Abstract
Purpose
To provide a comprehensive report of the experience gained in the prenatal treatment of congenital diaphragmatic hernia (CDH) using fetoscopic endoluminal tracheal occlusion (FETO) following its implementation at a newly established specialized fetal medicine center.
Methods
Mothers of fetuses with severe CDH were offered prenatal treatment by FETO.
Results
Between 2018 and 2021, 16 cases of severe CDH underwent FETO. The median gestational age (GA) at balloon insertion was 28.4 weeks (IQR 27.8–28.6). The median GA at delivery was 37 weeks (IQR 34.4–37.8). The survival rate was 8/16 cases (50%). None of the survivors required home oxygen therapy at 6 months of age. Comparison between the survivors and deceased showed that survivors had balloon insertion 1 week earlier (27.8 vs. 28.4 weeks, p = 0.007), a higher amniotic fluid level change between pre- to post-FETO (3.4 vs 1.3, p = 0.024), a higher O/E LHR change between pre- to post-FETO (50.8 vs. 37.5, p = 0.047), and a GA at delivery that was 2 weeks later (37.6 vs. 35.4 weeks, p = 0.032).
Conclusions
The survival rate at 6 months of age in cases of severe CDH treated with FETO in our center was 50%. Our new fetal medicine center matches the performance of other leading international centers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital diaphragmatic hernia (CDH) affects approximately 1 in 4000 births and left-side CDH accounts for about 85% of all cases [1, 2]. CDH is characterized by intrathoracic herniation of the abdominal viscera that may impair normal lung growth and pulmonary vascular development. This defect is associated with a high risk of neonatal death, mainly due to respiratory failure and pulmonary hypertension. Morbidity of the surviving infants may include respiratory, hemodynamic, gastrointestinal, and orthopedic complications, and developmental delays, necessitating a multidisciplinary follow-up [3, 4].
The diagnosis of CDH can be achieved during the routine second-trimester fetal sonographic anomaly scan. The presence of abdominal organs in the thoracic cavity (bowel, stomach, liver, etc.), displaced heart position, and interrupted diaphragm are the main diagnostic findings.
The overall survival of neonates with CDH is around 70% [5], and the main prognostic factor for survival is the degree of lung hypoplasia. The severity of lung hypoplasia is assessed by determining the ratio of the contralateral lung area-to-head circumference (LHR), and by the observed (measured)-to-expected lung size to head circumference (O/E LHR). In cases with O/E LHR below 25% for left-sided CDH (or LHR < 1), and below 35% for right-sided CDH, the condition is considered severe. Until recently, the survival rate of severe left-sided CDH was 10–25% and in some cases resulted in complications in the surviving newborn. Such complications include oxygen supplementation and a prolonged period to establish enteral feeding [6, 7].
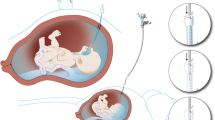
It has been shown that fetuses with congenital laryngeal obstruction have huge lungs due to lung hyperplasia, most likely due to active alveolar fluid secretion that accumulates in the lungs. This has led to a new therapeutic intervention in fetuses with severe CDH (hence having lung hypoplasia), aiming to stimulate lung growth by tracheal occlusion [8]. Pioneering work by Deprest, Gratacos, and Nicolaides, published in 2004, applied fetoscopic endoluminal tracheal occlusion (FETO) by introducing an inflatable balloon, which occludes the trachea. The balloon is removed after a few weeks (before delivery). Tracheal occlusion leads to the accumulation of lung fluid, which, in turn, causes lung stretch. This is followed by increased proliferation of the lung cells and decreased severity of the lung hypoplasia [9].
A randomized clinical trial of FETO for severe CDH cases published in 2021 [1] showed that among fetuses with isolated severe left-sided CDH undergoing FETO at 27–29 weeks’ gestation, the survival rate was 40% compared to 15% in the case of expectant management. The survivors also had reduced neonatal complications in the first 6 months post-delivery, and were less dependent on oxygen supplementation compared to cases treated by expectant care. This multi-center clinical trial demonstrated the benefits and safety, and depicted meticulous steps needed to reach positive results, as well as the risks associated with the FETO technique [10]. It also indicated the benefits are mainly for severe CDH and limited for the moderate cases [1, 10].
Fetal medicine fellows from around the world, who have been trained by members of the pioneering group, have established new fetal medicine centers in their homeland hospitals to spread the technique availability to the benefit of a larger patient population. This facilitates the access of pregnant women to this new clinical procedure. In fact, the group of Persico et al. [11] in Milano, Italy, has already published its experience from their center, covering 21 cases.
Our Fetal Medicine Center was established in 2018 at the Helen Schneider Hospital for Women at the Rabin Medical Center, following training at the Fetal Medicine Research Institute King's College Hospital in London. We work in very close cooperation with our on-campus Schneider’s Children Medical Center. Our center serves referrals from around the country and from the surrounding regions.
Here, we describe our experience with the first cases of severe CDH treated by FETO in our center.
Materials and methods
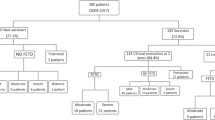
Since opening the Fetal Medicine Center in 2018 and until 2021, FETO was performed in 16 cases with severe CDH. During this period, we performed, on average, 33 in utero fetal surgeries per year, including fetoscopies for complicated monochorionic twins, total percutaneous spina bifida repair, and FETO for severe CDH.
Diagnosis of CDH was made by ultrasound using an RAB 4–8 transducer, Voluson E8, and E10 (GE Medical Systems, Milwaukee, WI, USA). The definition of severity was based on the combination of sonographic measurements of lung-to-head ratio (LHR) and the side of the defect (left, right).
The lung area was measured using the longest diameter method and the LHR was calculated. O/E LHR for the contralateral lung was calculated using the formula of Jani et al. [5]. Fetoscopic endoluminal tracheal occlusion (FETO) was offered if the O/E LHR was < 25% in left-side defects and < 35% in right-side hernias [12, 13]. An anatomical ultrasound scan was carried out to assess the presence of other major structural defects. All cases underwent genetic counseling and amniocentesis, including a comparative genomic hybridization (CGH) array to rule out genetic abnormalities that are known to have significant impact on postnatal survival. Each family met with a fetal medicine specialist, a neonatal intensive care unit (NICU) team member, and a pediatric surgeon to discuss the pros and cons of FETO compared to conservative management, as well as the potential benefits and adverse effects of the intrauterine intervention. Families were then given the choice of FETO surgery, conservative management, or termination of pregnancy. FETO was carried out after the mother provided her signed informed consent.
FETO was carried out using a miniature straightforward telescope (00, straight, diameter 3.3 mm, length 30 cm, 11,506 AAK, Karl Storz GmbH, Tuttlingen, Germany). After the administration of fetal intramuscular anesthesia (pancuronium, fentanyl, atropine), and maternal regional analgesia (spinal/epidural), a 3.4 mm diameter cannula (RCF-10.0–38-J, Cook Medical, Bloomington, IN, USA) was introduced transabdominally into the amniotic sac under ultrasound guidance. The cannula was intended to be placed in the upper region of the uterus to minimize the risk of premature rupture of the membranes (PROM), based on experience gained from other fetal surgeries (percutaneous spina bifida repair). The fetoscope was inserted through the fetal oral cavity to reach the trachea, where a balloon (GOLDBALL 2 and COAX catheter; Balt Extrusion, Montmorency, France) was inflated with saline solution and detached from the catheter between the carina and the vocal cords, as previously described [14]. Amniotic fluid drainage was performed routinely during FETO in cases with polyhydramnios (deepest pocket of amniotic fluid ≥ 8). All patients received a course of betamethasone (12 mg intramuscular daily for 2 days) prior to FETO.
The multidisciplinary supporting team included fetal medicine specialists, obstetricians, neonatologists, pediatric surgeons, anesthesiologists, high-risk pregnancy nurses, and a social worker. Following the FETO surgery, each patient was hospitalized for 24 h and tocolysis was given if indicated (in cases with premature contractions). Weekly clinical visits were then conducted for fetal and maternal assessment including evaluation of the lung response, assurance of the correct placement of the balloon, fetal growth, deepest pocket of amniotic fluid, and cervical length.
A second procedure to reverse the tracheal occlusion was performed 6 weeks after FETO using the same fetoscopic technique under anesthesia. By inserting a needle through the fetoscope, the balloon was punctured and then removed by a grasper (puncture needle and grasping forceps: Karl Storz GmbH, Tuttlingen, Germany). Weekly clinical visits were conducted, and delivery by induction or cesarean section was scheduled and planned to take place at term. The NICU team was alerted to be available at the delivery. For cases in which urgent removal of the balloon was required due to imminent preterm birth, a 24/7 on-call team performed emergency balloon removal by direct ultrasound-guided puncture immediately after delivery. Delivery of all patients took place at our center and the neonates were managed on-campus at the Schneider Children’s Medical Center according to the internationaly published standardized postnatal protocol [15]. Extracorporeal membrane oxygenation (ECMO) therapy of the neonate was used if required.
The study was approved by the Rabin Medical Center Ethics Committee.
Statistical analysis
The study database was built as cases came in and were treated. The analysis was performed using SPSS package version 24 (SPSS Inc., Chicago, IL, USA). Medians with inter-quartile range (IQR) were calculated for a continuous variable by the parametric Mann–Whitney U test. A χ2 test or a Fisher’s exact test was used for categorical variables. Groups of deceased and survivors at 6 months of age (or hospital-discharged patients) were compared. A p-value < 0.05 was considered significant for each parameter, and each adverse pregnancy outcome.
Results
Preoperative cohort characteristics
The median maternal age at enrollment was 28 years (IQR 23.0–29.5) and the median GA at enrollment was 24.5 weeks (IQR 20.4–27.0); four women (25%) were nulliparous (Table 1). Conception was spontaneous in 15 cases (94%) and 1 case conceived through IVF (6%). There were 13 cases of left-sided CDH (81%) and 3 right-sided ones (19%). The placental position was anterior in half [8] of the cases. The median cervical length at enrollment was 37.5 mm (IQR 34.0–43.5). There were two cases with short (< 25 mm) cervical length and one case with didelphic uterus, all considered to be at higher risk for preterm delivery. Pre-FETO median LHR was 0.75 (IQR 0.66–0.90), and the median O/E LHR was 21.7% (IQR 17.0–24.3) (Table 1).
FETO-related characteristics
The balloon was successfully placed in the fetal trachea in all cases. The median GA at FETO was 28.4 weeks (IQR 27.8–28.6) (Table 2). No maternal or fetal intraoperative complications occurred during the FETO procedure. The incidence of polyhydramnios at FETO insertion was 1/16 (6.3%), which required amniotic fluid drainage.
There was one spontaneous balloon deflation 1 week after the FETO, and a second procedure was not performed due to the patient’s high risk for preterm delivery (uterus didelphic and premature contractions). In 14 cases, the balloon was electively removed by fetoscopy, and in 1 case, successful urgent balloon removal was performed after delivery due to preterm birth. The median GA at balloon removal was 33.7 weeks (IQR 33.0–34.6) (Table 2). The median interval between balloon insertion and removal was 5.7 weeks (IQR 5.3–6.0).
The median LHR at balloon removal appeared to be approximately four times higher compared with the value before FETO, reaching 2.83 (2.23–3.23). Median O/E LHR % reached 71.6% (IQR 51.5–80.1), representing a median increase of the O/E LHR by 44.0% (IQR 34.2–54.6.), approximately 3.5 times higher as compared with baseline O/E LHR.
There were four cases of PROM (25%) and three before 34 weeks. The median GA at delivery was 37.0 weeks (IQR 34.4–37.8), 25% of patients delivered before 34 weeks, and 12.5% before 32 weeks. In 33% (5/15) of patients, delivery occurred within 24 h after balloon removal. Delivery was conducted vaginally for four cases (25%), ten (63%) were delivered through planned cesarean section (CS), and two cases (12%) required delivery by emergency CS.
Fetal outcome characteristics
Of the cohort’s 16 cases, 8 cases were discharged home (50%) (Table 3) and survived for at least 6 months after delivery. The median duration of survivors’ stay in the NICU was 57 days (IQR 48–122). The median newborn weight was 2591 g (IQR 2252–2850), and males accounted for 9 cases of the cohort of 16 (56%).
Only one newborn was placed on ECMO. All survivors underwent postnatal surgical repair between Day 0 and Day 3. Out of the eight survivors, seven were free of oxygen treatment at discharge, and none required oxygen at home at 6 months. Moreover, at discharge, six had no reflux, and only one suffered from reflux at 4 months. Furthermore, at 6 months of pediatric evaluation, growth below the lower third centile was observed in three cases. Among the eight cases who did not survive, five cases died from lung hypoplasia, one due to sepsis, one due to combined lung hypoplasia and sepsis, and one due to intraoperative complications.
Comparison between survivors and deceased
Comparison of obstetrics and fetal characteristics between the survivors and deceased revealed four significant differences (Table 4): 1. survivors had FETO surgery 1 week earlier at a median 27.8 weeks (IQR 27.4–28.1) vs. a median 28.4 (IQR 28.4–29.1) among the deceased (p = 0.007); 2. the median deepest pocket of amniotic fluid change (before balloon placement and before balloon removal) was higher among the survivors 3.4 cm (IQR 2.7–4.8) vs. 1.3 cm (IQR 1.0–2.5) (p = 0.024); 3. the median O/E LHR change between the pre- to post-FETO (before balloon placement and before balloon removal) was higher by a factor of 1.4 between survivors (50.8 IQR 43.1–76.8) and deceased (37.5 IQR 23.7–48.8) (p = 0.047); and 4. gestational week at delivery of survivors was 2 weeks later—37.6 weeks (IQR 37.0–38.1) compared to 35.4 weeks (IQR 32.6–36.6) for the deceased (p = 0.032). Other parameters such as the side of the defect, placenta position, cervical length, pre-FETO O/E LHR, duration of the tracheal occlusion, PROM, and neonatal weight did not affect the survival rate.
Discussion
In this study, we present the experience of a new fetal medicine center that introduced FETO to clinical practice in our region. The survival rate at 6 months of age after FETO in isolated cases of unilateral CDH was 50% and was identical to the survival rate at discharge. FETO was safe for both the mother and fetus. The significant prognostic factors for survival were an earlier insertion of the balloon, an increase of O/E LHR, an increase of amniotic fluid level at balloon removal compared to balloon placement, and higher gestational age at delivery.
Recently, two large, randomized, controlled, multi-center clinical trials were reported in the New England Journal of Medicine [1, 10]. In the randomized trial of fetal surgery for a severe left diaphragmatic hernia that included 80 women (the TOTAL trial), the fetal survival rate at discharge was 40% compared to 15% in the expectant care group. Survival at 6 months was identical [1]. Similar results were published in a previous large multi-center study [14] and in a smaller cohort study [11]. In the randomized TOTAL trial for moderate hypoplasia of left diaphragmatic hernia that included 196 women, fetal survival rate at discharge was 63% compared to 50% in the expectant care group, a finding considered not statistically significant. A secondary combined analysis of both TOTAL study arms (severe and moderate) revealed that FETO increases survival for both moderate and severe lung hypoplasia, although the difference in efficacy could be derived from the time of balloon insertion, which was 2 weeks later in moderate cases of CDH [16]. In our much smaller cohort of 16 cases, we reached a 50% neonatal survival rate at 6 months after delivery, which is in line with previously reported studies [1, 11, 14]. When comparing the subgroups of surviving fetuses to those who did not survive, we found that insertion of the balloon earlier in the recommended window (27.8 vs. 28.4 weeks’ gestation) increases the survival rate (p = 0.007). This is consistent with the results reported for the combined TOTAL trial [16].
Successful balloon placement was achieved in all cases, and the procedure was safe for both the mother and fetus. This is consistent with previously reported studies [1, 10, 11, 14]. There was no case of intrauterine fetal demise (IUFD) in our study, which is consistent with previous reports showing that FETO does not increase the risk of IUFD [10, 14].
A reported complication associated with FETO is PROM [14]. In our study, the PROM rate was relatively low (25% before 37 weeks and 19% before 34 weeks), compared to 48% and 35%, respectively, in the TOTAL trial [1]. It is estimated that the low rate of PROM in our study might be related to the upper uterine position of the inserted cannula [17]. This may also account for the relatively late gestational age at delivery in our study (37 weeks) and the few deliveries before 34 weeks (25%) compared to earlier delivery reported elsewhere [1, 11, 14].
The median deepest pocket of amniotic fluid change (before balloon placement and before balloon removal) was almost three times higher in the surviving group. This may reflect the efficacy of the tracheal occlusion achieved by the balloon. Interestingly, this increase in amniotic fluid level did not increase the risk for early delivery. The median O/E LHR change at balloon removal compared to balloon placement was 40% higher in the survivors compared with the deceased. This finding is consistent with lung growth response to tracheal occlusion as previously reported by 2D and 3D ultrasound and by MRI [11, 18, 19]. Finally, the median gestational age at delivery among survivors was at term (37.6 weeks) compared to preterm delivery (35.4 weeks) among the deceased. In our study, the prematurity appears to be a major risk factor for mortality, as previously indicated [14].
In conclusion, FETO is found to be a lifesaving in utero procedure which can be offered in cases of severe CDH who choose to continue their pregnancies. The results show that our new fetal medicine center compares with other leading international centers and meets their performance measures.
References
Deprest JA, Nicolaides KH, Benachi A, Gratacos E, Ryan G, Persico N, Sago H, Johnson A, Wielgoś M, Berg C et al (2021) Randomized trial of fetal surgery for severe left diaphragmatic hernia. N Engl J Med 385:107–118. https://doi.org/10.1056/NEJMoa2027030
Langham MR Jr, Kays DW, Ledbetter DJ, Frentzen B, Sanford LL, Richards DS (1996) Congenital diaphragmatic hernia: epidemiology and outcome. Clin Perinatol 23:671–688
Harting MT, Lally KP (2014) The Congenital Diaphragmatic Hernia Study Group registry update. Semin Fetal Neonatal Med 19:370–375. https://doi.org/10.1016/j.siny.2014.09.004
Raval MV, Wang X, Reynolds M, Fischer AC (2011) Costs of congenital diaphragmatic hernia repair in the United States—extracorporeal membrane oxygenation foots the bill. J Pediatr Surg 46:617–624. https://doi.org/10.1016/j.jpedsurg.2010.09.047
Zani A, Chung WK, Deprest J, Harting MT, Jancelewicz T, Kunisaki SM, Patel N, Antounians L, Puligandla PS, Keijzer R (2022) Congenital diaphragmatic hernia. Nat Rev Dis Primers 8(1):37. https://doi.org/10.1038/s41572-022-00362-w
Jani JC, Peralta CF, Nicolaides KH (2012) Lung-to head ratio: a need to unify the technique. Ultrasound Obstet Gynecol 39:2–6. https://doi.org/10.1002/uog.11065
Done E, Debeer A, Gucciardo L, Van Mieghem T, Lewi P, Devlieger R, De Catte L, Lewi L, Allegaert K, Deprest J (2015) Prediction of neonatal respiratory function and pulmonary hypertensiuses with isolated congenital diaphragmatic hernia in the fetal endoscopic tracheal occlusion era: a single-center study. Fetal Diagn Ther 37:24–32. https://doi.org/10.1159/000364805
Van der Veeken L, Russo FM, De Catte L, Gratacos E, Benachi A, Ville Y, Nicolaides K, Berg C, Gardener G, Persico N et al (2018) Fetoscopic endoluminal tracheal occlusion and reestablishment of fetal airways for congenital diaphragmatic hernia. Gynecol Surg 15:9. https://doi.org/10.1186/s10397-018-1041-9
Deprest J, Gratacos E, Nicolaides KH, FETO Task Group (2004) Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol 24:121–126. https://doi.org/10.1002/uog.1711
Deprest JA, Benachi A, Gratacos E, Nicolaides KH, Berg C, Persico N, Belfort M, Gardener GJ, Ville Y, Johnson A et al (2021) Randomized trial of fetal surgery for moderate left diaphragmatic hernia. N Engl J Med 385:119–129. https://doi.org/10.1056/NEJMoa2026983
Persico N, Fabietti I, Ciralli F, Gentilino V, D’Ambrosi F, Boito S, Ossola MW, Colnaghi M, Condò V, Macchini F et al (2017) Fetoscopic endoluminal tracheal occlusion in fetuses with severe diaphragmatic hernia: A three-year single-center experience. Fetal Diagn Ther 41:215–219. https://doi.org/10.1159/000448096
Jani J, Nicolaides KH, Keller RL, Benachi A, Peralta CF, Favre R, Moreno O, Tibboel D, Lipitz S, Eggink A et al (2007) Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol 30:67–71. https://doi.org/10.1002/uog.4052
DeKoninck P, Gomez O, Sandaite I, Richter J, Nawapun K, Eerdekens A, Ramirez JC, Claus F, Gratacos E, Deprest J (2015) Right-sided congenital diaphragmatic hernia in a decade of fetal surgery. BJOG 122:940–946. https://doi.org/10.1111/1471-0528.13065
Jani JC, Nicolaides KH, Gratacós E, Valencia CM, Doné E, Martinez JM, Gucciardo L, Cruz R, Deprest JA (2009) Severe diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol 34:304–310. https://doi.org/10.1002/uog.6450
Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, Gorett Silva M, Greenough A, Tibboel D, CDH EURO Consortium (2010) Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology 98:354–364. https://doi.org/10.1159/000320622
Van Calster B, Benachi A, Nicolaides KH, Gratacos E, Berg C, Persico N, Gardener GJ, Belfort M, Ville Y, Ryan G et al (2022) The randomized tracheal occlusion to accelerate lung growth (TOTAL)-trials on fetal surgery for congenital diaphragmatic hernia: reanalysis using pooled data. Am J Obstet Gynecol 226:560.e1-560.e24. https://doi.org/10.1016/j.ajog.2021.11.1351
Chmait RH, Chon AH, Korst LM, Llanes A, Kontopoulos EV, Quintero RA (2018) Risks of preterm premature rupture of membranes and preterm birth post fetoscopy based on location of trocar insertion site. Am J Perinatol 35:801–808. https://doi.org/10.1055/s-0037-1620268
Cannie MM, Jani JC, De Keyzer F, Allegaert K, Dymarkowski S, Deprest J (2009) Evidence and patterns in lung response after fetal tracheal occlusion: clinical controlled study. Radiology 252:526–533. https://doi.org/10.1148/radiol.2522081955
Ruano R, da Silva MM, Campos JA, Papanna R, Moise K Jr, Tannuri U, Zugaib M (2012) Fetal pulmonary response after fetoscopic tracheal occlusion for severe isolated congenital diaphragmatic hernia. Obstet Gynecol 119:93–101. https://doi.org/10.1097/AOG.0b013e31823d3aea
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The Center of Fetal Medicine for in utero surgery and procedure was established by YG and AW following training and supervision by KN. Patients referred to the center were diagnosed by YG, AI, KTG, DN and NRD who, together with AW, also participated in the FETO procedure, balloon removal, and patient delivery. RB, GK, and AA participated in the FETO procedure consultation to the parents and in the neonatal management and hernia correction post-delivery. SOZ was in charge of the anesthesiology of the mothers. HM and ASN, together with YG, built the database and carried out the analysis. All parties were engaged in writing and editing the manuscript. AI—project development, data management, manuscript writing and editing. KTG, DN, NRD, —data collection, data analysis, manuscript editing. RB—neonatal data collection, data analysis, manuscript editing—neonatal data collection, data analysis, manuscript editing. SOZ—anesthesia data collection, data analysis, manuscript editing. AA—neonatal data collection, data analysis, manuscript editing. HM—project development, data management, manuscript writing and editing. ASN—data analysis, manuscript editing. KN—project development, manuscript editing. AW—project development, manuscript editing. YG—project development, data management, data analysis, manuscript writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the Ethics Committee of the Rabin Medical Center, approval number RMC-19-0295.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Idelson, A., Tenenbaum-Gavish, K., Danon, D. et al. Fetal surgery using fetoscopic endoluminal tracheal occlusion for severe congenital diaphragmatic hernia: a single-center experience. Arch Gynecol Obstet 310, 345–351 (2024). https://doi.org/10.1007/s00404-023-07215-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07215-1