Abstract
Background
Pulmonary embolism (PE) is a life-threatening thromboembolic complication in patients who have undergone a Fontan procedure for augmenting pulmonary blood flow in the setting of single-ventricle physiology. In patients following a Fontan procedure, lack of proper contrast agent mixing in the right atrium and sluggish, low-velocity blood flow within the Fontan circulation often results in suboptimal contrast enhancement within the pulmonary artery for evaluating PE. Unfortunately, there is a paucity of information describing the optimal contrast-enhancement technique with multidetector CT (MDCT) for evaluating PE in children and young adults following a Fontan procedure.
Objective
We illustrate the MDCT imaging findings of suboptimal contrast enhancement within the pulmonary artery, which can be mistaken for PE, in patients following a lateral Fontan procedure, and we discuss MDCT techniques to optimize contrast enhancement within the pulmonary artery in these patients for evaluating PE.
Materials and methods
The MDCT imaging findings in pediatric and young adult patients following a lateral Fontan procedure and with clinically suspected PE are illustrated. We describe intravenous contrast agent injection techniques that can be used to optimize the contrast enhancement in the pulmonary artery in patients following a lateral Fontan procedure.
Results
The use of a suboptimal contrast-enhancement technique led to initial misdiagnosis and incomplete evaluation of PE in the three patients following a lateral Fontan procedure. Imaging in two patients showed that optimal evaluation of thrombosis in the Fontan pathway and PE in the pulmonary arteries can be successfully achieved with simultaneous upper- and lower-limb injections of contrast agent.
Conclusion
This series demonstrates that suboptimal contrast enhancement can result in misdiagnosis or incomplete evaluation of PE in children and young adults following a lateral Fontan procedure. Careful attention to optimizing contrast enhancement during MDCT examination for evaluation of PE in these patients is essential to prevent misdiagnosis and incomplete evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thromboembolism is a substantial cause of morbidity and mortality in patients who have undergone a Fontan procedure, a multiple-stage surgical reconstruction that diverts systemic venous return to the pulmonary arteries in the setting of single-ventricle physiology [1–3]. In the past, conventional pulmonary angiography was the gold standard for diagnosing pulmonary embolism (PE) [4]. However, with the advent of multidetector CT (MDCT), which has allowed high sensitivity and specificity in detecting PE in recent years, CT pulmonary angiography (CTPA) is emerging as a reliable, noninvasive diagnostic imaging modality for evaluation of PE in patients following a Fontan procedure. Unfortunately, because of the sluggish, low-velocity blood flow that occurs in the Fontan pathway and pulmonary arteries in patients following a lateral Fontan procedure, incomplete mixing of blood and intravenous contrast agent within the Fontan pathway and pulmonary artery can mimic PE and result in misdiagnosis or incomplete evaluation [5]. In order to prevent misdiagnosis or delay in correctly diagnosing PE in patients who have undergone a Fontan procedure, understanding the optimal contrast-enhancement technique during MDCT evaluation of PE is essential.
Materials and methods
Our hospital’s institutional review board (IRB) approved the review of radiological and clinical data for this study. Informed consent was waived by the IRB for this retrospective analysis, but patient confidentiality was protected. We reviewed CTPA studies performed for evaluation of PE in pediatric and young adult patients who had undergone a lateral tunnel Fontan procedure at our institution between September 2006 and December 2008. We identified three patients in whom suboptimal contrast enhancement within the pulmonary artery resulted in initial misdiagnosis or incomplete evaluation of PE. In addition, we identified two CTPA studies in children following a lateral Fontan procedure that showed optimal contrast agent opacification in the Fontan pathway and pulmonary arteries which had been performed with a simultaneous upper- and lower-limb contrast agent injection technique with MDCT.
Case 1
A 20-year-old woman presented with intermittent chest pain, shortness of breath, and nausea. The laboratory data showed an elevated D-dimer level. The woman had been diagnosed with tricuspid atresia and right pulmonary artery stenosis at birth. She subsequently underwent multiple cardiac surgery procedures at 3 years of age including: (1) an initial right Blalock-Taussig (BT) shunt followed by a lateral tunnel fenestrated Fontan, (2) balloon angioplasty of right pulmonary artery stenosis, and (3) Fontan fenestration closure with a 17-mm clamshell device.
Upon admission to our hospital, the woman underwent CTPA with a 64-slice MDCT scanner (Sensation 64; Siemens Medical Solutions, Erlangen, Germany) for evaluation of PE. CT parameters included 0.6-mm collimation with weight-based low-dose tube current and kilovoltage, high-speed mode, and a pitch of 1.5. Contrast agent (100 ml, Optiray-320) was administered via a right upper-extremity vein. The monitoring scan was set at the level of the main pulmonary artery bifurcation.
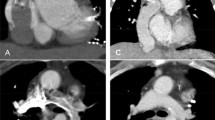
CT images showed a filling defect in the right main pulmonary artery, which was indicative of possible PE (Fig. 1). In addition, inhomogeneous contrast enhancement in the left branch pulmonary artery resulted in incomplete evaluation of possible PE (Fig. 1). The woman subsequently underwent MRI examination for confirmation of possible PE in the bilateral branch pulmonary arteries. The MRI showed completely patent bilateral main pulmonary arteries (Fig. 1). The woman’s symptoms spontaneously resolved within 24 h and she was discharged home.
A 20-year-old woman with a history of tricuspid atresia and right pulmonary artery stenosis who had undergone a lateral Fontan procedure. The woman presented with intermittent chest pain, shortness of breath, and nausea. CTPA was performed for evaluation of possible PE. a Enhanced axial CT image shows a filling defect (arrow) in the right main pulmonary artery and inhomogeneous contrast enhancement (curved arrow) in the left main pulmonary artery. b Enhanced axial MR image demonstrates patent right main (arrow) and left main (curved arrow) pulmonary arteries
Case 2
A 10-year-old boy was referred to our department with the complaint of chest pain and an elevated D-dimer level raising concern for PE. At birth, he was found to have tricuspid atresia, a double outlet left ventricle, and pulmonic stenosis. In the first year of life, the boy underwent a central shunt and main pulmonary artery ligation followed by central shunt takedown with right and left pulmonary artery patch-plasty. Additional cardiac surgery procedures at 2 years of age included: (1) bilateral bidirectional Glenn shunts, (2) takedown of a right BT shunt, and (3) formation of a fenestrated lateral tunnel Fontan.
Upon admission to our hospital, the child underwent CTPA with a 64-slice MDCT scanner (Sensation 64; Siemens Medical Solutions, Erlangen, Germany). CT parameters included 0.6-mm collimation with weight-based low-dose tube current and kilovoltage, high-speed mode, and a pitch of 1.5. Simultaneously, 30 ml of contrast agent (Optiray-320) was administered via a right lower-extremity vein using a power injector at a flow rate of 3 ml/s and 20 ml of contrast agent was administered via a right upper-extremity vein by hand injection at a rate of approximately 2 ml/s. The monitoring scan was set at the level of the Fontan pathway close to the level of the main pulmonary artery bifurcation.
CT images showed incomplete opacification of the hypoplastic left pulmonary artery (Fig. 2), resulting in incomplete evaluation of possible PE in the left pulmonary artery. The Fontan pathway and right main pulmonary artery were patent. Because of the possibility of PE in the left main pulmonary artery, an echocardiogram was immediately obtained after CT examination. The echocardiogram showed patent main pulmonary arteries and Fontan pathway without evidence of PE. The boy’s chest pain eventually resolved and he was discharged home in stable condition the next day.
A 10-year-old boy with complex congenital heart disease who had undergone a lateral Fontan procedure presented with chest pain and elevated D-dimer level. Enhanced axial CT image shows incomplete opacification of the hypoplastic left main pulmonary artery (arrows) with contrast agent resulting in incomplete evaluation of possible PE in the left main pulmonary artery. A subsequently obtained echocardiogram showed a patent left main pulmonary artery
Case 3
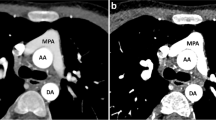
An 18-year-old girl with a history of tetralogy of Fallot and an additional conoventricular septal defect into inlet septum had a lateral tunnel fenestrated Fontan procedure performed at 1 year of age. When the girl developed shortness of breath, she underwent CTPA at an outside hospital for possible PE. At that hospital, CT images were interpreted as PE in the bilateral main pulmonary arteries (Fig. 3). The girl was subsequently transferred to our hospital for further evaluation.
An 18-year-old girl with complex congenital heart disease who had undergone a lateral Fontan procedure. CTPA was performed for evaluation of PE when she presented with shortness of breath. a Enhanced axial CT image obtained at an outside hospital demonstrates a filling defect in the right (arrow) and left (curved arrow) main pulmonary arteries. b Subsequently obtained enhanced CT image shows delayed but homogeneous contrast enhancement within the right (arrow) and left (curved arrow) main pulmonary arteries without evidence of PE
Upon admission to our hospital, the outside hospital CT images (Fig. 3) were reviewed by a pediatric cardiac radiologist and pediatric chest radiologist. Further investigation of the girl’s outside hospital CTPA protocol revealed a single contrast agent injection via a right upper-extremity vein for the CTPA study. The pediatric radiologists and cardiologist taking care of this patient concluded in consensus that filling defects observed in the bilateral main pulmonary arteries observed in the outside hospital CT images might have resulted from incomplete opacification of the contrast agent rather than a real PE. Because of the serious need for clarification of a possible large PE, the girl underwent a repeat CTPA study with a 64-slice MDCT scanner (Sensation 64; Siemens Medical Solutions, Erlangen, Germany) at our institution. CT parameters included 0.6-mm collimation with weight-based low-dose tube current and kilovoltage, high-speed mode, and a pitch of 1.5. Simultaneously, 80 ml of contrast agent (Optiray-320) was administered via a right lower-extremity vein using a power injector at a flow rate of 3 ml/s and 40 ml of contrast agent was administered via a right upper-extremity vein by hand injection at a flow rate of approximately 2 ml/s. The monitoring scan was set at the level of the Fontan pathway at the level of the main pulmonary artery bifurcation. Initial CT scanning was performed 50 s after contrast agent injection. Delayed-phase CT images were then obtained approximately 3 min after the initial contrast agent administration.
Initial repeat CT images demonstrated inhomogeneous contrast enhancement in the bilateral main pulmonary arteries that was less prominent than findings seen on the outside hospital CT images. However, delayed repeat CT images showed much improved homogeneous contrast enhancement within the bilateral main pulmonary arteries without PE (Fig. 3). The absence of PE in the bilateral main pulmonary arteries was confirmed on echocardiography. The girl became asymptomatic after overnight observation and was discharged home the following day.
Case 4
A 4-year-old boy with complex congenital heart disease (including heterotaxy, unbalanced common atrioventricular canal, pulmonary atresia, and total anomalous pulmonary venous connection) and cardiac surgical history (including Glenn and lateral tunnel Fontan procedures) was referred to our hospital for evaluation of PE after recent detection of nonocclusive thrombus in the Fontan pathway on transesophageal echocardiography.
Upon admission to our hospital, the child underwent CTPA with a 64-slice MDCT scanner. CT parameters included 0.6-mm collimation with weight-based low-dose tube current and kilovoltage, high-speed mode, and a pitch of 1.5. Simultaneously, 15 ml of contrast agent was administered via a left lower-extremity vein using a power injector at a flow rate of 1 ml/s and 15 ml of contrast agent was administered via a left upper-extremity vein by hand injection at a flow rate of approximately 1 ml/s. The monitoring scan was set at the level of the Fontan pathway near the level of the main pulmonary artery bifurcation.
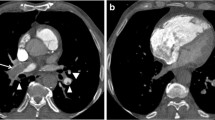
CT images demonstrated nonocclusive thrombus in the inferior vena cava portion of the Fontan pathway and PE in the right branch pulmonary artery (Fig. 4). No thrombus or PE was detected in the superior vena cava portion of the Fontan pathway. Upon diagnosis of the thrombus in the Fontan pathway, the child was started on anticoagulation therapy.
A 4-year-old boy with complex congenital heart disease and single-ventricle physiology who had undergone a lateral Fontan procedure. CTPA was performed with simultaneous upper- and lower-limb injections of contrast agent for evaluation of PE after detection of nonocclusive thrombosis in the Fontan pathway with transesophageal echocardiography. a Enhanced axial CT image obtained at the level of the inferior vena cava demonstrates a filling defect (curved arrow) within the Fontan pathway consistent with nonocclusive thrombus. Note the low-density delineating part of the surgical Fontan baffle (straight arrow). b Axial-enhanced CT image at the level of the main pulmonary artery shows a filling defect (arrow) in the right main pulmonary artery (A aorta)
Case 5
A 2-year-old girl with heterotaxy syndrome (including dextrocardia, common atrium, atrioventricular canal, double outlet right ventricle with pulmonary stenosis, and asplenia) was admitted to our hospital with shortness of breath and desaturation for evaluation of PE. The child’s surgical history included a right BT shunt, a left bidirectional Glenn shunt and left pulmonary artery patch-plasty, coil embolization of aortopulmonary collateral vessels, and a lateral tunnel Fontan procedure.
Upon admission, the child underwent CTPA with a 64-slice MDCT scanner. CT parameters included 0.6-mm collimation with weight-based low-dose tube current and kilovoltage, high-speed mode, and a pitch of 1.5. Simultaneously 20 ml of contrast agent (Optiray-320) was administered via a right lower-extremity vein using a power injector at a flow rate of 1 ml/s and 10 ml of contrast agent was administered via a right upper-extremity vein by hand injection at a flow rate of approximately 1 ml/s. The monitoring scan was set at the level of the Fontan pathway near the level of the main pulmonary artery bifurcation.
CT images were mildly limited by a beam hardening artefact resulting from multiple intrathoracic embolization coils; however, it was diagnostic for evaluation of thrombosis in the Fontan pathway and PE in the pulmonary arteries. The Fontan pathway and pulmonary arteries were well opacified with intravenous contrast agent without evidence of thrombus or PE (Fig. 5). Based on the CTPA findings, the girl was not subsequently treated with anticoagulation therapy and her symptoms eventually resolved spontaneously.
A 2-year-old girl with complex congenital heart disease including dextrocardia and pulmonary atresia status who had undergone a Fontan procedure presented with shortness of breath and desaturation. CTPA was performed with simultaneous upper- and lower-limb injections of contrast agent for evaluation of PE. Enhanced axial CT image demonstrates patent right (arrow) and left (curved arrow) main pulmonary arteries. Also noted is a metallic artefact from embolization coils
Discussion
The Fontan procedure, initially described by Fontan and Baudet [6] in 1971, is a multistage surgical procedure aimed at diverting systemic venous blood to the pulmonary arteries in patients with a single-ventricle physiology. Thrombosis and thromboembolism are well-known early and late complications after the Fontan operation, with a reported incidence ranging from 5% to 17% [3, 7–11]. The exact etiology of thrombosis and thromboembolism in patients following a Fontan procedure is not known; however, it is assumed to be multifactorial, with possible causative factors including atrial arrhythmias, abnormal liver function tests, protein-losing enteropathy, coagulation abnormalities, and slow nonlaminar blood flow patterns in the Fontan pathway [2, 10, 12]. Cross-sectional studies in which the prevalence of thrombus in the right atrium was evaluated after a Fontan procedure have shown a prevalence of thrombi between 9% and 33% [7, 10, 11, 13–15]. Unfortunately, there are no published data regarding the prevalence of PE in regard to the different pulmonary artery levels (e.g., central, main, lobar, segmental and subsegmental pulmonary arteries) in children.
The number of individuals with congenital heart disease who survive into adulthood has been steadily increasing. With improved surgical technique and postoperative management, an increasing number of patients who have undergone a Fontan procedure might present with clinical symptoms raising concern for PE. In the past, conventional pulmonary angiography was the gold standard for diagnosing PE [16–18]. In recent years, MDCT has emerged as a reliable, noninvasive diagnostic imaging modality for evaluating PE, with a high sensitivity and specificity for detecting PE in adults without congenital heart disease. When the CTPA protocol involves MDCT with four or more detector rows, the sensitivity and specificity for correctly detecting PE (in adults without congenital heart disease) has been reported to be 83–100% and 89–97%, respectively [19–22]. Superb diagnostic capability combined with noninvasiveness and wide availability makes MDCT with CTPA the test of choice for evaluating PE. The diagnostic capability of MDCT with CTPA depends primarily on the degree of contrast enhancement within the pulmonary artery to reliably differentiate thrombus from blood incompletely opacified with contrast agent. It is well known that suboptimal contrast enhancement within the pulmonary artery is the one of the most common technical limitations in the evaluation of PE with MDCT [23, 24].
In one of our patients who had undergone a lateral Fontan procedure (patient 1), suboptimal contrast enhancement within the pulmonary arteries that mimicked PE resulted from a single injection of contrast agent via the upper-extremity vein. In patients who have not undergone a Fontan procedure, there is complete mixing of contrast agent with blood within the right atrium and right ventricle before it enters the pulmonary arteries. If contrast agent is only administered via an upper-extremity vein in patients with a lateral tunnel Fontan procedure, contrast agent from the upper-extremity vein is incompletely mixed with blood from the inferior vena cava within the Fontan pathway and pulmonary artery. Furthermore, blood flow within the Fontan pathway and the branch pulmonary arteries is merely passive and largely depends on the transpulmonary gradient between the pulmonary artery and left atrium. The blood flow is therefore sluggish, which further compromises optimal opacification in the Fontan pathway and pulmonary artery [25]. If the CT scan acquisition is initiated when there is incomplete mixing of blood and contrast agent within the Fontan pathway and the branch pulmonary arteries, the incompletely opacified blood can be misdiagnosed as a pulmonary embolus.
In patient 2, although both upper- and lower-limb veins were simultaneously used for contrast agent injection, there was incomplete opacification of the left branch pulmonary artery. The reason for the incomplete contrast agent opacification of the branch pulmonary arteries with simultaneous injection of upper- and lower-limb veins in our patient 2 was the presence of a left superior vena cava, which supplied unopacified blood into the left branch pulmonary artery that was not recognized prior to performing the study. Contrast agent injected into the right upper limb vein filled the right superior vena cava that was anastomosed with the right pulmonary artery and did not opacify the left superior vena cava. A delayed scan obtained to allow opacification of the left superior vena cava would have avoided the diagnostic problem. This case illustrates the importance of reviewing the details of the prior surgical procedure and the postoperative anatomy before planning the CT scan.
Our patients 4 and 5 showed that optimal evaluation of thrombosis in the Fontan pathway and PE in the pulmonary arteries can be achieved with simultaneous upper- and lower-limb injections of contrast agent. Patient 4 demonstrated thrombosis in the Fontan pathway and PE in the right branch pulmonary artery while patient 5 showed a patent Fontan pathway and the branch pulmonary arteries without PE. Although the scan in patient 3 was performed with simultaneous upper- and lower-limb injections of contrast agent, we emphasize that a delayed second phase CT scan was necessary for the uniform opacification of the pulmonary arteries because of the markedly sluggish circulation in this particular patient.
The timing of the CT acquisition is an important technical factor that determines the quality of the contrast enhancement on MDCT images. Three main methods for determining the timing of CT scanning include: (1) predetermined empirical delay, (2) bolus tracking, and (3) the use of a test bolus [26]. We believe that the bolus tracking method, which employs a low-dose monitoring scan at a predetermined location of interest to determine the exact timing for the CT scan, is the most effective method in patients who have undergone a Fontan procedure. Because of the unpredictable degree of contrast enhancement secondary to variable blood flow velocity in the Fontan pathway and pulmonary artery, using a predetermined empirical delay for timing the CT scan acquisition is not advisable in this group of patients. Although the test bolus method is a promising technique, there are no published data for its efficacy, particularly in pediatric patients who have undergone a Fontan procedure.
Based on our experience with the five patients in this report and previously published reports [5, 27], we believe that diagnostic-quality contrast enhancement for evaluation of PE in patients who have undergone a lateral Fontan procedure can be achieved by optimizing three CT technical factors during CTPA studies with MDCT: (1) employing simultaneous injections of contrast agent via catheters placed in both upper- and lower-extremity veins; (2) performing a delayed second-phase CT scan in patients with a bilateral Glenn shunt and markedly sluggish blood in the Fontan pathway or pulmonary artery, if there is suboptimal contrast agent opacification on the first-phase CT scan; and (3) utilizing a monitoring scan with contrast agent bolus tracking to initiate CT scanning when optimal contrast enhancement is observed within the Fontan pathway and pulmonary artery.
If the MDCT study results are still inconclusive after optimizing the technical factors listed above, there are other somewhat limited noninvasive imaging modalities for evaluation of PE in this patient population including echocardiography, MRI, and ventilation-perfusion scintigraphy (V/Q scan). Although transthoracic echocardiography can be used to evaluate PE in the main and proximal branch pulmonary arteries, it has limitations for a complete evaluation of PE because the evaluation of distal pulmonary arteries is markedly limited with this technique [14, 28]. MRI has been described as a useful technique for evaluating PE [29]. However, the relatively high cost, lack of wide availability, and need for sedation in infants and young children makes this imaging modality limited as a practical alternative to MDCT. Last, V/Q scan with modified technique is a possible alternative imaging modality for evaluation of PE in patients who have undergone a Fontan procedure [30–32]. Similar to the technique involving dual administration of contrast agent used in our patients 3, 4 and 5, simultaneous injections of radiolabeled macroaggregates into the upper- and lower-extremity veins has been shown to be possible [31, 32]. This might also prevent false-positive diagnosis of PE resulting from preferential blood flow from the superior vena cava to the right pulmonary artery and the inferior vena cava to the left pulmonary artery.
Conclusion
The cases in our series illustrate that suboptimal contrast agent opacification of the branch pulmonary arteries during evaluation of PE with MDCT can result in misdiagnosis and incomplete evaluation of PE in patients who have undergone a lateral Fontan procedure. We believe that diagnostic-quality contrast enhancement for evaluation of PE in patients who have undergone a lateral Fontan procedure can be achieved by: (1) employing simultaneous injections of contrast agent via upper- and lower-extremity veins; (2) obtaining a delayed second-phase CT scan if there is suboptimal contrast agent opacification on the first-phase CT scan; and (3) utilizing a monitoring scan with contrast agent bolus tracking to initiate CT scanning when optimal contrast enhancement is observed within the Fontan pathway and pulmonary artery. Understanding the techniques for optimization of contrast enhancement with MDCT for evaluation of PE in patients who have undergone a Fontan procedure is essential to prevent misdiagnosis or delay in diagnosis. This, in turn, will result in optimal management of patients who have undergone a lateral Fontan procedure with clinically suspected PE.
References
Bull K (1998) The Fontan procedure: lessons from the past. Heart 79:213–214
Monagle P, Karl TR (2002) Thromboembolic problems after the Fontan operation. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 5:36–47
Varma C, Warr MR, Hendler AL et al (2003) Prevalence of ‘silent’ pulmonary emboli in adults after the Fontan operation. J Am Coll Cardiol 41:2252–2258
Russo V, Piva T, Lovato L et al (2005) Multidetector CT: a new gold standard in the diagnosis of pulmonary embolism? State of the art and diagnostic algorithms. Radiol Med 109:49–61; quiz 62–63
Singh HR, Forbes TJ, Humes RA (2008) CT artifact mimicking pulmonary embolism in a patient with single ventricle. Pediatr Cardiol 29:241–242
Fontan F, Baudet E (1971) Surgical repair of tricuspid atresia. Thorax 26:240–248
Coon PD, Rychik J, Novello RT et al (2001) Thrombus formation after the Fontan operation. Ann Thorac Surg 71:1990–1994
Dobell AR, Trusler GA, Smallhorn JF et al (1986) Atrial thrombi after the Fontan operation. Ann Thorac Surg 42:664–667
Fletcher SE, Case CL, Fyfe DA et al (1991) Clinical spectrum of venous thrombi in the Fontan patient. Am J Cardiol 68:1721–1722
Rosenthal DN, Friedman AH, Kleinman CS et al (1995) Thromboembolic complications after Fontan operations. Circulation 92(9 Suppl):II287–293
Jacobs ML (2005) The Fontan operation, thromboembolism, and anticoagulation: a reappraisal of the single bullet theory. J Thorac Cardiovasc Surg 129:491–495
Monagle P, Cochrane A, McCrindle B et al (1998) Thromboembolic complications after fontan procedures − the role of prophylactic anticoagulation. J Thorac Cardiovasc Surg 115:493–498
Balling G, Vogt M, Kaemmerer H et al (2000) Intracardiac thrombus formation after the Fontan operation. J Thorac Cardiovasc Surg 119:745–752
Fyfe DA, Kline CH, Sade RM et al (1991) Transesophageal echocardiography detects thrombus formation not identified by transthoracic echocardiography after the Fontan operation. J Am Coll Cardiol 18:1733–1737
Jahangiri M, Ross DB, Redington AN et al (1994) Thromboembolism after the Fontan procedure and its modifications. Ann Thorac Surg 58:1409–1413; discussion 1413–1414
Gottschalk A, Sostman HD, Coleman RE et al (1993) Ventilation-perfusion scintigraphy in the PIOPED study. Part II. Evaluation of the scintigraphic criteria and interpretations. J Nucl Med 34:1119–1126
The PIOPED Investigators (1990) Value of the ventilation/perfusion scan in acute pulmonary embolism: results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA 263:2753–2759
Wells PS, Ginsberg JS, Anderson DR et al (1998) Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med 129:997–1005
Qanadli SD, Hajjam ME, Mesurolle B et al (2000) Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 217:447–455
Remy-Jardin M, Pistolesi M, Goodman LR et al (2007) Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology 245:315–329
Stein PD, Hull RD (2007) Multidetector computed tomography for the diagnosis of acute pulmonary embolism. Curr Opin Pulm Med 13:384–388
Winer-Muram HT, Rydberg J, Johnson MS et al (2004) Suspected acute pulmonary embolism: evaluation with multi-detector row CT versus digital subtraction pulmonary arteriography. Radiology 233:806–815
Gosselin MV, Rassner UA, Thieszen SL et al (2004) Contrast dynamics during CT pulmonary angiogram: analysis of an inspiration associated artifact. J Thorac Imaging 19:1–7
Gotway MB, Patel RA, Webb WR (2000) Helical CT for the evaluation of suspected acute pulmonary embolism: diagnostic pitfalls. J Comput Assist Tomogr 24:267–273
Shannon FL, Campbell DN, Clarke DR (1986) Right atrial thrombosis: rare complication of the modified Fontan procedure. Pediatr Cardiol 7:209–212
Frush DP, Herlong JR (2005) Pediatric thoracic CT angiography. Pediatr Radiol 35:11–25
Greenberg SB, Bhutta ST (2008) A dual contrast injection technique for multidetector computed tomography angiography of Fontan procedures. Int J Cardiovasc Imaging 24:345–348
Stumper O, Sutherland GR, Geuskens R et al (1991) Transesophageal echocardiography in evaluation and management after a Fontan procedure. J Am Coll Cardiol 17:1152–1160
Takawira F, Ayer JG, Onikul E et al (2008) Evaluation of the extracardiac conduit modification of the Fontan operation for thrombus formation using magnetic resonance imaging. Heart Lung Circ 17:407–410
Matsushita T, Matsuda H, Ogawa M et al (1990) Assessment of the intrapulmonary ventilation-perfusion distribution after the Fontan procedure for complex cardiac anomalies: relation to pulmonary hemodynamics. J Am Coll Cardiol 15:842–848
Pruckmayer M, Zacherl S, Salzer-Muhar U et al (1999) Scintigraphic assessment of pulmonary and whole-body blood flow patterns after surgical intervention in congenital heart disease. J Nucl Med 40:1477–1483
Tayama M, Hirata N, Matsushita T et al (1999) Pulmonary blood flow distribution after the total cavopulmonary connection for complex cardiac anomalies. Heart Vessels 14:154–160
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prabhu, S.P., Mahmood, S., Sena, L. et al. MDCT evaluation of pulmonary embolism in children and young adults following a lateral tunnel Fontan procedure: optimizing contrast-enhancement techniques. Pediatr Radiol 39, 938–944 (2009). https://doi.org/10.1007/s00247-009-1304-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-009-1304-8