Abstract
Background
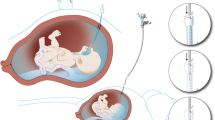
Fetal endoscopic tracheal occlusion (FETO) is a promising treatment for severe congenital diaphragmatic hernia, a condition that carries significant morbidity and mortality. It is hypothesised that balloon occlusion of the fetal trachea leads to an improvement in lung growth and development. The major documented complications of FETO to date are related to preterm delivery.
Objective
To report a series of five infants who developed tracheomegaly following FETO.
Materials and methods
Review of all children referred with tracheomegaly to the paediatric intensive care and tracheal service at two referral centres.
Results
Five neonates presented with features of respiratory distress shortly after birth and were subsequently found to have marked tracheomegaly. Two neonates had tracheomalacia in addition.
Conclusion
There are no previous reports in the literature describing tracheomalacia, or more specifically, tracheomegaly, as a consequence of FETO. We propose that the particularly compliant fetal airway is at risk of mechanical damage from in utero balloon occlusion. This observation of a new problem in this cohort suggests a thorough evaluation of the trachea should be performed in children who have had FETO in utero. It may be that balloon occlusion of the trachea earlier in utero (before 26 weeks’ gestation) predisposes to this condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital diaphragmatic hernia (CDH) is a relatively common disorder, with an incidence between 1 in 2,000 and 1 in 5,000. Its aetiology is unknown [1, 2]. There is significant associated morbidity and mortality secondary to pulmonary hypoplasia and pulmonary hypertension [3]. Other complications include recurrent herniation, repeated hospitalisations, growth failure and gastro-oesophageal reflux disease [3, 4].
In addition to primary surgical repair, several other treatments have been used in the past, including prenatal corticosteroids, high-frequency oscillatory ventilation, liquid ventilation, surfactant therapy, extracorporeal membrane oxygenation and delayed surgical repair. Despite these efforts, the morbidity and mortality remain high. In fact, each apparent improvement in survival over the last decade was associated with an increase in antenatal death rate [5]. Fetal surgery has been reported to be of no benefit [6].
It is hypothesised that antenatal obstruction of the trachea improves lung growth and development. Effective and sustained obstruction can be achieved by endoscopic deployment of a detachable balloon between the carina and vocal cords, a procedure known as fetal endoscopic tracheal occlusion (FETO). Intrauterine reversal of obstruction improves surfactant production [7, 8].
Occlusion of the fetal trachea has been shown to stimulate fetal lung growth in animal models [9]. Although FETO carries the risk of amniorrhexis and consequent preterm delivery, no serious maternal complications or direct adverse effects on the fetus have been reported to date. In particular, no tracheal damage has been demonstrated in experimental studies [10, 11]. Data on long-term morbidity continue to be collected. There is a single report of a baby with severe developmental delay following FETO [12]. We now report five infants who developed tracheomegaly subsequent to FETO.
Materials and methods
We found five infants with a history of tracheomegaly and prior in utero FETO referred to the tracheal service and the paediatric intensive care units at two large children’s centres. See Table 1 for a summary of the major clinical details.
Results
Case 1
This baby girl was diagnosed with left-side CDH, an intra-thoracic liver and cystic adenomatoid malformation (CCAM) at 21 weeks’ gestation. The lung-to-head ratio (LHR) was 0.6. FETO was performed at 23.7 weeks. Elective balloon removal by fetoscopy was performed at 34.4 weeks. Delivery occurred at 35.4 weeks, followed by surgical repair of the hernia at 2 days. There was good postoperative lung expansion, and she was extubated at day 3. Several days later, she represented with cough, noisy breathing and increasing respiratory distress. The chest radiograph showed a dilated distal trachea, confirmed on CT, with a transverse diameter of 11 mm (Fig. 1). The cross-sectional area of the trachea was larger than an adjacent vertebral body (Fig. 1). The mid-section of the trachea was wider than the carina. No CCAM was in fact present. Tracheomalacia was diagnosed clinically. There was bilateral, perihilar, peribronchial airspace shadowing extending into both lower lobes, but the lungs were otherwise normal. She initially required CPAP for a few months, and was admitted on two occasions to intensive care with severe respiratory distress requiring intubation and ventilation. Thereafter, her condition gradually improved during the first year of life. She is currently well, aged 1 year, and is not requiring any supplemental oxygen or ventilation.
Case 1. a Axial CT on lung window settings at the thoracic inlet shows a normal calibre trachea with an endotracheal (ET) tube in situ (W 1169, L 256). b Axial CT section at the level of the major head and neck vessels on soft-tissue settings shows marked tracheal dilatation (W 433, L 55). Note artefact from the same ET tube and nasogastric (NG) tubes
Case 2
A left-side CDH was diagnosed on US at 21 weeks’ gestation. This boy had an LHR of 0.7 with the liver also herniated. FETO was performed at 25.9 weeks. Vaginal delivery took place after induction of labour at 37.9 weeks. The balloon was removed following delivery. The birth weight was 2.28 kg. The boy was intubated and ventilated, and surgical repair of the hernia was performed at day 4. During the next few days, feeding difficulties were noted and a contrast study revealed gastric outlet obstruction due to abnormal anatomical orientation of the stomach, which necessitated a duodenoplasty followed by gastrostomy and feeding jejunostomy. Further fluoroscopic procedures demonstrated tracheomegaly and tracheomalacia (Fig. 2). The baby failed extubation; a chest radiograph and later CT again confirmed a dilated trachea (Fig. 2). A long-term tracheostomy was required. He remained on continuous CPAP after this, has had numerous ICU admissions, and at the age of 27 months required further surgery for adhesive bowel obstruction and a second patch repair for a recurrent diaphragmatic hernia. He is currently on overnight CPAP, aged 32 months.
Case 2. a Tracheal dilatation is seen on an upper GI study with b tracheal collapse in expiration denoting tracheomalacia. c Volume-rendered CT coronal reformatted images of the trachea in inspiration and d expiration again show tracheomegaly with inspiration and tracheomalacia, respectively. The arrow points to an air-filled oesophagus, not seen on the previous inspiratory image
Case 3
A left-side CDH was diagnosed at 20 weeks’ gestation on antenatal US. The liver was included in the hernia and the LHR was 0.9. FETO was performed on this female fetus at 24.4 weeks, with the balloon being punctured in utero at 33.7 weeks. Delivery occurred at 38.1 weeks. The birth weight was 2.7 kg. Surgical repair of the CDH was performed at day 2. A fundoplication and gastrostomy were necessary at age 3 months. Post-operatively, the girl suffered episodes of hypoxia and noisy breathing, was ventilated for 3 days and had transient left lung collapse. Following reintubation, bronchoscopy showed tracheomegaly and tracheomalacia, and normal bronchi distally. CT in expiration revealed exaggerated AP narrowing and anterior convexity of the posterior tracheal wall suggestive of tracheomalacia (Fig. 3). Bronchography confirmed tracheomalacia and, in addition, showed massive tracheal dilatation with only 5 cmH2O CPAP (Fig. 3). She required CPAP for the first few months of life, had frequent noisy breathing, but later improved. She was well at last follow-up and is now over 2 years of age.
Case 3. a Axial CT shows anteroposterior narrowing of the upper trachea suggesting tracheomalacia (W 412, L 55). b Massive tracheal dilatation is seen at bronchography with only 5 cmH2O CPAP (arrows)
Case 4
A right-side CDH was diagnosed on antenatal US at 20 weeks’ gestation. The liver was displaced superiorly as part of the hernia and the LHR was 0.6. FETO was performed on this female fetus at 25.9 weeks. Emergency delivery for pre-eclampsia occurred at 31.3 weeks, with a birth weight of 905 g. The trachea was noted to be dilated on chest radiographs (Fig. 4). No cross-sectional imaging was performed as the girl was too unwell. Surgical repair of the CDH was undertaken at day 2. Unfortunately, the operation was complicated by pulmonary haemorrhage and cardiovascular instability, which continued into the postoperative period. By day 7, sepsis developed. A left-side pneumothorax occurred after an attempt at left internal jugular central venous access, and there was rapid respiratory compromise, not responding to intervention. Death followed cardiac arrest on day 8.
Case 4. Chest radiograph on day 3 of life shows ET and NG tubes, a right chest drain and an umbilical venous catheter. Marked tracheal dilatation is evident on this film and was also seen on all other chest radiographs (white arrows). The small radiodense opacity on the left side, which was present on all films, was assumed to be due to a collapsed tracheal occlusion catheter in the left main bronchus (black arrow). This child died aged 8 days and did not have an autopsy
Case 5
A female fetus was noted to have an intrathoracic liver, CCAM and left CDH at 20 weeks’ gestation. Her LHR was 0.5. FETO was performed at 24.1 weeks with later additional insertion of a thoraco-amniotic shunt to drain the CCAM. At 28 weeks’ gestation polyhydramnios was treated with amnioreduction. Vaginal bleeding and spontaneous rupture of membranes occurred 5 days later. Emergency cord prolapse necessitated emergency caesarean section, with delivery at 29.4 weeks. This infant had not undergone in utero deflation of the tracheal balloon. Immediately after delivery, after a period of difficult ventilation, it was realised the balloon was still inflated and it was then punctured and removed. A chest radiograph had shown a large trachea and the radio-opaque marker of the balloon catheter above the carina. Chest CT on day 5 showed tracheal dilatation up to four times the width of a thoracic vertebral body extending into the right main bronchus (Fig. 5). CDH repair was performed, with difficulty, on day 8, due mainly to the absence of diaphragmatic tissue. The patch dehisced on day 24 requiring a repeat repair using a latissmus dorsi flap. A second recurrence of the left CDH occurred on day 60. A third left CDH repair took place on day 80, but a recurrent hernia was evident again by day 86. On day 100, further deterioration was seen with loops of bowel again in the chest. During all this time the baby required CPAP in addition to frequent ventilator support. A tracheostomy was performed on day 130. No further attempts to repair the diaphragm were thought possible. Pulmonary hypertension, chronic lung disease and cardiac failure ensued, resulting in death at age 6 months.
Case 5. a Axial CT following contrast medium enhancement, on lung window settings, on day 5 of life shows marked tracheal dilatation. The trachea measures 14 mm in maximal axial dimension (W 1490, L 263). b Axial image at the level of the carina, on soft-tissue settings, shows enlargement of the proximal right main bronchus (W 378, L 40). c Coronal reformat also shows the tracheal dilatation extending into the right main bronchus
Discussion
Tracheomegaly, which generally manifests as diffuse dilatation of the trachea, typically occurs as a result of weakness of the airway wall or abnormal collapsibility. Causes of tracheomegaly include connective tissue disorders, lung fibrosis and prior intubation, but true tracheomegaly is rarely seen in childhood [13]. Tracheomegaly is characterised by atrophy or absence of elastic fibres and thinning of smooth muscle layers in the trachea but, unfortunately, our two babies who died did not have autopsies [14]. Tracheomalacia refers to a weakness of the tracheal wall, resulting in expiratory collapse, typically with >50% decrease in tracheal diameter. Tracheomalacia is usually due to focal or diffuse cartilage deficiency. It may be congenital, manifesting in neonates, when it is commonly seen in association with oesophageal atresia or cardiovascular anomalies. Alternatively, tracheomalacia may be acquired later in life as a result of diffuse tracheal disease, prolonged intubation or extrinsic compression.
There are no reports to date of tracheomegaly as a complication of FETO. However, we have now described five such cases. Two infants also had tracheomalacia proven radiologically. Our five infants with tracheomegaly suggest that the tracheal cartilage can be damaged by the prolonged intratracheal balloon deployment method of FETO. An alternative explanation for the tracheal abnormality would be damage to the trachea from balloon removal, but we believe this is unlikely as two of our children had tracheomegaly documented prior to removal of the balloon.
Mounier-Kuhn syndrome is a rare but noteworthy congenital abnormality that manifests with marked widening of the trachea and major bronchi [14]. Although it has been reported in children as young as 18 months of age, the condition is usually seen in adults [14]. Our cohort does not, strictly speaking, fit the criteria for this syndrome as only one (case 5) had involvement of a bronchus in addition to tracheal dilatation.
The tracheal pathology was detected in these cases by worsening respiratory function several days after birth and confirmed by chest radiography, bronchography, bronchoscopy, and with CT in four cases. The tracheomegaly was marked in all the infants, with the tracheal lumen up to three times the diameter of an adjacent vertebral body on CT. Diffuse dilatation of the intrathoracic trachea was seen in all infants. Some tracheal measurements have been added to the figures provided, but it should be noted that measured tracheal dimensions at CT are entirely dependent on the CT windowing and levels used. Tracheomalacia is a dynamic process, often under-appreciated at routine (inspiratory) CT, but was clearly shown with fluoroscopy, bronchography and with combined inspiratory/expiratory CT in two of our cases. Excessive tracheal collapse with expiration can result in a crescent-shaped, “frown-like” appearance of the airway lumen on CT (Fig. 3) [15].
FETO is an invasive procedure with an inherent risk for amniorrhexis, and hence a high chance of preterm delivery, as occurred in three of our five cases [16]. Indications for FETO include a singleton pregnancy, an anatomically and chromosomally normal fetus, gestation at randomization prior to 32+5 weeks, observed/expected (O/E) LHR 25–34.9% (irrespective of the position of the liver) or O/E 35–44.9% with intrathoracic liver herniation [17]. Of note, all the fetuses had intrathoracic liver herniation (Table 1).
Although tracheal damage is not known to be a complication, the sites of the tracheal abnormalities in these cases correspond to the previous sites of the in utero tracheal balloons. The proximal airways are known to be very compliant early in gestation. A balloon can stretch the tracheal circumference by 15% [10]. Studies in animal models have revealed changes to the tracheal architecture at a microscopic level, with loss of the typical epithelial folding pattern, squamous metaplasia and elongation of the pars membranacea, but without obvious flattening or structural damage to the cartilage [11]. Changes also occur distal to the balloon, probably due to higher airway pressures [11]. One fetal lamb sacrificed early had tracheal dilatation, but this study concluded that these changes disappear almost completely for the remainder of gestation following in utero unplugging [11]. No cartilaginous effects were reported [11]. Harrison et al. [16] reported a small cohort who had stridor and vocal cord paralysis secondary to in utero intervention. Two of their children also had tracheomalacia, but that group had undergone tracheal dissection, transtracheal occluding clips (and tracheal lacerations) rather than simpler balloon placement [16].
The inflated balloon for FETO has a standard diameter of 7 mm. To date, 96 FETO procedures have been performed in London at a median gestation of 26 weeks, with a range of 23–33 weeks (K. Nicolaides, personal communication). Thus it would appear that our small cohort with tracheomegaly had FETO performed slightly earlier in gestation than is probably typical, as they all had FETO prior to 26 weeks’ gestational age. The individual prognosis of these children was poor without antenatal intervention as they all had a LHR of <1 [18]. In fact, when the LHR is <1 and the liver is herniated into the chest, the predicted survival rate is less than 10% [19]. It could be speculated that the intratracheal balloons used were relatively large for the gestational age, or that performing FETO earlier in gestation runs a higher risk of tracheomegaly. It is also likely these complications of FETO, namely tracheomalacia and tracheomegaly, are uncommon, as over 150 FETO procedures have been performed in Europe, and we are not aware of any other cases of tracheomegaly subsequent to in utero FETO [17]. Of note, one North American FETO trial was stopped, not because of lack of efficacy of FETO, but rather after interim analysis had shown better than expected results were achieved with conventional management [7, 9]. It remains to be seen whether the potential pulmonary benefits of tracheal occlusion are superior to the adverse effects of earlier delivery on pulmonary function [9].
Conclusion
We present five cases of tracheomegaly subsequent to in utero FETO. Two infants died from respiratory complications (one in the newborn period, the other at 6 months), and two required tracheostomy. All these babies had a poor prognosis without in utero treatment and it is difficult to know how significant the tracheal enlargement was on an individual basis, taking into account their other postnatal problems. Although FETO is a promising technique, we believe the observation of a new problem in this cohort prompts further careful consideration of the risks involved with the procedure. This will presumably be addressed in on-going randomised controlled trials [12, 17]. We propose that the compliant trachea of the fetus in the second trimester is at risk of mechanical damage from in utero balloon occlusion. It would therefore seem sensible that those surviving FETO procedures should have close paediatric respiratory follow-up and specific tracheal assessments. It may be that balloon occlusion of the trachea earlier in utero (before 26 weeks’ gestation) predisposes to tracheal dilatation. The paediatric and obstetric community need to be aware of these findings, reported here for the first time.
References
Torfs CP, Curry CJ, Bateson TF et al (1992) A population-based study of congenital diaphragmatic hernia. Teratology 46:555–565
Saura L, Castanon J, Prat A et al (2007) Impact of fetal intervention on postnatal management of congenital diaphragmatic hernia. Eur J Pediatr Surg 17:404–407
West SD, Wilson JM (2005) Follow up of infants with congenital diaphragmatic hernia. Semin Perinatol 29:129–133
Cortes RA, Keller RL, Townsend T et al (2005) Survival of severe congenital diaphragmatic hernia has morbid consequences. J Pediatr Surg 40:36–46
Deprest J, Jani J, Gratacos E et al (2005) Fetal intervention for congenital diaphragmatic hernia: the European experience. Semin Perinatol 29:94–103
Harrison MR, Adzick NS, Bullard KM et al (1997) Correction of congenital diaphragmatic hernia in utero. VII: a prospective trial. J Pediatr Surg 32:1637–1642
Laberge JM, Flageole H (2007) Fetal tracheal occlusion for the treatment of congenital diaphragmatic hernia. World J Surg 31:1577–1586
Flageole H, Evrard V, Piedboef B et al (1998) The plug-unplug sequence: an important step to achieve type II pneumocyte maturation in the fetal lamb model. J Pediatr Surg 33:299–303
Harrison MR, Keller RL, Hawgood SB et al (2003) A randomised trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Eng J Med 349:1916–1924
Chiba T, Albanese CT, Farmer DL et al (2000) Balloon tracheal occlusion for congenital diaphragmatic hernia: experimental studies. J Pediatr Surg 35:1566–1570
Deprest J, Evrard V, Verbeken EK et al (2000) Tracheal side effects of endoscopic balloon tracheal occlusion in the fetal lamb model. Eur J Obstet Gynecol Reprod Biol 92:119–126
Doné E, Gucciardo L, Van Mieghem T et al (2008) Prenatal diagnosis, prediction of outcome and in utero therapy of isolated congenital diaphragmatic hernia. Prenat Diagn 28:581–591
Adam A, Dixon AK (eds) (2008) Grainger and Allison’s diagnostic radiology: A textbook of medical imaging, 5th edn. Elsevier Churchill Livingstone, Philadelphia, p 420
Menon B, Aggarwal B, Iqbal A (2008) Mounier-Kuhn syndrome: report of 8 cases of tracheobronchomegaly with associated complications. South Med J 101:83–87
Boiselle PM, Feller-Kopman D, Ashiku S et al (2003) Tracheobronchomalacia: evolving role of dynamic multislice helical CT. Radiol Clin North Am 41:627–636
Harrison MR, Sydorak RM, Farrell JA et al (2003) Fetoscopic temporary tracheal occlusion for congenital diaphragmatic hernia: prelude to a randomized, controlled trial. J Pediatr Surg 38:1012–1020
Randomized control trial of fetoscopic endoluminal tracheal occlusion with a balloon versus expectant management during pregnancy in fetuses with left sided congenital diaphragmatic hernia and moderate pulmonary hypoplasia. University Hospital, Gasthuisberg (2008) ClinicalTrials.gov Identifier: NCT00763737. http://clinicaltrials.gov/ct2/show/NCT00763737
Deprest J, Gratacos E, Nicolaides KH et al (2004) Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol 24:121–126
Cannie MM, Jani JC, De Keyzer F et al (2009) Evidence and patterns in lung response after fetal tracheal occlusion: clinical controlled study. Radiology 252:526–533
Acknowledgements
We are particularly grateful to Professor Kypros Nicolaides for furnishing much of the antenatal detail of the children and for the information regarding FETO. We are also grateful to innumerable paediatricians in Nottingham and Leeds for allowing us to report two of their patients. We are also grateful to the other members of the tracheal team at Great Ormond Street Hospital for Children, London, for their assistance with these difficult cases. These other team members include Ben Hartley, Colin Wallis, Mike Broadhead, Quen Mok, Alex Barnacle, Clare McLaren, Catherine Dunne, Clair Noctor and Caroline Doyle.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McHugh, K., Afaq, A., Broderick, N. et al. Tracheomegaly: a complication of fetal endoscopic tracheal occlusion in the treatment of congenital diaphragmatic hernia. Pediatr Radiol 40, 674–680 (2010). https://doi.org/10.1007/s00247-009-1437-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-009-1437-9