Abstract
The dentate nuclei lie deep within the cerebellum and play a vital role in the pathways involved in fine motor control and coordination. They are susceptible to a variety of diseases. Some pathological processes preferentially affect the dentate nuclei, while concomitant basal ganglia or white matter involvement can be a striking finding in others. A familiarity with the normal appearance of the dentate nuclei at different ages in combination with the radiological distribution of pathology in the brain allows the paediatric radiologist to develop a logical approach to the interpretation of MR imaging of these deep cerebellar nuclei. In this article we review the normal appearance and MR features of the dentate nuclei, including changes that are seen with myelination. We describe the specific imaging characteristics of childhood diseases that involve the dentate nuclei, and develop a systematic approach to the differential diagnosis of dentate nucleus abnormalities on MR imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
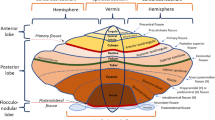
The dentate nucleus is the largest and most lateral of the deep cerebellar nuclei. Named because of its toothed appearance, the dentate forms part of the functional neo-cerebellum, which is involved in the coordination and timing of fine and skilled voluntary movements including speaking, writing and dancing. It receives its afferents from the premotor cortex and the supplementary motor cortex via the pontocerebellar system. Efferent fibres from the dentate nucleus pass in the superior cerebellar peduncles and cross over at the pontomesencephalic junction to synapse in the red nucleus, then on to the ventrolateral thalamus and project to the motor cortex (Fig. 1).
A variety of disease processes can affect the dentate nuclei; some preferentially involve the dentate nucleus, while others are associated with basal ganglia and white matter changes. Familiarity with the normal appearance of the dentate nuclei at different ages, combined with the radiological distribution of pathology in the brain, suggests a differential diagnosis in children with MR abnormalities identified in these deep cerebellar nuclei.
MR imaging parameters
MR imaging was performed on a 1.5-T Signa system (General Electric, Milwaukee, Wis.). The MR imaging protocols used in our department included the following sequences and parameters: axial T1-weighted (T1-W) spin-echo (neonates to 6 months TR 500 ms, TE 9 ms; >6 months TR 405 ms TE 9 ms), axial T2-weighted (T2-W) fast spin-echo (neonates TR 4,525 ms, TE 200 ms; 3–6 months TR 4,000 ms, TE 140 ms; >6 months TR 4,050 ms, TE 85 ms), T2 FLAIR (TR 9,000 ms, TI 2,200 ms, TE 120 ms) and T1-W inversion recovery (TR 2,000 ms, TI 750 ms, TE 9 ms).
MR appearance of the normal dentate nucleus
The normal appearance of the dentate changes as myelination progresses in the first years of life [1]. We have reviewed a large number of normal MRI scans at various ages and can describe the following findings.
T1-W MR
At term the normal dentate nucleus on T1-W images is hypointense to adjacent myelinated white matter (Fig. 2). As myelination progresses both the central part of the dentate and the peridentate white matter become brighter on T1-W images by the third month of life [2]. The dentate nucleus becomes difficult to distinguish from the surrounding cerebellar peduncles on T1 images by 6 months. Hyperintensity of the central part of the dentate compared with surrounding white matter is always abnormal.
T2-W MR
At term the normal dentate nuclei are hyperintense on T2-W MR with a dark outer serrated border [1]. Over the first year the central part of the dentate nuclei becomes isointense with grey matter, while the outer border blends with gradually myelinating middle cerebellar peduncles and the white matter of the cerebellum. By 2 years of age the dentate nuclei become difficult to distinguish from the medial borders of the adjacent cerebellar white matter (Fig. 3).
Diffusion-weighted imaging (DWI)
The normal dentate nuclei are not distinguishable from surrounding white matter on DWI. On apparent diffusion coefficient (ADC) images in the first 6 months of life the normal dentate may be detectable by very slightly less restricted diffusion than adjacent white matter (Fig. 4). Brightness of the dentate on DWI and significant hypointensity on ADC images is therefore always abnormal.
DWI images at (a) 12 days, (b) 3 months (c) 6 months and (d) 1 year of age show that normal dentate is not distinguishable from surrounding white matter. On corresponding ADC images at (e) 12 days, (f) 3 months (g) 6 months and (h) 1 year of age the normal dentate may be detectable by very slightly less restricted diffusion than adjacent white matter
The dentate and disease
A wide variety of pathological processes can involve the dentate nuclei including metabolic, genetic and neurodegenerative disorders, ischaemia and some infections. In the next section we will review the salient imaging features that have been observed when the dentate nucleus is involved in these diseases.
Metabolic disorders
Mitochondrial disorders is a collective term for a wide variety of overlapping conditions involving defects in the respiratory chain; a complex multiunit system within the inner membrane of mitochondria involved in the process of oxidative phosphorylation. Mitochondrial disorders exhibit a broad spectrum of clinical manifestations, imaging features and inheritance patterns. There is often overlap between different disease entities with regards to both clinical and imaging features [3, 4].
Leigh disease (subacute necrotising encephalomyelopathy) refers to a symptom complex with characteristic but variable clinical and pathological manifestations, which usually presents in the first year of life with psychomotor retardation and brain stem dysfunction. There is progressive neurological deterioration with lactic acidaemia. Several biochemical and genetic abnormalities cause Leigh disease. However four main groups of defects appear to be responsible for the majority of cases—defect of pyruvate dehydrogenase complex, cytochrome oxidase (COX, complex IV), complex V and complex I deficiencies [5]. On T2-W and fluid-attenuated inversion recovery (FLAIR) sequences, focal high-signal intensities, which are often bilaterally symmetric, can be seen in the peri-aqueductal grey matter, the remainder of the brain stem, basal ganglia, thalami, subthalamic and dentate nuclei (Fig. 5) [3–7].
Glutaric aciduria type 1 (GA1), an autosomal-recessive (AR) inborn error of metabolism, is due to mitochondrial glutaryl-co-enzyme A dehydrogenase deficiency. This is not a respiratory chain disorder but an organic aciduria. It is characterized clinically by episodic acute encephalopathic crises. Frontotemporal atrophy and dilatation of the sylvian fissures is seen on MR imaging in 93% of GA1 patients. Signal abnormalities in the basal ganglia progress to atrophy over time (Fig. 6). Other findings in GA1 include white matter and dentate nuclei abnormalities (Fig. 6) and reduced NAA peak on MR spectroscopy (MRS) [8]. Elevated lactate on MRS may be seen during a crisis [9].
An 18-month-old girl with GA1. a Axial T2-W images through the cerebrum at the level of the basal ganglia shows high signal in the lentiform and caudate nuclei. Abnormal mixed signal is also seen in the thalami. b The dentate nuclei demonstrate abnormal high signal (arrows). The medial lemnisci in the posterior pons also have abnormal increased signal intensity
L-2 hydroxyglutaric aciduria is an AR organic aciduria of unknown aetiology. As the clinical presentation is often non-specific, the distinctive MR brain appearance of this disorder can be key in initiating the appropriate metabolic workup. Both grey and white matter are involved. The white matter changes follow a centripetal distribution with the subcortical U-fibres being the most severely affected (Fig. 7). The basal ganglia and dentate nuclei are always abnormal and are often swollen (Fig. 7). Gradual cerebral and cerebellar atrophy develop over time with progression of disease [10, 11].
A 22-year-old man (who presented in childhood) with L-2 hydroxyglutaric aciduria. Axial T2-W images. a Image demonstrates high signal abnormality and swelling affecting the superficial cerebral white matter and sub-cortical U-fibres. There is relative sparing of the deep white matter. b Abnormal high signal is shown in the dentate nuclei bilaterally (arrows)
Mucopolysaccharidoses (MPS) are inherited disorders of metabolism characterised by enzyme deficiency that results in inability to break down glycosaminoglycan. MPS are classified according to the specific enzyme deficiency. The perivascular (Virchow-Robin) spaces become dilated by accumulated glycosaminoglycan producing a cribriform appearance of the white matter (Fig. 8), corpus callosum and basal ganglia on MRI. The dentate nuclei can demonstrate abnormal signal (Fig. 8). Other neuroimaging features seen in MPS include craniocervical junction compression and occasional arachnoid cysts [12].
An 18-year-old child with MPS 1, Hurler –Scheie phenotype. a Axial T1-W image showing cyst-like lesions within basal ganglia, thalami and external capsule (arrow). b T2 FLAIR image shows abnormal white matter signal. There is also cyst formation in the external capsules (arrow). c The dentate has low signal on T2 FLAIR and (d) mixed signal on T2-W images
Aicardi-Goutieres syndrome Aicardi and Goutieres [13] in 1984 reported eight cases of progressive familial encephalopathy in infancy, with basal ganglia calcification and chronic cerebrospinal fluid lymphocytosis. There was rapid deterioration to a vegetative state [13]. Aicardi-Goutieres syndrome is an AR disorder and is phenotypically similar to in-utero viral infection. There is cerebral atrophy, white matter abnormality and cerebral calcification (Fig. 9). Dentate nuclei may be affected [14].
Peroxisomal disorders are subdivided into two major categories. Peroxisome biogenesis disorders are disorders resulting from a failure to form normal peroxisomes, resulting in multiple metabolic abnormalities. The second category includes those disorders resulting from the deficiency of a single peroxisomal enzyme. The dentate nucleus may be affected in both types of peroxisomal disorders including infantile Refsum disease, X-linked adrenoleukodystrophy and acyl CoA oxidase deficiency (Fig. 10) [15].
Maple syrup urine disease (MSUD) is an inherited disorder of branched chain amino acid metabolism presenting in newborns with neurological deterioration, ketoacidosis and hyper-ammonemia. There is marked fluid restriction seen in DWI with reduced ADC map signal in keeping with myelin oedema. The oedema usually involves the cerebellum including the dentate nuclei, the brain stem and corticospinal tracts more than the cerebral hemispheres (Fig. 11) [16, 17].
A 1-month-old infant girl with MSUD. a, b Axial T2-W images through the cerebellum and at sylvian fissure level show hyperintensity in (a) both dentate nuclei (white arrows) and cerebellar white matter, (b) the thalami (arrowheads) and white matter. c DWI through the basal ganglia shows abnormal hyperintensity in both thalami, globi pallidi and posterior limb of internal capsule. d ADC confirms restricted diffusion in the same areas in keeping with myelin oedema
White matter diseases
Canavan disease is a progressive AR leukodystrophy due to aspartoacyclase deficiency resulting in N-acetyl aspartic aciduria. MR imaging reveals diffuse symmetric cerebral white matter abnormalities with low T1-W and high T2-W signal intensity. There is early preferential involvement of the subcortical white matter, thalami and globus palladi. Deep white matter and dentate nuclei abnormalities occur later [10]. Distinctive elevation of N-acetyl-aspartate (NAA) is visible on MRS (Fig. 12).
A 6-month-old girl with Canavan disease. a–c T2-W imaging shows hyperintensities in the cerebellar white matter, dentate nuclei, brain stem, thalami (arrowhead) and globi pallidi (arrow). d MRS (centered on left thalamus and adjacent lentiform nucleus, TE 35 ms) shows marked NAA peak. e Normal MRS in this location at the same age. Note how the NAA peak is 1.5 times higher than the creatine (Cr) peak, compared with three times higher in the child with Canavan disease
Globoid cell leukodystrophy (Krabbe disease) is a progressive AR degenerative leukodystrophy due to a deficiency of galactocerebroside beta-galactosidase. In the very early stages MR imaging can be normal but CT may demonstrate hyperdense basal ganglia, thalami and dentate nuclei. As the disease develops the deep cerebral white matter becomes hypointense on T1-W and hyperintense on T2-W sequences. Abnormal signal is also seen in the basal ganglia and thalami as well as the dentate nuclei (Fig. 13) [18, 19].
A 1-year-old boy with Krabbe disease. a, b Axial CT images show calcification in the thalami, basal ganglia and dentate. c Axial T2-W images confirm abnormality in the dentate with high signal centrally, surrounding low signal and abnormal high signal in peridentate white matter. Abnormal high signal can also be seen in the medial longitudinal fasciculus in the anterior pons. d The cerebral deep white matter also has abnormal high signal
Megalencephalic leukoencephalopathy with cysts (previously known as Van Der Knaap leukoencephalopathy) is an autosomal-recessive neurodegenerative leukoencephalopathy. Affected children have macrocephaly from birth or develop it in the first year of life. There is usually mild developmental delay and cerebellar ataxia develops early in childhood. By their teens, MR of the brain shows swollen white matter with sub-cortical cysts especially anterior temporal and fronto-parietal regions (Fig. 14). Dentate nucleus involvement has been described (Fig. 14) [20].
A 3-year-old child with megalencephalic leukoencephalopathy with cysts. a Axial FLAIR and (b, c) axial T2-W imaging show (a, b) abnormal high signal in the dentate nuclei and swollen white matter with sub-cortical cysts especially in the anterior temporal (arrows) and (c) frontal lobe white matter (arrowheads)
Merosin-negative muscular dystrophy comprises only 30% of congenital muscular dystrophies. This heterogeneous group of AR myopathies present at birth with hypotonia. There is significant hypomyelination and high T2 signal of white matter in the merosin-negative type. High signal may also be seen in the dentate nuclei and in the cerebellar white matter (Fig. 15). Additional MRI findings include polymicrogyria and callosal, vermian or septal hypogenesis [21].
Miscellaneous disorders
In the preceding few sections we have described the abnormal MR appearance of the dentate nuclei in a variety of rare genetic and metabolic disorders. We have also observed dentate nuclei abnormalities in more commonly encountered childhood diseases including ischaemic insults and infection, phakomatoses and Langerhans cell histiocytosis (LCH).
Ischaemia
Particular susceptibility of the dentate nuclei to ischaemia has not been described but we have found that the dentate nuclei may be particularly involved in diffuse acute ischaemia while other posterior fossa structures are relatively spared (Fig. 16). The dentate nuclei are supplied by the superior cerebellar artery but selective involvement cannot be explained by vascular distribution and is more likely related to a high metabolic rate comparable to that seen in the thalami and basal ganglia.
An 8-month-old girl with severe acute cerebral ischaemia secondary to cardiac arrest. a DWI demonstrates diffuse abnormal cerebral diffusion. b DWI of the posterior fossa shows markedly abnormal diffusion in both dentate nuclei (arrowheads). c, d Right middle cerebral artery (MCA) infarction in a 16-month-old girl. On T2-W sequences there is severe cystic encephalomalacia in the right MCA territory. d. FLAIR image demonstrates abnormal high signal in the contralateral left dentate nucleus (arrow)
We have also seen unilateral changes in the dentate nuclei associated with prior contralateral cerebral ischaemia (Fig. 16). This is a form of crossed cerebellar diaschisis. Predominant involvement of the dentate in diaschisis has not been described. In fact a study of 67 patients with crossed cerebellar diaschisis with positron emission tomography (PET) showed glucose metabolism in the contralateral dentate nucleus was relatively preserved [22].
Periventricular leukomalacia (PVL)
We have also noted involvement of the dentate nuclei in PVL. The pathogenesis of PVL, which occurs in pre-term infants, was previously believed to be related to ischaemia in watershed zones in the periventricular white matter of immature brains. It is now believed that PVL is probably related to the selective vulnerability of cells of oligodendrocyte lineage to changes of hypoxia-ischaemia.
Early MR changes include abnormal T1 hypointensity and T2 hyperintensity in the periventricular white matter, most frequently in the peritrigonal regions with or without cyst formation [23]. A triad of imaging findings in end-stage PVL include abnormally increased T2 signal and loss of volume in the peritrigonal periventricular white matter bilaterally as well as compensatory focal ventriculomegaly with mild atrial wall irregularity (Fig. 17) [24].
Axial T2 FLAIR through the posterior fossa, basal ganglia and centrum semi-ovale in a 2-year-old girl with PVL, who was born at 27 weeks’ gestation. a The dentate nuclei show abnormal high signal. b, c Abnormal high signal, cyst formation and volume loss can be seen in the periventricular white matter
Varicella infection
CNS complications are seen in 1% of cases of childhood varicella zoster infections. These include acute cerebellar ataxia, encephalitis, and vasculitis. In acute cerebellar ataxia MRI reveals diffuse or focal areas of decreased T1- and increased T2-signal in the cerebellum and cerebellar peduncles. The dentate nuclei may be involved. Varicella encephalitis may have a nonspecific pattern similar to other encephalitides, have multifocal cortical and subcortical abnormalities or a clear arterial distribution of abnormal signal. These are all due to varicella vasculitis with different appearances depending on whether small peripheral, medium-size central or larger vessels are affected [25, 26]. Sometimes the encephalitis associated with varicella infection is clinically less severe and is responsive to steroids consistent with an acute disseminated encephalomyelitis (ADEM) and MRI may show multiple supratentorial and infratentorial white matter lesions as well as lesions of the basal ganglia and dentate nuclei (Fig. 18).
A 4-year-old girl with varicella zoster infection who presented with encephalitis as the skin lesions were crusting. The encephalitis responded to steroids as expected in ADEM. FLAIR imaging demonstrates abnormal high signal in (a) both thalami and lentiform nuclei and (b) in right crus cerebri of midbrain. c T2-W FLAIR image shows high signal in both dentate nuclei
Langerhans cell histiocytosis (LCH)
LCH is an aggressive non-neoplastic disorder of unknown aetiology characterised by the intense proliferation of Langerhans cell histiocytes. Diabetes insipidus is a common clinical finding secondary to involvement of the hypothalamus and pituitary infundibulum. Dentate nuclei involvement occurs in up to 40% of patients. Focal T1 hyperintensity in the dentate nuclei can be an early imaging finding with later T2 hyperintensity (Fig. 19) [27, 28].
Neurofibromatosis type 1 (NF1)
NF1 or von Recklinghausen disease is an autosomal-dominant neurocutaneous disorder or phakomatosis. The hallmark of NF1 is the development of multiple tumours. Characteristic paediatric cerebral imaging findings include neurofibromatosis bright objects (NBO) and optic nerve gliomas (Fig. 20). NBOs are discrete areas of altered signal intensity without mass effect due to foci of myelin vacuolisation. Typical locations include the globus pallidus, cerebellar peduncles, midbrain or pons, internal capsule and splenium of the corpus callosum. Unusual locations include the dentate nucleus and periventricular white matter. Cerebellar white matter and dentate lesions usually occur in children <10 years old and never in the third decade [29]. These areas of myelin vacuolisation are considered benign and most regress or disappear with age [30, 31]. Proliferation and tumour formation in NBOs have been reported especially in those patients with a large number of NBOs. There are however no reported cases of tumour formation in the dentate nuclei [32].
An approach to dentate nuclei abnormalities
Dentate nucleus abnormalities can be seen in a wide spectrum of disease entities. Involvement of the dentate nucleus can be a specific feature in a number of disorders such as organic acidurias (L-2 hydroxyglutaric aciduria and glutaric aciduria type I), infantile Refsum disease, Krabbe disease, LCH and NF1. In other pathological processes the dentate nuclei are infrequently affected or changes are observed only in the later stages of the disease. Concomitant brain stem involvement may also help narrow the differential diagnosis (Fig. 21).
Once the dentate nuclei are deemed abnormal on imaging, a simple schematic approach (Fig. 22) can be used to develop an appropriate differential diagnosis that can act as a guide to clinicians regarding suitable further investigations.
Conclusion
In this article we have reviewed the imaging appearances of normal dentate nuclei as myelination occurs. Armed with this knowledge one is able to more accurately diagnose abnormalities in these deep cerebellar nuclei. As we have demonstrated, a wide variety of pathological processes can affect the dentate nuclei; however, careful assessment of the radiological distribution of disease within the remainder of the brain can help produce a clinically useful differential diagnosis.
References
Barkovich AJ (1998) MR of the normal neonatal brain: assessment of deep structures. AJNR 19:1397–1403
Kizildag B, Dusunceli E, Fitoz S et al (2005) The role of classic spin echo and FLAIR sequences for the evaluation of myelination in MR imaging. Diagn Interv Radiol 11:130–136
Valanne L, Ketonen L, Majander A et al (1998) Neuroradiologic findings in children with mitochondrial disorders. AJNR 19:369–377
Hendriksz CJ (2009) Inborn errors of metabolism for the diagnostic radiologist. Pediatr Radiol 39:211–220
Finsterer J (2008) Leigh and Leigh-like syndrome in children and adults. Pediatr Neurol 39:223–235
Lee HF, Tsai CR, Chi CS et al (2009) Leigh syndrome: clinical and neuroimaging follow-up. Pediatr Neurol 40:88–93
Rossi A, Biancheri R, Bruno C et al (2003) Leigh Syndrome with COX Deficiency and SURF-1 gene mutations: MR imaging findings. AJNR 24:1188–1191
Twomey EL, Naughten ER, Donoghue VB et al (2003) Neuroimaging findings in glutaric aciduria type 1. Pediatr Radiol 33:823–830
Oguz KK, Ozturk A, Cila A (2005) Diffusion-weighted MR imaging and MR spectroscopy in glutaric aciduria type 1. Neuroradiology 47:229–234
Takanashi J, Fujii K, Sugita K et al (1999) Neuroradiologic findings in glutaric aciduria type II. Pediatr Neurol 20:142–145
Topçu M, Erdem G, Saatçi I et al (1996) Clinical and magnetic resonance imaging features of L-2-hydroxyglutaric acidemia: report of three cases in comparison with Canavan disease. J Child Neurol 11:373–377
Kara S, Sherr EH, Barkovich AJ (2008) Dilated perivascular spaces: an informative radiologic finding in Sanfilippo syndrome type A. Pediatr Neurol 38:363–366
Aicardi J, Goutières F (1984) A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol 15:49–54
Uggetti C, La Piana R, Orcesi S et al (2009) Aicardi-Goutières syndrome: neuroradiologic findings and follow-up. AJNR 30:1971–1976
Choksi V, Hoeffner E, Karaarslan E et al (2003) Infantile Refsum disease: case report. AJNR 24:2082–2084
Di Rocco M, Biancheri R, Rossi A et al (2004) MRI in acute intermittent maple syrup urine disease. Neurology 63:1078
Patay Z (2005) Diffusion-weighted MR imaging in leukodystrophies. Eur Radiol 15:2284–2303
Provenzale JM, Peddi S, Kurtzberg J et al (2009) Correlation of neurodevelopmental features and MRI findings in infantile Krabbe’s disease. AJR 192:59–65
Nagar VA, Ursekar MA, Krishnan P et al (2006) Krabbe disease: unusual MRI findings. Pediatr Radiol 36:61–64
Sener RN (2000) van der Knapp syndrome: MR imaging findings including FLAIR, diffusion imaging and proton MR spectroscopy. Eur Radiol 10:1452–1455
van der Knaap MS, Smit LM, Barth PG et al (1997) Magnetic resonance imaging in classification of congenital muscular dystrophies with brain abnormalities. Ann Neurol 42:50–59
Fulham MJ, Brooks RA, Hallett M et al (1992) Cerebellar diaschisis revisited: pontine hypometabolism and dentate sparing. Neurology 42:2267–2273
Huang BY, Castillo M (2008) Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. Radiographics 28:417–439
Baker LL, Stevenson DK, Enzmann DR (1988) End-stage periventricular leucomalacia: MR evaluation. Radiology 168:809–815
Baskin HJ, Hedlund G (2007) Neuroimaging of herpes infections in children. Pediatr Radiol 37:949–963
Montenegro MA, Santos SL, Li LM et al (2002) Neuroimaging of acute cerebellitis. J Neuroimaging 12:72–74
Prayer D, Grois N, Prosch H et al (2004) MR imaging presentation of intracranial disease associated with Langerhans cell histiocytosis. AJNR 25:880–891
Martin-Duverneuil N, Idbaih A, Hoang-Xuan K et al (2006) MRI features of neurodegenerative Langerhans cell histiocytosis. Eur Radiol 16:2074–2082
Itoh T, Magnaldi S, White RM et al (1994) Neurofibromatosis type 1: the evolution of deep gray and white matter MR abnormalities. AJNR 15:1513–1519
Herron J, Darrah R, Quaghebeur G (2000) Intra-cranial manifestations of neurocutaneous syndromes. Clin Radiol 55:82–98
Jacques C, Dietemann JL (2005) Imaging features of neurofibromatosis type 1. Neuroradiology 32:180–197
Griffiths PD, Blaser S, Mukonoweshuro W et al (1999) Neurofibromatosis bright objects in children with Neurofibromatosis type 1: a proliferative potential? Pediatrics 104:e49
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McErlean, A., Abdalla, K., Donoghue, V. et al. The dentate nucleus in children: normal development and patterns of disease. Pediatr Radiol 40, 326–339 (2010). https://doi.org/10.1007/s00247-009-1504-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-009-1504-2