Abstract
Purpose
This study was done to evaluate the role of fetal magnetic resonance imaging (MRI) in the study of gastrointestinal malformations in comparison to prenatal ultrasound (US).
Materials and methods
A prospective (2010–2012) study of 38 fetal MRI scans was performed on 38 fetuses between 24 and 38 weeks of gestation. All the fetuses had a US diagnosis of gastrointestinal anomalies. T2-weighted HASTE, T1-weighted fast gradient echo, TrueFISP and diffusion-weighted images of the fetal abdomen were obtained on a 1.5-Tesla magnet. All fetal MRI diagnoses were compared with postnatal US findings, autopsy or surgical reports.
Results
Fetal MRI was able to confirm the sonographic findings in nine of 38 fetuses (23.7 %), to provide additional information in 23 of 38 fetuses (60.6 %), to exclude the US diagnosis in five cases (5.2 %) and to change it in two cases (5.2 %). It was not able to characterize a case of gastric duplication and a case of abdominal cystic lymphangioma (5.2 %).
Conclusions
Fetal MRI can be used as a complementary imaging modality to US in prenatal evaluation of gastrointestinal anomalies and can be considered a valuable tool not only for confirming or excluding but also for providing additional information to fetal ultrasonographic findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The number of studies performed on the use of fetal magnetic resonance imaging (MRI) in the evaluation of gastrointestinal (GI) anomalies is still limited [1–3]. Ultrasound (US) is the modality of choice in the prenatal screening of these pathologies for its wide availability and its relative low cost [4]. However, its utility could be limited in some cases, such as oligohydramnios, maternal obesity or unfavorable fetal position [5]. In addition, its sensitivity in the detection of GI anomalies depends on the features of the pathology, and it is not always possible to reach an exact prenatal diagnosis, such as in cases of abdominal masses, which are often difficult to characterize during US examination [6–9].
Fetal MRI is, therefore, necessary either when a US examination is inconclusive or when additional information is necessary for prognostic or therapeutic findings [7, 10]. Although MRI is not recommended in the first trimester of pregnancy, there is no evidence of adverse effects of this technique on fetal development [11].
The introduction of ultrafast sequences provided an excellent evaluation of fetal anatomy with marked reduction of motion artifacts [12]. Due to its multiparametricity, MRI allows an optimal tissue characterization, which is useful for determining the content of abdominal masses and distinguishing between meconium and urine [13].
In our study, we evaluated the role of MRI in confirming, providing additional information about US findings, and changing or excluding the US diagnosis of fetal GI anomalies.
Materials and methods
Patients and study
From December 2010 to March 2012, we performed 224 fetal MRI examinations at our university hospital. Of these, we prospectively enrolled in the study 38 cases with suspicious findings of GI abnormalities on previous obstetric US.
In particular, obstetric US identified or suspected: congenital diaphragmatic hernia (n = 8); bowel dilation (n = 15); intra-abdominal cysts of suspected GI origin (n = 6); esophageal atresia (n = 5); fetal echogenic bowel (FEB) without any abdominal anomalies (n = 3); and isolated ascites (n = 1). All the obstetric ultrasound examinations were performed by an obstetric specialist with more than 9-year experience in high-risk obstetric US on a high-resolution scanner, GE Voluson 730 Expert (General Electric, Waukesha, Wisconsin). The mean maternal age was 30 years (range 23–42 years) and the mean gestational age was 27 weeks (range, 24-38 weeks).
This single-institution study project was approved by the ethics committee of our hospital and written informed consent was obtained from all mothers.
Our inclusion criteria were: visualized or suspected fetal GI pathologies at a recent prenatal US (from 0 to 7 days before MRI), the agreement of the mothers to give information about the course of the pregnancy, the delivery and the newborn, as well as a gestational age at the time of the MRI examination of more than 19 weeks; the gestational age >19 weeks was adopted as an inclusion criterion because obtaining sufficient spatial and contrast resolution to provide diagnostic or additional information with respect to US examinations is not considered possible before 19 weeks and all major developmental steps have taken place only by this age of gestation [5].
Exclusion criteria were fetal abdominal abnormalities of suspected genitourinary (GU) origin (e.g., cystic kidney disease, hydrometrocolpos, ovarian cyst), abdominal solid masses of non-GI origin (e.g., sacrococcygeal teratoma, neuroblastoma) on US as well as general contraindications to MRI of the mother, claustrophobia and gestational age <19 weeks. We did not include in our study fetuses with defects of the abdominal wall (e.g., omphalocele, gastroschisis) because to date there is no evidence of greater accuracy than US, and the indication for fetal MRI may be for surgical planning and defining the manner of birth rather than for diagnostic purposes per se [5].
Amniocentesis was performed on 15/38 patients, resulting in a normal karyotype in all the 15 cases (nine fetuses 46, XY; six fetuses 46, XX).
Of the 38 fetuses included in the study, 33 (87 %) were brought to term, with 27/33 fetuses being born with natural birth and 6/33 with Cesarean section; 5/38 pregnancies (13 %) were voluntarily terminated due to the presence of cloacal malformation in one case and diaphragmatic hernia with severe cardiomediastinic shift and lung damage in four cases. Three cases of diaphragmatic hernia and two cases of esophageal atresia died after birth due to the presence of respiratory complications.
Of the 38 fetuses in the study, 22 (58 %) were males and 16 (42 %) were females.
Follow-up was available for all fetuses. Fetal MRI findings were compared to after-birth US in six cases, to autoptic data in 10 cases and to surgical findings in 22 cases (Table 1).
MRI Technique
All fetal MRI examinations were performed on a 1.5-T superconducting magnet (Siemens Avanto, Erlangen, Germany) using one or two phased-array surface coils, depending on the maternal abdomen size. The women were placed in the supine or lateral decubitus position and no contrast agent or maternal sedation was used.
A common scan protocol included:
-
T2-weighted half-Fourier acquisition single-shot turbo spin echo (HASTE) sequences (TR 1000 ms, TE 119 ms, acquisition thickness 3 mm, FOV 260 × 350 mm, matrix 256 × 144; TA 14-19 s) in three orthogonal planes of the fetal thorax and abdomen (axial, coronal and sagittal). T2-weighted cholangiographic coronal MR images were also acquired to better evaluate the biliary tract.
-
True–fast imaging with steady-state precession (TrueFISP) sequences (TR/TE = 3/1, flip angle 60°, FOV 300 × 300 mm, matrix 256 × 144, acquisition thickness 4 mm) in three orthogonal planes of the fetal thorax and abdomen (axial, coronal and sagittal).
-
FLASH (fast low angle shot) T1-weighted gradient echo (GRE) breath-hold sequences with and without fat saturation (FS) (TR 362 ms; TE 4.8 ms; acquisition thickness 4 mm; flip angle 70°; FOV 350 × 300 mm; matrix 256 × 192; mean TA 30 s) in three orthogonal planes of the fetal thorax and abdomen (axial, coronal and sagittal).
-
Diffusion-weighted echo-planar imaging (DWI-EPI) sequences in the axial plane (TR 8,000 ms; TE 90 ms; TI 185 ms; acquisition thickness: 5 mm; FOV 420 × 300 mm; matrix 192 × 192; TA 45 s; b-factor 0, 200 and 700 s/mm2).
The total examination time was less than 30 min.
Analysis of the MR images
MR images were reviewed by a radiologist with 10-year experience in fetal MR imaging who was blinded to the US findings. In the images, the following parameters and anatomical structures were assessed:
-
1.
Presence and location of the stomach;
-
2.
Appearance and caliber of small bowel loops (normal value <3 mm), colon and rectum (normal value <5 mm);
-
3.
Presence or absence of meconium;
-
4.
Liver, bile ducts and gallbladder;
-
5.
Presence of ascites;
-
6.
Location, extension, morphology and structure (cystic, complex cystic-solid or solid mass) of abdominal space-occupying lesions;
-
7.
Diaphragm.
Results
Fetal MRI confirmed the US findings of GI anomalies in nine of the 38 fetuses (23.7 %), provided additional information in 23 (60.5 %), excluded the US diagnosis in five (5.2 %), changed it in two (5.2 %) and failed in two cases (5.2 %) (Table 2).
Fetal MRI correctly diagnosed: bowel atresia in 11 cases, cloacal malformation in one case, meconium pseudocyst in one case, posterior urethral valve (PUV) in one case, esophageal atresia in five cases, isolated ascites in one case, intra-abdominal cysts in three cases and diaphragmatic hernia in eight cases.
In 12 of the 15 fetuses with a US diagnosis of bowel dilation, MRI provided more information than US because it identified the level of obstruction by evaluating the caliber of the bowel loops and the meconium and/or amniotic fluid content. In particular, we diagnosed: one case of duodenal atresia, one case of both jejunal and distal ileal atresia (Fig. 1), three cases of jejunal atresia (Fig. 2), three cases of ileal atresia, three cases of colonic atresia and one case of cloacal malformation. In the three cases of colonic atresia and in the only case of multiple atresia MRI also revealed the presence of microcolon, whereas all the other cases of bowel atresia presented normal post-atresic bowel loops and rectum.
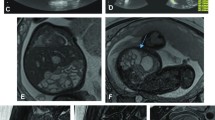
A 34-week fetus with dilated bowel loops due to the presence of both jejunal and distal ileal atresia. a Axial sonogram of the fetal abdomen performed 7 days before MR examination shows anechoic tubular structures (white arrows). b, c A TrueFISP axial MR image shows hypointense dilated bowel loops (b, white arrows), which are hyperintense on a fat-saturated T1-weighted axial MR image due to the meconium content (c, black arrows). d, e A TrueFISP coronal MR image (d) and a T2-weighted HASTE sagittal image (e) show the subhepatic (d, open arrow) and anterior (e, white arrow) location of the dilated bowel loops in the fetal abdomen. f Colon of reduced caliber, distal to the obstruction, on a fat-saturated T1-weighted axial MR image (black arrow)
A 34-week fetus with dilated stomach, duodenum and jejunal loops due to the presence of jejunal atresia. a Axial sonogram of the fetal abdomen performed 4 days before MR examination shows dilated stomach (open arrow) and duodenum (white arrow). b–f Axial (b), sagittal (d), coronal (f) TrueFISP MR images and a coronal T2-weighted MR image (e) show hyperintense dilated stomach (open arrow) and duodenum (white arrow), which are hypointense on a T1-weighted MR axial image (c), due to the presence of amniotic fluid. Dilated small bowel loops (asterisks), hyperintense on TrueFISP (b, f) and T2-weighted (e) images and slightly hyperintense on the T1-weighted image (c) due to the poor content of meconium, present thin wall because of the increased intraluminal tension (f, black arrow)
In two cases, MRI changed the US diagnosis of bowel dilatation, revealing a meconium pseudocyst in one case (Fig. 3) and PUV with the presence of the typical “keyhole sign” associated with marked ureteral dilation in the other fetus (Fig. 4); bowel loops were normal in both cases.
A 36-week fetus with meconium pseudocyst. a Axial sonogram of the fetal abdomen performed 6 days before MR examination shows hypoechoic tubular structures. b–d A cystic formation of about 2.5 cm is located in the left iliac fossa (white arrow) and presents inhomogeneous hyperintensity on T2-weighted axial (b) and coronal (c) MR images, and high hyperintensity on a T1-weighted axial FS MR image (d). The small bowel loops are not dilated (b, circle). The MRI findings are highly suggestive of a meconium pseudocyst
A 37-week fetus affected by posterior urethral valve (PUV) associated with bilateral ureteral dilation. a Axial sonogram of the fetal abdomen performed 7 days before MR examination shows dilated tubular anechoic structures. b–e The dilated ureters (white arrows) are hyperintense on a T2-weighted HASTE axial (b) and a TrueFISP coronal (d) image and hypointense on T1-weighted axial (c) and coronal (e) MR images, because of the presence of urine; the rectosigmoid colon (open arrow) is located in the middle of the fetal abdomen and presents low signal intensity on T2-weighted (b) and TrueFISP (d) images and high signal intensity on T1 (c, e), due to its meconium content. f The typical “keyhole sign” (open arrow) on a T2-weighted HASTE sagittal image suggests the diagnosis of PUV
In one case MRI excluded any GI malformation, which was also excluded by postnatal US. A gastric dilation was detected in three of the 15 fetuses with US diagnosis of bowel dilation (Fig. 2), whereas a small stomach was observed in the five cases with a US suspicion of esophageal atresia, which MRI confirmed without providing any additional information.
In all the eight fetuses with a US diagnosis of diaphragmatic hernia, MRI provided more information than US because of a more accurate evaluation of the diaphragm and a more precise detection of the abdominal structures herniated inside the thorax (Fig. 5). In particular, we studied the presence of stomach, colon, small bowel loops, liver (liver up vs. liver down) and kidney (kidney up vs. kidney down) in the thorax and the presence of lung damage and/or cardiomediastinic shift (Fig. 5), which was detected in 4/8 cases. In all cases, there was small bowel and stomach herniation, in 5/8 cases also liver up and colon herniation and in 2/8 cases also kidney up, liver up and colon herniation (Fig. 5).
A 28-week fetus with diaphragmatic hernia on the left side of the diaphragm. a An axial sonogram of the fetal abdomen performed 2 days before MR examination shows an increased echogenicity of the left side of the thorax suggesting the presence of a diaphragmatic hernia (black arrow). b–d Axial (b) and coronal (c) T2-weighted MR images show the presence of small and large bowel loops in the left emithorax (b, c, circle) associated with shift of the heart to the right side of the thorax (white arrow). A T1-weighted sagittal MR image shows the presence of hyperintense large bowel into the thorax (d, black arrow). e–f Coronal (e) and sagittal (f) HASTE T2-weighted MR images show the presence of the upper pole of the left kidney (open arrow) and of the right lobe of the liver (white arrow) in the thorax along with compressed parenchyma of the right lung (e, asterisk)
We also evaluated the presence of abdominal space-occupying lesions, making a correct diagnosis in 3/6 abdominal cystic masses diagnosed on US. In one fetus with US findings of an abdominal cyst in the right upper quadrant of the abdomen, the cyst was detected neither on fetal MRI examination nor on postnatal US. It correctly identified the organ of origin in three fetuses with choledochal cyst, postnatally confirmed by surgical data (Fig. 6). In these three cases, MRI provided more information than US because it determined the fluid content of the mass and its connection to the biliary tract. It was not possible to characterize the cystic lesion in 2/6 fetuses: in one case, the MR findings led us to suspect a choledochal cyst, but after birth a careful surgical evaluation made a diagnosis of gastric duplication; in the other, MR only confirmed the sonographic finding of a cystic mass, but the absence of septa led us to suspect a fluid collection. The final diagnosis of abdominal cystic lymphangioma was made on postnatal surgical evaluation.
A 32-week fetus with a choledochal cyst. a Axial sonogram of the fetal abdomen performed 2 days before MR examination shows an anechoic cystic mass (white arrow) adjacent to the liver with significant posterior acoustic enhancement. b–c A subhepatic cystic formation of 3 cm is hyperintense on a T2-weighted HASTE axial MR image (b, open arrow) and hypointense on a T1-weighted FS image (c, white arrow) due to its fluid content. d–e A T2-weighted cholangiographic coronal image and a T2-weighted HASTE coronal image show the cyst (open arrow) and the stomach (white arrow). f A T2-weighted HASTE sagittal image confirms the subhepatic location of the cyst (white arrow) and the connection to the biliary tract (black arrow). The bladder is located below the abovementioned cystic formation (open arrow)
MRI confirmed the US finding of isolated ascites, excluding the presence of any GI or genitourinary (GU) tract anomalies. Postnatal US examination showed disappearance of the ascites one month after birth.
In the three cases of FEB without any abdominal anomalies, fetal MRI and postnatal US examinations confirmed the absence of bowel dilation or other fetal GI abnormalities.
Discussion
Several authors have compared the efficacy of US and MRI in the diagnosis of fetal anomalies, revealing that MRI provides additional information in comparison to US in 36–57 % of cases [14–17]. Most of them evaluated the accuracy of MRI in the diagnosis of central nervous system diseases, which are the most common indications for fetal MRI [18–20], but little has been written on the use of fetal MRI in confirming or excluding US diagnosis of fetal GI abnormalities [1, 2, 10, 21].
Thanks to a wide field of view, its intrinsic multiparametricity and multiplanarity MRI provides a detailed evaluation of the whole fetal GI tract and could be helpful in identifying the various intestinal segments [1, 2, 10]. In cases of bowel obstruction, sonography usually detects only the dilated loops proximal to the atresia [22] without providing any significant information about the presence of meconium and/or amniotic fluid. In our study, in 12 of the 15 fetuses with a US diagnosis of bowel dilation, MRI detected the level of obstruction, determining the content and the caliber of both the pre- and post-atresic bowel loops and rectum. As previously reported, the signal of the bowel content depends on the site of the atresia. In a proximal atresia, only fluid will accumulate, with a high T2 and a low T1 signal, whereas in a distal atresia MRI shows high pre-atresic bowel loops signal on T1- and low signal on T2-weighted images, due to the presence of more or less hydrated meconium [1]. In our study, MRI showed high T2 and low T1 signal intensity proximally to the obstruction, due to presence of amniotic fluid, in fetuses with jejunal and duodenal atresia and intermediate T1 and T2 signal, for the simultaneous presence of meconium and amniotic fluid, in fetuses with ileal atresia. In the case of multiple atresia and in the three fetuses with colonic atresia, the pre-atresic bowel loops had meconium-like signal, high on T1- and from low to intermediate on T2-weighted images; in these fetuses MRI also detected a microcolon, showing a reduced caliber of the bowel distal to the obstruction and rectum along with a poor meconium content with low to intermediate T1 signal intensity. This last finding is often undetected on US although its diagnosis is important for optimal surgical planning [1].
In fetuses with cloacal malformation, the urinary tract, the vagina and the rectum converge above the perineum, create a common channel with a single external opening. This condition is often associated with intestinal dilation, a high distal bowel position and anomalies of the genitourinary (GU) system [23]. US often leads to a misdiagnosis of this anomaly as other abnormal abdominal structures [1]. In our case, MRI provided additional information revealing a dilated rectum and colon with abnormal increased T2 and decreased T1 signals, indicating communication between the urinary tract and rectum, which was separated from the bladder wall by a third structure with intermediate T1 and T2 signal.
MRI also distinguished between bowel and ureteral dilation in the case of PUV described, because urine has high signal intensity on T2-weighted and low on T1-weighted sequences [13, 24] and showed the typical “keyhole” sign, which refers to the appearance of the dilated posterior urethra associated with thick-walled distended bladder [25].
Meconium peritonitis is caused by a bowel perforation linked to various conditions, such as the presence of intestinal atresia, meconium ileus [26] or intussusception [27]. Calcifications or pseudocyst may be seen on US due to meconium peritonitis and localized ascites. Tissue characterization is usually inferior with MRI because of low sensitivity of MR for detection of calcification [7]. However, in our study US did not detect any cystic formation, which MRI was able to identify in one case. MRI also showed the meconium content of the cyst due to the high T1 signal, allowing differentiation of other intra-abdominal cysts, such as mesenteric or duplication cysts.
In a case of US diagnosis of bowel dilation, fetal MRI did not show any abnormality. This could be explained by fetal intestinal peristalsis with sonographic image of transient bowel dilation, as previously reported by Bronshtein [28]. Also in the three cases of isolated FEB, no fetal GI anomalies were detected by MRI. FEB is a US finding which can be observed in cases of cystic fibrosis, prenatal infections or other fetal pathologies, where it is often associated with other US findings, such as ascites or bowel dilatation [29]. According to Carcopino, we demonstrated that fetal MRI does not add significant data to US in cases of isolated FEB. However, because FEB is associated with 33.3 % of poor perinatal outcome and 5.5 % of perinatal mortality [29], it is important to exclude other fetal associated anomalies in case it tends to persist during the pregnancy.
Some studies have demonstrated the usefulness of fetal MRI in determining the origin [1, 2, 14, 30] and in providing an accurate characterization of abdominal fetal cystic masses [7, 14, 21]. However, in our study, MRI was not able to characterize 2/5 cases of abdominal cystic masses. In the first, the intraperitoneal location and the fluid content of the lesion were not sufficient to make the diagnosis of cystic lymphangioma. Some authors reported the MRI diagnosis of this abnormality [14, 31], but in our case the absence of septa, which are usually present in this kind of anomaly, led us to wrongly diagnose a fluid collection. In the second, the proximity of the cystic formation to the biliary tract directed us to the incorrect diagnosis of choledochal cyst, but the postnatal examination revealed a gastric duplication. In the other three cases, the evident connection of the cystic lesion to the biliary tract, not visualized at US, and the fluid content of the lesion, led us to correctly diagnose the presence of a choledochal cyst, confirming the role of MRI in recognizing this fetal malformation, as previously reported by Chen [32]. In one fetus, the US detection of an abdominal cyst located in the right upper abdomen was not confirmed by MRI and was likely to be of hepatic origin. The intrauterine disappearance of a hepatic cyst can be expected especially when the cysts are peripheral [33].
In the eight cases of diaphragmatic hernia MRI detected the site of the defect, the extent, the content of the herniation, and the mass effect of the herniated organs on the thoracic structures, with the presence of cardiomediastinic shift and lung damage [34, 35]. On T2-weighted sequences, structures with fluid content, such as the stomach or the proximal small bowel, showed high signal intensity. T1-weighted sequences allowed us to distinguish the liver, relatively hyperintense compared to the heart, from the colon, typically hyperintense because of the presence of meconium. Furthermore, MRI was superior to US in the assessment of the position of the liver and the kidney in relation to the diaphragm, which is considered an independent predictive factor and defined as liver up versus liver down and kidney up versus kidney down [36].
Another frequent abdominal finding is the presence of ascites, usually caused by an obstructive uropathy or a GI disorder. Some authors believe that if no factor is etiologically correlated with the presence of ascites, this could be related to the presence of a chylous peritoneal fluid, caused by the obstruction of lymphatic vessels or the presence of a lymphatic dysplasia [37]. In our case of isolated ascites, fetal MRI showed a hyperintense abdominal fluid collection on T2-weighted sequences with no associated GI or GU abnormalities. This finding disappeared without any intervention and no abnormality was present at birth [38].
Finally, our study revealed that MRI can confirm the US suspicion of esophageal atresia showing the presence of a small stomach and polyhydramnios, although Matsuoka demonstrated that MRI can also provide several additional findings to US diagnosis of this fetal abnormality [39].
There were some limitations in the present study. One main limit was that only fetuses with a sonographic suspicion of GI anomalies underwent MR examination and these cases probably benefit more from the advanced imaging. Furthermore, the greater number of patients in some anomaly groups—bowel dilation and diaphragmatic hernia—where MRI is particularly able to refine the US diagnosis, could have determined the ability of MRI to provide additional information in our study.
In conclusion, our study demonstrated that Fetal MR imaging can be used as a complementary method in prenatal imaging to improve diagnosis in cases of complex GI abnormalities or if US could not easily visualize the GI tract, providing additional information to prenatal US in 60.5 % of cases.
In particular, we confirmed its role in adding information about the level of obstruction in cases of bowel atresia, by determining the caliber and content of bowel loops, and in providing a more detailed evaluation of the herniated organs in cases of diaphragmatic hernia and the exact characterization of some abdominal cystic masses.
References
Saguintaah M, Couture A, Veyrac C et al (2002) MRI of the fetal gastrointestinal tract. Pediatr Radiol 32:395–404
Veyrac C, Couture A, Saguintaah M et al (2004) MRI of fetal GI tract abnormalities. Abdom Imaging 29:411–420
Barnewolt CE (2004) Congenital abnormalities of the gastrointestinal tract. Semin Roentgenol 39:263–281
Corteville JE, Gray DL, Langer JC (1996) Bowel abnormalities in the fetus—correlation of prenatal ultrasonographic findings with outcome. Am J Obstet Gynecol 175:724–729
Triulzi F, Manganaro L, Volpe P (2011) Fetal magnetic resonance imaging: indications, study protocols and safety. Radiol Med 116:337–350
Sherwood W, Boyd P, Lakhoo K (2008) Postnatal outcome of antenatally diagnosed intra-abdominal cysts. Pediatr Surg Int 24:763–765
Gupta P, Sharma R, Kurma S et al (2010) Role of MRI in fetal abdominal cystic masses detected on prenatal sonography. Arch Gynecol Obstet 281:519–526
Goldberg JD (2004) Routine screening for fetal anomalies: expectations. Obstet Gynecol Clin North Am 31:35–50
Pugash D, Bruggerb PC, Bettelheimc D et al (2008) Prenatal ultrasound and fetal MRI: the comparative value of each modality in prenatal diagnosis. Eur J Radiol 68:214–226
Rubesova E (2012) Fetal bowel anomalies—US and MR assessment. Pediatr Radiol 42(Suppl 1):S101–S106
Amini H, Wikstrom J, Ahlstrom H et al (2011) Second trimester fetal magnetic resonance imaging improves diagnosis of non-central nervous system anomalies. Acta Obstet Gynecol Scand 90:380–389
Kubik-Huch RA, Huisman TA, Wisser J et al (2000) Ultrafast MR imaging of the fetus. AJR Am J Roentgenol 174:1599–1606
Farhataziz N, Engels JE, Ramus RM et al (2005) Fetal MRI of urine and meconium by gestational age for the diagnosis of genitourinary and gastrointestinal abnormalities. AJR Am J Roentgenol 184:1891–1897
Breysem L, Bosmans H, Dymarkowski S et al (2003) The value of fast MR imaging as an adjunct to ultrasound in prenatal diagnosis. Eur Radiol 13:1538–1548
Santos XM, Papanna R, Johnson A et al (2010) The use of combined ultrasound and magnetic resonance imaging in the detection of fetal anomalies. Prenat Diagn 30:402–407
Frates MC, Kumar AJ, Benson CB et al (2004) Fetal anomalies: comparison of MR imaging and US for diagnosis. Radiology 232:398–404
Perrone A, Savelli S, Maggi C et al (2008) Magnetic resonance imaging versus ultrasonography in fetal pathology. Radiol Med 113:225–241
Manganaro L, Savelli S, Francioso A et al (2009) Role of fetal MRI in the diagnosis of cerebral ventriculomegaly assessed by ultrasonography. Radiol Med 114:1013–1023
Rajeswaren R, Chandrasekharan A, Joseph S et al (2009) Ultrasound versus MRI in the diagnosis of fetal head and trunk anomalies. J Matern Fetal Neonatal Med 22:115–123
Blondiaux E, Garel C (2013) Fetal cerebral imaging—ultrasound vs. MRI: an update. Acta Radiol 54:1046–1054
Hill BJ, Joe BN, Quayyum A et al (2005) Supplemental value of MRI in fetal abdominal disease detected on prenatal sonography: preliminary experience. AJR Am J Roentgenol 184:993–998
Boyd PA, Chamberlain P, Gould S et al (1994) Hereditary multiple intestinal atresia—ultrasound findings and outcome of pregnancy in an affected case. Prenat Diagn 14:61–64
Chauvin NA, Epelman M, Victoria T et al (2012) Complex genitourinary abnormalities on fetal MRI: imaging findings and approach to diagnosis. AJR Am J Roentgenol 199:W222–W231
Zizka J, Elias P, Hodik K et al (2006) Liver, meconium, haemorrhage: the value of T1-weighted images in fetal MRI. Pediatr Radiol 36:792–801
Bernardes LS, Aksnes G, Saada J et al (2009) Keyhole sign: how specific is it for the diagnosis of posterior urethral valves? Ultrasound Obstet Gynecol 34:419–423
Reynolds E, Douglass B, Bleacher J (2000) Meconium peritonitis. J Perinatol 3:193–195
Yang J, Kim H, Chang K et al (2004) Intrauterine intussusception presenting as fetal ascites at prenatal ultrasonography. Am J Perinatol 21:241–246
Bronshtein M, Zimmer EZ (1996) Early sonographic detection of fetal intestinal obstruction and possible diagnostic pitfalls. Prenat Diagn 16:203–206
Carcopino X, Chaumoitre K, Shojai R et al (2007) Foetal magnetic resonance imaging and echogenic bowel. Prenat Diagn 27:272–278
Inaoka T, Sugimori H, Sasaki Y et al (2007) VIBE MRI for evaluating the normal and abnormal gastrointestinal tract in fetuses. AJR Am J Roentgenol 189:W303–W308
Cozzi DA, Olivieri C, Manganaro F et al (2010) Fetal abdominal lymphangioma enhanced by ultrafast MRI. Fetal Diagn Ther 27:46–50
Chen CP, Cheng SJ, Sheu JC et al (2004) Third-trimester evaluation of choledochal cyst using magnetic resonance imaging. Prenat Diagn 24:838–839
Bronstein M, Nizar K, Weiner Z (2009) Significance of early prenatal diagnosis of fetal hepatic cyst. J Clin Ultrasound 37:65–68
Walsh DS, Hubbard AM, Olutoye OO et al (2000) Assessment of fetal lung volumes and liver herniation with magnetic resonance imaging in congenital diaphragmatic hernia. Am J Obstet Gynecol 183:1067–1069
Manganaro L, Perrone A, Sassi S et al (2008) Diffusion-weighted MR imaging and apparent diffusion coefficient of the normal fetal lung: preliminary experience. Prenat Diagn 28:745–748
Lazar DA, Ruano R, Cass DL et al (2012) Defining “liver-up”: does the volume of liver herniation predict outcome for fetuses with isolated left-sided congenital diaphragmatic hernia? J Pediatr Surg 47:1058–1062
Nose S, Usui N, Soh H et al (2011) The prognostic factors and the outcome of primary isolated fetal ascites. Pediatr Surg Int 27:799–804
We JS, Young L, Park IY et al (2012) Usefulness of additional fetal magnetic resonance imaging in the prenatal diagnosis of congenital abnormalities. Arch Gynecol Obstet 286:1443–1452
Matsuoka S, Takeuchi K, Yamanaka Y et al (2003) Comparison of magnetic resonance imaging and ultrasonography in the prenatal diagnosis of congenital thoracic abnormalities. Fetal Diagn Ther 18:447–453
Acknowledgments
The material contained in the manuscript has not been previously published and is not under consideration for publication elsewhere. Each author has participated sufficiently in any submission to take public responsibility for its content. Publication is approved by all authors.
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manganaro, L., Saldari, M., Bernardo, S. et al. Role of magnetic resonance imaging in the prenatal diagnosis of gastrointestinal fetal anomalies. Radiol med 120, 393–403 (2015). https://doi.org/10.1007/s11547-014-0464-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-014-0464-2