Abstract
The technical quality of prenatal US and fetal MRI has significantly improved during the last decade and allows an accurate diagnosis of bowel pathology prenatally. Accurate diagnosis of bowel pathology in utero is important for parental counseling and postnatal management. It is essential to recognize the US presentation of bowel pathology in the fetus in order to refer the patient for further evaluation or follow-up. Fetal MRI has been shown to offer some advantages over US for specific bowel abnormalities. In this paper, we review the normal appearance of the fetal bowel on US and MRI as well as the typical presentations of bowel pathologies. We discuss more specifically the importance of recognizing on fetal MRI the abnormalities of size and T1-weighted signal of the meconium-filled distal bowel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the technical advances in US imaging and in fetal MRI, prenatal diagnosis has gained accuracy and improved the quality of prenatal and postnatal management of a large number of fetal pathologies. An accurate prenatal diagnosis of bowel pathologies is important for parental counseling and postnatal management. US remains the first-line examination for fetal bowel abnormalities and in the hands of an experienced sonographer or radiologist it gives excellent information on the bowel wall, bowel content and abnormalities such as the degree of dilatation or abnormal peristalsis. However, it has some limitations and fetal MRI has been shown to be superior to US for the imaging of some abnormalities. MRI offers a larger field of view and a better differentiation between small bowel and colon, allows multiplane post-processing of images and is less operator-dependant [1, 2]. This paper summarizes the main advantages and disadvantages of both modalities for the imaging of fetal bowel pathology.
Normal and abnormal fetal bowel on US
Although the fetal bowel demonstrates a relatively isoechogenic appearance to other abdominal structures and the lumen is difficult to visualize in the first and second trimesters of pregnancy, the bowel wall and some fluid-filled bowel loops can be more clearly identified in the third trimester [3]. Normal peristalsis can also be noticed. In the third trimester, the fetal colon is filled with a larger amount of meconium, which is usually slightly less echogenic than the liver. Haustrations of the wall of the colon can be noticed.
Proximal accumulation of meconium in the small bowel can be seen in distal bowel atresia or meconium ileus and can be mistaken for normal meconium-filled colon on US. Therefore, fetal MRI should be considered in cases of suspicion of distal ileal obstruction [4–6].
Echogenic bowel has been largely described as a marker for chromosomal and congenital anomalies [7, 8]. Echogenic bowel is usually defined as being as echogenic as the surrounding bone and is seen approximately in 1.8% of fetuses [8] (Fig. 1). Usually, the hyperechogenicity resolves after 20 weeks of gestation but can persist beyond that [7]. Most common anomalies associated with echogenic bowel are chromosomal abnormalities, infection, cystic fibrosis, IUGR, premature delivery, bowel abnormalities and multiple malformations. The pathophysiological explanation for the increased echogenicity in the bowel remains for the most part speculative [10]. The diagnosis of echogenic bowel justifies a series of additional testing [8]. The etiologies of echogenic bowel are multiple. It is important to differentiate the echogenic appearance of the bowel as the result of increased echogenicity of the bowel content as it is seen in cystic fibrosis, chromosomal abnormalities or swallowed blood and in the case of increased echogenicity of the bowel wall [9]. Echogenic bowel wall has also been associated with bowel dilatation, and it has been speculated that it is the result of an ischemic event in utero. Fetuses with only dilated and echogenic bowel wall but no other associated abnormalities have been described as having a good outcome [9].
Persistent echogenic bowel, when chromosomal abnormalities and viral infections have been ruled out, might justify an MRI evaluation of the fetal bowel to rule out bowel anomalies or other congenital malformations. Larger studies are necessary to confirm the degree of additional information brought by MRI in the setting of echogenic bowel.
Assessment of the fetal bowel on MRI
MR imaging of the fetal bowel requires both T1- and T2-weighted sequences. It is important to know the normal distribution of meconium in the fetal bowel at different gestational ages in order to understand bowel anomalies and to make a correct diagnosis. The high T1 signal of meconium is the result of accumulation of material composed of dry residual of swallowed amniotic fluid, desquamated cells from the fetal bowel wall and bile salts [12, 13]. Since meconium has high signal on T1-weighted images, T1-weighted images are crucial for diagnosis of any bowel pathology [11].
Most authors use 3D gradient echo images because these images provide a fast T1-weighted acquisition in a fetus where fetal motion interferes with the quality of images, especially at early gestational ages and in the case of polyhydramnios. At later gestational ages (>30 weeks), motion is limited. The 3D GRE can be done with breath-hold, which improves the quality of images. The breath-hold of approximately 25 sec is usually well tolerated by the patient even at advanced gestational ages. Ideally, T1 acquisitions should be performed in all three planes, in order to assess the diameter of the colon and avoid any misinterpretation of partial volume acquisitions.
At our institution, we perform 3D gradient echo TR/TE: 175 ms/min, flip angle 90 degrees. We use a slice thickness of 4 mm, spacing of 0.4 mm. Most authors use a slice thickness of 3–5 mm. Other sequences providing ultrafast T1-weighted imaging could be used in the future.
Meconium is seen in the small bowel and colon beginning at 18 weeks of gestation. Then, with progression of pregnancy and onset of bowel peristalsis, meconium accumulates first in the rectum and progressively in the more proximal colon [1, 2, 14, 15]. Several studies show evidence of defecation of meconium in the amniotic fluid, mainly between 24 and 33 weeks of gestation, but it is not completely clear how much meconium is retained in the amniotic fluid of a normal fetus [16]. The fetal rectum is consistently the largest area of the fetal bowel through the entire gestation [17]. This is an essential sign to assess on fetal MRI, and identification of a high T1 signal in the rectum should be the starting point of any evaluation of the fetal bowel [1, 2, 11, 18]. Therefore, lack of high T1-weighted signal in the rectum should be considered a sign of bowel pathology. Measurements of the normal size of the rectum have been reported, as well as measurements of the fetal colon and rectal diameter [17, 19]. Both types of measurements have some limitations. The bowel is a soft and tortuous structure that peristalses and therefore, it is challenging to obtain reproducible measurements of the colon diameter in two different patients or in the same patient at different times. Additionally, the degree of bladder filling may compress the rectum and modify its diameter. Measurements in three planes are required to obtain accurate data. A volumetric measurement of the fetus can be estimated on three-dimensional acquisition images by multiplying the area containing high signal intensity meconium by the slice thickness. Volumetric measurement of the colon provides reproducible data but is more time-consuming. Volumetric measurements are also subject to volume averaging in cases such as Hirschprung disease and colonic atresia, where proximal colon dilatation and microcolon coexist [19]. Therefore, the subjective overall appearance of the colon and the size of the rectum should be taken in consideration while using this method of measurements.
In the second trimester, small bowel is filled with meconium and appears high signal on T1-weighted images. In the third trimester, it is common to see some residual meconium in the distal ileum up to 30 weeks of gestational age but the proximal loops of small bowel are filled with amniotic fluid. The wall of the proximal bowel and specifically, any bowel wall thickening can be better evaluated in the third trimester [1, 2, 11, 12].
Microcolon and associated anomalies
Absence of high signal at the level of the rectum on T1-weighted images should raise the concern for microcolon or pathology that interferes with meconium formation or meconium accumulation in the rectum.
The most clearly defined pathology associated with microcolon is distal ileal obstruction, as is seen in neonates. Many cases of ileal atresia can be diagnosed in utero [5, 6, 18]. US images can be misleading in some cases, because prenatal US is not as accurate as MRI in the differentiation between meconium-filled distal ileum and normal colon filled with meconium. This is partly because shadowing from the pelvic bones on US limits the visualization of the rectum. MRI is superior to US for visualization of microcolon [20].
Differentiating distal ileal atresia and meconium ileus is difficult because both demonstrate meconium-filled distal ileum and microcolon.
Proximal bowel obstruction is not typically associated with microcolon. However, we have found that some fetuses with clear proximal bowel obstruction also have abnormal signal intensity or anomalies of size of the colon or rectum. Abnormal signal intensity of the meconium can be explained by decreased concentration of the components that give meconium its high T1 signal such as bile salts. Abnormally small rectum or microcolon is seen in proximal bowel obstruction when there is associated distal atresia. Concomitant signs of proximal bowel obstruction and microcolon should always raise the concern of multiple atresias.
Severe vascular insult to bowel in utero is believed to be at the origin of bowel atresia. Some studies have speculated that a less severe vascular insult results in transiently dilated bowel and echogenic bowel wall. The latter is usually associated with resolution of the bowel abnormalities and good outcome if the fetus had no other abnormalities or malformations [9, 21]. We have made similar observations in fetuses with a small diameter of the colon. In our observation of a small series of fetal patients with microcolon in the second trimester, in more than half the diameter of the colon normalized on a follow-up MRI at 32 weeks or at delivery. The effects of vascular insult in utero remain for the most part just speculative and need to be confirmed by pathophysiological studies (Fig. 2).
Finally, other abnormalities must be considered when the diagnosis of microcolon is made. These include microcolon-megacystis-hypoperistalsis, meconium plug syndrome and Hirschprung disease [20, 22].
Anomalies of the signal of meconium
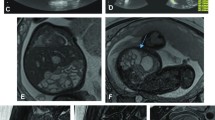
In the absence of high signal intensity at the level of the rectum on T1-weighted images, fluid-sensitive images should be reviewed to evaluate the content of the rectum. Fluid-filled rectum is seen most frequently in cases of urorectal septum malformations and in particular in cases of high anal atresia with urorectal fistula [23]. Urine from the fetal bladder fills the rectal pouch and mixes with the meconium. In the presence of urine, meconium precipitates into intraluminal calcifications, also called enteroliths, most likely secondary to pH changes in the fetal bowel [23]. Enteroliths can be easily recognized on US and fetal MRI. On US, the typical appearance of calcified balls floating in fluid-distended bowel should always raise concern for the diagnosis of urorectal communication. On MRI, the fluid-filled rectum is usually more prominent and the low T2-weighted signal enteroliths are more difficult to see than on US, especially at early gestational age (Fig. 3).
Anal atresia and urorectal fistula in a fetus at 32 weeks of gestational age. a Axial US image of the fetal abdomen shows dilated bowel loops with fluid (urine) and multiple enteroliths (arrow). b Sagittal T2-W image of the same fetus demonstrates fluid in the rectum and some low signal enteroliths (arrowhead)
Fluid-filled colon and rectum can be also seen in cases of congenital diarrhea [24, 25]. Congenital chloride diarrhea is a rare autosomal-recessive disorder associated with severe electrolyte disturbances secondary to abnormal chloride-bicarbonate channels. Intraluminal chloride concentrations are very high, with the patient suffering severe hypochloremia and hypokalemia causing metabolic alkalosis [26]. The prenatal diagnosis is essential to allow immediate correction of the electrolyte unbalance and dehydration at delivery. Another form of congenital diarrhea is sodium diarrhea, which has worse prognosis than chloride diarrhea [27].
Fetuses with congenital diarrhea demonstrate moderately fluid-dilated small bowel as seen in some small bowel obstruction, but in congenital diarrhea there is also fluid in the colon and in the rectum. No high signal meconium is seen on T1-weighted images and there is associated polyhydramnios. The diagnosis can be made by prenatal US [28, 29] but is better assessed by MRI, which confirms the presence of fluid-filled colon and rectum [11]. Analysis of the amniotic fluid can help confirm the diagnosis of congenital diarrhea but the final diagnosis is usually made postnatally (Fig. 4).
Conclusion
Although US remains the main modality for the diagnosis of fetal bowel anomalies, fetal MRI provides important information regarding meconium distribution in the fetal bowel. The understanding of meconium formation and distribution in the bowel at different gestational ages helps to provide accurate diagnosis in cases of bowel abnormality. However, we are limited in our understanding of bowel abnormalities; despite improving image quality, fetal MRI doesn’t allow accurate localization of the level of distal bowel obstruction. The mechanisms of transient bowel distension and transient microcolon are also not clearly understood. Additional studies and ongoing improvement of MRI technique might improve our understanding of the congenital bowel abnormalities.
References
Brugger PC, Prayer D (2006) Fetal abdominal magnetic resonance imaging. Eur J Radiol 57:278–293
Huisman TA, Kellenberger CJ (2008) MR imaging characteristics of the normal fetal gastrointestinal tract and abdomen. Eur J Radiol 65:170–181
Hertzberg BS (1994) Sonography of the fetal gastrointestinal tract: anatomic variants, diagnostic pitfalls, and abnormalities. AJR 162:1175–1182
Hill BJ, Joe BN, Qayyum A (2005) Supplemental value of MRI in fetal abdominal disease detected on prenatal sonography: preliminary experience. AJR 184:993–998
Garel C, Dreux S, Philippe-Chomette P (2006) Contribution of fetal magnetic resonance imaging and amniotic fluid digestive enzyme assays to the evaluation of gastro-intestinal tract abnormalities. Ultrasound Obstet Gynecol 28:282–291
Benachi A, Sonigo P, Jouannic JM (2001) Determination of the anatomical location of an antenatal intestinal occlusion by magnetic resonance imaging. Ultrasound Obstet Gynecol 18:163–165
Kesrouani AK, Guibourdenche J, Muller F (2003) Etiology and outcome of fetal echogenic bowel. Ten years of experience. Fetal Diagn Ther 18:240–246
Nyberg DA, Dubinsky T, Resta RG (1993) Echogenic fetal bowel during the second trimester: clinical importance. Radiology 188:527–531
Grignon A, Dubois J, Ouellet MC (1997) Echogenic dilated bowel loops before 21 weeks’ gestation: a new entity. AJR 168:833–837
Nyberg DA, Resta RG, Mahony BS et al (1993) Fetal hyperechogenic bowel and Down’s syndrome. Ultrasound Obstet Gynecol 3:330–333
Colombani M, Ferry M, Garel C et al (2010) Fetal gastrointestinal MRI: all that glitters in T1 is not necessarily colon. Pediatr Radiol 40:1215–1221
Zizka J, Elias P, Hodik K et al (2006) Liver, meconium, haemorrhage: the value of T1-weighted images in fetal MRI. Pediatr Radiol 36:792–801
Deroches A, Galerne D, Baudon J (1974) Biochemical composition of meconium (carbohydrates, lipids, proteins and related substances). J Gynecol Obstet Biol Reprod (Paris) 3:543–552, In French
Zalel Y, Perlitz Y, Gamzu R (2003) In utero development of the fetal colon and rectum: sonographic evaluation. Ultrasound Obstet Gynecol 21:161–164
Veyrac C, Couture A, Saguintaah M (2004) MRI of fetal GI tract abnormalities. Abdom Imaging 29:411–420
Ramón y Cajal CL, Martínez RO (2003) Defecation in utero: a physiologic fetal function. Am J Obstet Gynecol 188:153–156
Saguintaah M, Couture L, Veyrac C (2002) MRI of the fetal gastrointestinal tract. Pediatr Radiol 32:395–404
Shinmoto H, Kuribayashi S (2003) MRI of fetal abdominal abnormalities. Abdom Imaging 28:877–886
Rubesova E, Vance CJ, Ringertz HG (2009) Three-dimensional MRI volumetric measurements of the normal fetal colon. AJR 192:761–765
Munch EM, Cisek LJ Jr, Roth DR (2009) Magnetic resonance imaging for prenatal diagnosis of multisystem disease: megacystis microcolon intestinal hypoperistalsis syndrome. Urology 74:592–594
Ghi T, Tani G, Carletti A (2005) Transient bowel ischaemia of the fetus. Fetal Diagn Ther 20:54–57
Farhataziz N, Engels JE, Ramus RM (2005) Fetal MRI of urine and meconium by gestational age for the diagnosis of genitourinary and gastrointestinal abnormalities. AJR 184:1891–1897
Lubusky M, Prochazka M, Dhaifalah I (2006) Fetal enterolithiasis: prenatal sonographic and MRI diagnosis in two cases of urorectal septum malformation (URSM) sequence. Prenat Diagn 26:345–349
Colombani M, Ferry M, Toga C (2010) Magnetic resonance imaging in the prenatal diagnosis of congenital diarrhea. Ultrasound Obstet Gynecol 35:560–565
Husu S, Nelson N, Selbing A (2001) Prenatal bowel dilatation: congenital chloride diarrhoea. Arch Dis Child Fetal Neonatal 85:F65
Höglund P, Sormaala M, Haila S (2001) Identification of seven novel mutations including the first two genomic rearrangements in SLC26A3 mutated in congenital chloride diarrhea. Hum Mutat 18:233–242
Orlowski J, Grinstein S (2004) Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pfluger Arch 447:549–565
Kim SH, Kim SH (2001) Congenital chloride diarrhea: antenatal ultrasonographic findings in siblings. J Ultrasound Med 20:1133–1136
Tsukimori K, Nakanami N, Wake N et al (2007) Prenatal sonographic findings and biochemical assessment of amniotic fluid in a fetus with congenital chloride diarrhea. J Ultrasound Med 26:1805–1807
Disclaimer
The supplement this article is part of is not sponsored by the industry. Dr. Rubesova has no financial interest, investigational or off-label uses to disclose.