be delineated, or the termination cannot be located by echocardiography [21].
Other differential diagnosis
Meandering pulmonary vein (MPV), anomalous unilateral single pulmonary vein (AUSPV), scimitar vein (SV), intrapulmonary vertical vein (IPVV), and TAPVC can be mistaken for MITAPVC [27,28,29,30]. Table 2 sets out the differentiating features of each of these conditions. As the origin, course, and termination of the channel form the main distinguishing features, a CT scan remains the investigation of choice.
The confluence and intrapulmonary vein in MITAPVC
Delisle [8] has emphasised on the presence of a “surgeons friend”, a common confluence, which carries the total pulmonary venous return. This confluence is posterior to the left atrium, oriented horizontally and located just inferior to the branch pulmonary arteries, running parallel to them. It was also recognised that in TAPVC directly opening into the right atrium and mixed type [31], the typical confluence may not be present. In cardiac type, the confluence is present but communicates with the coronary sinus. In infracardiac type, the confluence has a vertical orientation, classically described as a tree type [32]. But in all of them, each vein carries only a part of the venous return. In all the cases reviewed here, there was no confluence in the usual location, a horizontal component collected the pulmonary venous return in a sequential manner, one lung drained into the other, and hence, it was better termed as interpulmonary communicating channel.
In a regular TAPVC, the left vertical vein is normally in the pleuropericardial space, but on the right side, it is found in the space between SVC and the ascending aorta. In MITAPVC, however, the right vertical vein was to the right of the SVC and in pleuropericardial space. This intrapleural component was invariably dilated. The combination of interpleural communication, dilated intrapulmonary component, sequential drainage of pulmonary veins, and one lung draining into the other form the diagnostic features of MITAPVC. These features were present in all the cases under review; additionally, the interpleural channel was mostly single and displaced caudally, but occasionally multiple; however, all of them showed features of obstruction.
Surgical implications
The basic principle in TAPVC surgery is to ensure an adequate confluence and a wide unobstructed anastomosis of this confluence with the left atrium. As there is no confluence in this condition, it is imperative that a neo-confluence be created. The transverse portion is often hypoplastic and using this segment to anastomose to the left atrium results in incomplete correction of TAPVC. The transverse portion is displaced caudally and may result in distortion when directly anastomosed to the LA. The communication between the IPV and the SVC may be obstructed, and hence, unobstructed routing across the atrial septal defect may not always be possible, or may require additional procedures. The location of the IPV mandates that the pleural cavities should be explored to avoid a partial correction. In the infradiaphragmatic connection, the dissection should be complete to show all the four veins before anastomosing to the LA. In our review, non-recognition, partial correction, and inadequate tracing of the MITAPVC have been associated with fatal outcome. The condition may be associated with other complex intracardiac lesions, which may need staged palliation into the single ventricle pathway.
Development insights
In the developing secondary heart field, the dorsal mesentery gets canalised to form the common pulmonary vein (CPV) [33, 34]. The cephalad part of the splanchnic plexus invades the developing lung bud, and separates into left and right components. The left and right lungs have intercommunicating channels, which coalesce to form two channels on the right and single on the left side. The developing CPV has two ends, the cephalad end balloons into the interpulmonary channels to form the common chamber. The caudal end establishes connection with the LA and is ultimately absorbed giving the normal pulmonary venous pathway. The communication to the splanchnic system is lost by the time central connections are established. If the caudal end of CPV does not canalise into the LA, a classical TAPVC results. The splanchnic connection is retained; hence, the various types of TAPVC form. When the cephalad part of CPV fails to meet the interpulmonary veins, it results in MITAPVC, which have lost their splanchnic connection and hence are dilated, but carry the entire pulmonary venous return in a sequential manner. This connects to the closest systemic vein, which is either superior vena cava, inferior vena cava, portal vein or the brachiocephalic vein. Partial canalisation into the interconnecting pulmonary veins result in the other variants, for example, canalisation into the left leads to right intrapulmonary vertical vein. The interpulmonary channels, being lateral structures, are thus extrapericardial and intrapulmonary in nature. Cardiac variant with drainage into coronary sinus is not expected, unless this happens to be the only SVC. This could be directed by the lateralisation gene [34], given the close association with the high incidence of asplenia and heterotaxy isomerism. The tortuous and thin-walled nature of the vessel, cephalad displacement, and sequential drainage of pulmonary venous return probably indicate origin from the interpulmonary plexus.
Conclusion
The MITAPVC needs to be considered when the echocardiography does not demonstrate a usually located confluence or when the entire course and termination of the pulmonary venous return cannot be delineated. A thorough evaluation of MITAPVC by computerised tomography is essential to optimise surgical plan. Meticulous dissection and demonstration of the entire channel with creation of a neo-confluence are the key to ensuring good outcomes in this rare type of TAPVC. Clinical suspicion of this abnormal morphology along with good pre-operative imaging, has led to an increasing detection of the isolated variant and its successful management. The obstructed nature of MITAPVC may be responsible for the mortality if it is not recognised early. This condition gives new insights into development of the pulmonary venous system which needs to be corroborated by further embryological studies. This is not a new entity, but certainly deserves a separate subclassification under the TAPVC. Irrespective of whether this is just an unusual variant or a distinctly new entity, the outcomes depend on recognition and appropriate modification of surgical techniques to ensure a good outcome.
Availability of data and material
All references are shown and transparent; no statistical analysis other than numbers have been used.
Code availability
Not applicable.
References
Craig JM, Darling RC, Rothney WB. Total pulmonary venous drainage into the right side of the heart; report of 17 autopsied cases not associated with other major cardiovascular anomalies. Lab Invest. 1957;6:44–64.
Herlong JR, Jaggers JJ, Ungerleider RM. Congenital heart surgery nomenclature and database project: pulmonary venous anomalies. Ann Thorac Surg. 2000;69:S56-69.
Williams GR, Richardson WR, Campbell GS. Repair of total anomalous pulmonary venous drainage in infancy. J Thorac Cardiovasc Surg. 1964;47:199–204.
Shumacker HB Jr, King H. A modified procedure for complete repair of total anomalous pulmonary venous drainage. Surg Gynecol Obstet. 1961;112:763–5.
Shone JD, Edwards JE. Mitral atresia associated with pulmonary venous anomalies. Br Heart J. 1964;26:241–9.
Everhart FJ, Korns ME, Amplatz K, Edwards JE. Intrapulmonary segment in anomalous pulmonary venous connection Resemblance to scimitar syndrome. Circulation. 1967;35:1163–9.
Sutherland RD, Korns ME, Pyle RR, Edwards JE. Intrapulmonary vein contributing a segment of venous supply of contralateral lung. Chest. 1970;57:182–4.
Delisle G, Ando M, Calder AL, et al. Total anomalous pulmonary venous connection: Report of 93 autopsied cases with emphasis on diagnostic and surgical considerations. Am Heart J. 1976;91:99–122.
Brenner JI, Bharati S, Berman MA, Lev M. Rare type of intrapulmonary drainage of one lung by the other with total anomalous pulmonary venous return. J Am Coll Cardiol. 1983;2:1174–7.
Nagami H, Yamauchi M, Harada T, et al. A case of unusual total anomalous pulmonary venous drainage (supra cardiac type). Nihon Geka Gakkai Zasshi. 1988;89:1738–43.
Matsui M, Arai T, Horikoshi S, et al. Successful repair of a rare type of total anomalous pulmonary venous drainage. Ann Thorac Surg. 1991;52:131–3.
Fujimatsu T, Tsunemoto M, Kazuma H, et al. Successful surgical treatment of total anomalous pulmonary venous connection (supracardiac Ib type) showing an unusual shape of the pulmonary veins in an infant. Kyobu Geka. 1991;44:93–6.
Wang JK, Chiu IS, How SW, et al. Anomalous pulmonary venous pathway traversing pulmonary parenchyma. Diagnosis and implication. Chest. 1996;110:1363–6.
Chung JH, Suh YL, Lee HJ, Kang IS, Choe YH, Ree HJ. Rare variant of total anomalous pulmonary venous connection: intrapulmonary drainage of one lung by the other–a case report and review of the literature. Pediatr Pathol Lab Med. 1997;17:133–40.
Watanabe N, Ishihara S, Sugiyama Y, Iwasa S. Total anomalous pulmonary venous connection showing an unusual shape of the pulmonary vein successfully repaired in a neonate; report of a case. Kyobu Geka. 2006;59:244–6.
Malankar D, Talwar S, Makhija N, Sharma S, Choudhary SK. Surgical management of univentricular heart with total anomalous pulmonary venous drainage and intrapulmonary vertical vein. J Card Surg. 2010;25:84–6.
Talwar S, Rajashekar P, Anderson RH, et al. Totally anomalous pulmonary venous connection draining through an intrapulmonary vertical vein. World J Pediatr Congenit Heart Surg. 2012;3:521–4.
Imoto Y, Ochiai Y, Jooh K. Intrapulmonary vertical vein associated with an infracardiac type of totally anomalous pulmonary venous connection. Ann Thorac Surg. 2014;98:e7–9.
Kalantre AA, Champaneri B, Kottayil B, Vaidyanathan B. “Hemodynamic vice” of the right-sided ascending vertical vein in the setting of supracardiac total anomalous pulmonary venous connection in a neonate: Anatomic-embryological correlation. Ann Pediatr Cardiol. 2017;10:104–6.
Bhat V, Gadabanahalli K, Maiya S. Meandering vessel on a chest radiograph in a cyanotic neonate: rare presentation of intrapulmonary course of right vertical vein in patients with supracardiac total anomalous pulmonary venous connection. Quant Imaging Med Surg. 2018;8:447–51.
Jiang L, Xie L-J, Yang Z-G, et al. Preoperative evaluation of anomalous pulmonary venous connection using dual-source computed tomography: Comparison with echocardiography. Eur J Radiol. 2017;94:107–14.
Shen Q, Pa M, Hu X, Wang J. Role of plain radiography and CT angiography in the evaluation of obstructed total anomalous pulmonary venous connection. Pediatr Radiol. 2013;43:827–35.
Ali F, Qureshi S, Amanullah M, Atiq M. Accuracy of echocardiography in diagnosing total anomalous pulmonary venous return. Pak J Med Sci. 2018;34:1094–8.
Han F, Kiparizoska S, Campbell W, et al. The case of the missing pulmonary vein: A focused update on anomalous pulmonary venous connection in congenital cardiovascular disease. Echocardiography. 2019;36:1930–5.
Shaheen F, Gojwari TA, Andrabi M, Sofi S, Singh M. 64-slice CT imaging in a case of total anomalous pulmonary venous circulation. Indian J Radiol Imaging. 2009;19:54–6.
Mitchell FM, Prasad SK, Greil GF, Drivas P, Vassiliou VS, Raphael CE. Cardiovascular magnetic resonance: Diagnostic utility and specific considerations in the pediatric population. World J Clin Pediatr. 2016;5:1–15.
Odenthal C, Sarikwal A. Anomalous unilateral single pulmonary vein versus scimitar syndrome: Comparison of two paediatric cases and a review of the literature. J Med Imaging Radiat Oncol. 2012;56:247–54.
Tortoriello TA, Vick GW 3rd, Chung T, Bezold LI, Vincent JA. Meandering right pulmonary vein to the left atrium and inferior vena cava: the first case with associated anomalies. Tex Heart Inst J. 2002;29:319–23.
Lee M, Jeon KN, Park MJ, Bae K. Meandering pulmonary veins: Two case reports. Medicine (Baltimore). 2020;99:e19815.
Rodrigues MA, Ritchie G, Murchison JT. Incidental meandering right pulmonary vein, literature review and proposed nomenclature revision. World J Radiol. 2013;5:215–9.
Delius RE, de Leval MR, Elliott MJ, Stark J. Mixed total pulmonary venous drainage: still a surgical challenge. J Thorac Cardiovasc Surg. 1996;112:1581–8.
Kawashima Y, Matsuda H, Nakano S, Miyamoto K, Fujino M. Tree-shaped pulmonary veins in infracardiac total anomalous pulmonary venous drainage. Ann Thorac Surg. 1977;23:436–41.
van den Berg G, Moorman AFM. Development of the pulmonary vein and the systemic venous sinus: an interactive 3D overview. PLoS One. 2011;6:e22055.
Douglas YL, Jongbloed MRM, Deruiter MC, Gittenberger-de Groot AC. Normal and abnormal development of pulmonary veins: state of the art and correlation with clinical entities. Int J Cardiol. 2011;147:13–24.
Funding
None.
Author information
Authors and Affiliations
Contributions
The paper was conceived and authored by Dr Jagannath; Dr Balaji made the surgical illustrations. All authors were involved in critical review of the paper, collection of material and preparation of tables.
Corresponding author
Ethics declarations
Ethics approval
Being a retrospective case sheet and literature review, the Star Hospital ethics committee has waived the need for individual consent and has approved the study.
Informed consent
Waived by ethics committee of Star Hospital and Star MIMS Hospital, as the study is a retrospective case sheet review, vide letter no. SH/OS/IEC (42)2020–21/01.
Consent for publication
The article is not under consideration for publication elsewhere, and is being submitted for publication in Indian Journal of Cardiovascular and Thoracic Surgery.
Statement of human and animal rights
Being a retrospective case sheet review, and no study involving participation of humans or animals, in a formal study, this is not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Palaparthi, S., Ramkinkar, S., Jayanthi, K.V.K. et al. The meandering intrapulmonary total anomalous pulmonary venous channel (MITAPVC)—old wine in new bottle or a new variant?. Indian J Thorac Cardiovasc Surg 38, 382–393 (2022). https://doi.org/10.1007/s12055-021-01290-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-021-01290-2