Abstract
The Fontan procedure is the final stage in the palliative surgical approach to patients with single-ventricle physiology. These patients have an increased risk for thromboembolic disease in the Fontan circuit, which can be evaluated by chest computed tomography angiography (CTA) in acute settings. However, false-positive results are common secondary to unusual streaming patterns in the Fontan circuit. A biphasic CTA protocol was evaluated for the capability to clearly identify structures of the Fontan circuit that are critical for the evaluation of thromboembolic disease. The study was a retrospective chart review of Fontan patients with a chest CTA scan obtained between 2011 and 2017. Two pediatric cardiologists with additional training in cardiac CT imaging independently reviewed each CTA and awarded one point for each of 5 Fontan circuit structures clearly identified resulting in a score range of 0–5. A score of 0–2 considered not capable, 3–4 partially capable, and 5 capable to clearly identify critical structures of the Fontan circuit. During the study period, 46 CTA scans were performed on 21 patients. Of the CTA scans using a biphasic protocol, 62.5% (10/16) were considered capable to clearly identify all 5 critical structures of the Fontan circuit vs 27% (8/30) of the CTA scans using a monophasic protocol (p = 0.027). Overall our results suggest that the single-site biphasic CTA protocol has greater diagnostic capability to detect the presence of Fontan thromboembolic disease when compared to the more traditional monophasic CTA protocol. Future prospective studies are needed to confirm these findings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients with complex congenital heart disease (CHD) with single-ventricle physiology often undergo a series of staged palliative surgical procedures that typically end with a final-stage Fontan procedure which connects the systemic and pulmonary circulation in series via a Total Cavopulmonary Connection [1,2,3,4,5]. The intermediate-stage Glenn procedure re-routes blood typically from the superior vena cava (SVC) to the pulmonary arteries via an anastomosis of the SVC to the superior aspect of the right pulmonary artery (RPA). The Fontan procedure completes the separation of oxygenated from de-oxygenated blood by re-routing blood returning from the inferior vena cava (IVC) to the pulmonary arteries. Currently, the Fontan procedure typically involves either a lateral tunnel or an extracardiac Fontan completion with the latter being favored at our institution. The lateral tunnel Fontan incorporates the posterior wall of the right atrium with a patch to create a conduit from the IVC to the pulmonary arteries through the right atrium [2,3,4]. The extracardiac Fontan utilizes an extracardiac conduit to connect the IVC to the pulmonary arteries directly and bypasses the right atrium completely [5]. Despite advancements in surgical techniques, the anastomosis of the IVC to the pulmonary circulation in the extracardiac Fontan procedure is typically made to the under-surface of the RPA in a position that is slightly offset from the SVC anastomosis thus creating preferential blood flow from the SVC versus the IVC to the different branch pulmonary arteries [6, 7].
After the Fontan procedure, the patient’s pulmonary blood flow is dependent on low velocity, passive drainage of blood from the superior and inferior vena cavae. Patients with this physiology have been found to be at increased risk for pulmonary thromboembolic disease compared to the general population [8,9,10,11,12,13,14]. The exact cause for this increased risk for thromboembolic disease is not completely known. However, there are multiple suspected mechanisms that likely contribute to this increased risk: slow venous flow, ventricular dysfunction, cardiac arrhythmias, portal hypertension with subsequent hepatic dysfunction, alterations in the coagulation cascade, prosthetic material used in the Fontan circuit, lack of pulsatility in the pulmonary circulation with subsequent alterations in the endothelium, and cyanosis [8,9,10,11,12,13,14]. Thromboembolism in these patients has been found to be one of the leading causes of Fontan circuit failure or death outside the perioperative period [8,9,10,11,12]. This makes the ability to accurately evaluate for Fontan thromboembolic disease a vital necessity in caring for this population.
Physicians with access to an MRI machine will often obtain a cardiac MRI for non-emergent evaluation of Fontan thromboembolic disease. For urgent evaluation of Fontan thromboembolic disease, a transesophageal echocardiogram (TEE) can be performed. However, this is an invasive procedure that requires sedation and expertise that is often unavailable in an urgent fashion and detection of such lesions is often difficult with TEE imaging. More commonly, physicians utilize computed tomography angiography scans (CTAs) to evaluate for Fontan thromboembolic disease as this has the additional advantage of clearly defining the distal pulmonary architecture and extracardiac structures too. However, when a traditional CTA pulmonary embolus (PE) protocol is utilized, there is differential streaming of pulmonary blood flow that results in preferential filling of the right or the left pulmonary artery and related distal pulmonary arterial circulation depending on whether the intravenous (IV) contrast is injected from the upper or lower extremity. The corresponding CTA can then mistakenly be interpreted as being non-diagnostic or positive for flow-limiting thrombus in the other or poorly attenuated pulmonary artery or Fontan connection thus initiating a cascade of expensive and unnecessary testing and treatment protocols.
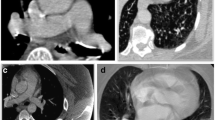
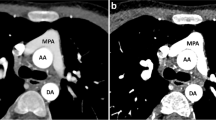
Various alterations to the traditional CTA PE protocol have been developed to overcome the differential streaming of contrast medium by focusing on the number of sites for contrast medium injection and the number of phases of acquisition [15, 16]. One technique utilizes dual sites of injection, one in an upper and one in a lower extremity vein, with a single acquisition. While another technique utilizes dual sites of injection and dual phases of acquisition. A third approach utilizes a single-site injection and single, late phase of acquisition. The fourth approach and one that is now favored by our team utilizes a single-site injection with dual phases of acquisition, also referred to as a biphasic CTA (Fig. 1). For biphasic CTAs, the initial acquisition typically evaluates the distal branch pulmonary arteries while the second acquisition evaluates the proximal Fontan circuit by allowing the contrast enough time for passive venous return. All four techniques have shown improvement over utilizing a single-site injection with single, early phase of acquisition. However, utilizing two IV sites to inject contrast is typically associated with double the discomfort with the added difficulty of obtaining venous access in a lower extremity that is of a sufficient caliber to allow for a power injection. Our study evaluated the use of a single-site injection with dual phases of acquisition compared to a single-site injection with a single, early phase of acquisition for optimal opacification of structures important for the evaluation of thromboembolic disease in the Fontan patient’s pulmonary circulation: SVC, IVC, Fontan, as well as the right and left branch pulmonary arteries.
Methods
The study was an IRB-approved retrospective chart review of Fontan patients with a chest CTA obtained between 2011 and 2017. This spanned the time that the UF Congenital Heart Center was adopting a biphasic CTA protocol for the evaluation of Fontan patients. The UF Congenital Heart Center Computed Tomography database was queried for the term “Fontan” creating a list of patients. These patients’ medical records were reviewed for chest CTAs with images available for review. Patients’ medical records were also reviewed for type of CHD, date and type of Fontan surgery, anticoagulation history, indication for CTA, route of contrast medium injection, phases of acquisition, resulting diagnosis and treatment plan after CTA, and follow-up imaging.
CTAs were performed on Toshiba-Aquilion machines with 64, 128, and 320 slice capabilities. Patients undergoing a biphasic CTA utilized a single-site injection via at least a 22 g peripheral IV and two acquisition phases. Patients received 0.65 milliliters (mL) per pound, up to a total of 150 mL, of iohexol contrast medium (350 milligram iodine/mL; Omnipaque 350, GE Healthcare, Ireland). If the patient weighed less than 75 lb, they received all of their contrast prior to the initial acquisition and their second acquisition was initiated 70 s after the initial contrast injection. If the patient weighed greater than 75 lb, half of the patient’s contrast volume was given via a power injection prior to the first acquisition. This first acquisition was triggered by bolus tracking that started 10 s following the start of the initial injection of contrast. The region of interest was placed on the main pulmonary artery and the Hounsfield units were set at 150. The second acquisition was also triggered by bolus tracking that started 50 s following the initial injection with the region of interest placed on the Fontan connection. If bolus tracking did not trigger an acquisition, the second acquisition was obtained manually no later than 70 s after the initial injection of contrast. The second injection of contrast was performed immediately prior to the second acquisition. For comparison, the study included monophasic CTAs of Fontan patients utilizing CTA protocols with only a single-site injection via at least a 22 g peripheral IV and a single early phase of acquisition. The monophasic CTA protocol involved using 0.5 mL per pound, up to a total of 150 mL, of iohexol contrast (350 mg iodine/mL; Omnipaque 350, GE Healthcare, Ireland). The contrast would be hand injected at a rate of 0.5 mL per second (mL/s) if the patient weighed less than 50 lb. The contrast would be infused at a rate of 1–2 mL/s for patients weighing 50–100 lb and at a rate of 2 mL/s for patients weighing greater than 100 lb. The acquisition would then occur immediately following the hand injection or, if the contrast was infused, 60 s following the start of contrast infusion. Neither the biphasic or the monophasic CTA protocol adjusted the rate of injection nor the timing of acquisition on an individual basis.
A scoring system was created to assess the diagnostic capability of each CTA for detecting thromboembolic disease based on the ability to clearly identify critical structures of the Fontan circuit (SVC, IVC, central Fontan circuit, the right pulmonary artery, and the left pulmonary artery). For the right and left pulmonary arteries to receive a point, contrast needed to fill the vessel up to the onset of the first set of branches from the right and left main pulmonary arteries. Two pediatric cardiologists with additional training in cardiac CT imaging independently reviewed each CTA and awarded one point for each structure clearly identified resulting in a score range of 0–5. A score of 0–2 was considered not capable, 3–4 partially capable, and 5 capable to clearly identify critical structures of the Fontan circuit.
Results
The database identified 46 CTAs performed during the study period on a total of 21 patients. The patients’ original CHD included six different diagnoses: hypoplastic left heart syndrome (9 patients, 16 CTAs), tricuspid atresia (3 patients, 7 CTAs), pulmonary atresia (1 patient, 5 CTAs), unbalanced atrioventricular septal defect (4 patients, 14 CTAs), double-inlet left ventricle (1 patient, 3 CTAs), and double-outlet right ventricle (1 patient, 1 CTA). There was no significant difference as to the type of Fontan between monophasic and biphasic populations, 63% lateral tunnel and 37% extracardiac vs 44% lateral tunnel and 56% extracardiac, respectively (Table 1).
There was no statistically significant difference between acute and non-acute indications for chest CTA, p = 0.36 (Table 2). Acute indications for a chest CTA included clinical concern for Fontan thromboembolic disease (n = 14), respiratory infection/hemoptysis (n = 5), thromboembolic disease on prior monophasic CTA (n = 4), renal artery thrombus on CTA at referring hospital (n = 1), failing Fontan with protein losing enteropathy (n = 1), and bleeding concern (n = 1). The non-acute indications for a chest CTA included vascular/anatomic evaluation as part of cardiac transplant evaluation, pre-surgical planning, or concern for a vascular abnormality (n = 19) and chronic hemoptysis (n = 1).
Of the 46 CTAs, 65% were monophasic and 35% were biphasic. Of the biphasic CTAs, 62.5% were considered capable to clearly identify the critical structures of the Fontan circuit by scoring 5/5 as opposed to 27% of the monophasic CTAs (Table 3). While the inter-observer variability for the scoring system was not significant (p = 0.71), some structures received differing scores by the two reviewers that resulted in an average that included partial numbers. This unfortunately limited the ability to perform formal statistics on the individual structures evaluated.
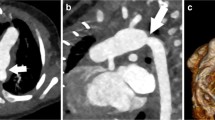
There were three patients who had a biphasic CTA performed due to the diagnosis of thromboembolic disease on a prior monophasic CTA, one of which had been performed at an outside institution. Two of these three patients’ diagnoses were reversed based on follow-up biphasic CTAs. One of the two patients whose diagnosis was reversed presented to clinic with a 9-day history of chest pain and shortness of breath brought on by stress at work. An outpatient, monophasic CTA was obtained that was concerning for a Fontan thrombus due to suboptimal opacification of the central left pulmonary artery while there was good opacification of the distal left pulmonary artery (Fig. 1a, b). The other patient whose diagnosis was reversed had a history of chronic mycobacterium avium-intracellulare with associated chronic hemoptysis. This patient presented to an outside hospital with a more severe episode of hemoptysis. As part of the evaluation, a monophasic CTA was obtained that was reported to be concerning for “complete occlusion of the stent extending from the inferior vena cava to the pulmonary artery outflow tract.” The third patient was admitted to the hospital with plastic bronchitis awaiting heart transplantation while on room air. They developed poor perfusion with associated emesis and acute worsening of their ventricular function on echocardiogram that ultimately led to respiratory failure requiring intubation. On chest X-ray, there were findings consistent with pneumonia in the right lower lobe. However, there were persistent abnormalities on follow-up chest X-rays that could be attributed to a thromboembolic etiology as opposed to a right lower lobe pneumonia. Therefore, a monophasic CTA was obtained to evaluate for thromboembolic disease. On this CTA, there was concern for Fontan thrombus due to a filling defect in the distal right pulmonary artery and the branches to the right lower lobe. In addition to the filling defect, there was evidence of pulmonary infarction due to a triangular parenchymal opacity that abutted the pleural margin with internal air bronchograms peripheral to the branches of this right lower lobe pulmonary artery. This clinical presentation was felt to be consistent with Fontan thromboembolic disease and the patient was treated with anticoagulation. This patient’s follow-up biphasic CTA was performed one week after the initial monophasic CTA and its negative finding was attributed to anticoagulation treatment. There was one patient with Fontan circuit thrombus noted on a monophasic CTA, whose diagnosis was reversed based on follow-up cardiac MRI. This patient presented to the emergency department, while pregnant at 32 weeks of gestational age, with sharp sternal chest pain. The monophasic CTA that was obtained was unable to rule out thromboembolic disease due to streaming of contrast. Therefore, of the 5 positive monophasic CTAs, two were subsequently found to be falsely positive (Table 3).
One patient was diagnosed with a Fontan thrombus by a biphasic CTA due to a contrast filling defect in the left lower lobar pulmonary artery. In addition to the contrast filling defect noted on the CTA, the presence of heterogeneous lung attenuation and ground glass opacity in the left lower lobe also supported the diagnosis of a pulmonary embolism. The CTA was obtained due to clinical symptoms of acute onset of chest tightness and shortness of breath. As such, there were no patients diagnosed with Fontan thrombus on a biphasic CTA, whose diagnosis was later reversed.
Discussion
As the population of patients having had a Fontan operation increases, the frequency of Fontan patients presenting with emergent or urgent symptoms concerning for thromboembolic disease will continue to rise. For centers without ready access to cardiac MRI or TEE or for those centers that prefer CTA as their primary diagnostic option for this disease, the need for an optimal diagnostic CTA protocol is imperative. The Fontan operation creates complex pulmonary flow dynamics resulting in streaming effects that alter the ability for a traditional monophasic CTA PE protocol to accurately diagnose Fontan thromboembolic disease [6, 7].
There have been multiple published iterations to the traditional monophasic CTA PE protocol that seeks to improve the ability to diagnose thromboembolic disease in Fontan patients [15, 16]. One iteration utilizes a single site for contrast injection with a delayed phase of acquisition. In our institution’s experience, utilizing this single, delayed acquisition leads to poor filling of the distal branch pulmonary arteries. Other iterations also involve a single acquisition but include two sites for contrast injection. However, utilizing a second site for contrast injection increases the time involved in obtaining the study as well as the patient’s risk for complications and discomfort associated with the need for a second IV. In order to avoid the need for a second IV, our institutional CTA Fontan protocol includes a second acquisition. The initial acquisition is obtained at a time that contrast has filled the distal branch pulmonary arteries while the second evaluates the proximal Fontan circuit by giving the contrast enough time for passive venous return.
In this study, we compared monophasic versus biphasic CTA protocols’ abilities to identify anatomical structures that are critical for evaluating thromboembolic disease in the Fontan circuit. Of the CTA scans using a biphasic protocol, 62.5% (10/16) were considered capable to clearly identify the critical structures of the Fontan circuit vs 27% (8/30) of the CTA scans using a monophasic protocol (p value = 0.027). Of the critical structures evaluated, using monophasic CTAs has given us the most difficulty evaluating the IVC, Fontan, and LPA (Table 4). With 70% of monophasic CTAs in our study utilizing a right upper extremity IV for contrast injection, these structures would be expected to be the least adequately filled as the typical streaming of contrast from the SVC is preferentially directed into the RPA due to the angle of anastomosis at the time of the Glenn procedure. Monophasic CTAs also led to three patients being inappropriately diagnosed with a thrombus in their Fontan circuit, two of which received inappropriate anticoagulation based on a flawed diagnosis. There were no biphasic CTAs that inappropriately diagnosed patients with a thrombus in their Fontan circuit.
We compared two of our institutional CTA protocols’ capability to clearly identify anatomical structures that are critical for evaluating Fontan circuit thromboembolic disease. There are limitations to this study. This being a retrospective study we lacked the ability to test each protocols detection accuracy for thromboembolic disease against other CTA protocols or other imaging modalities for a majority of the patients in the study. Also, there currently is no agreed upon gold standard for evaluating thromboembolic disease in the Fontan circuit. Furthermore, if we did wish to compare serial studies with the data available, of the 46 CTAs, only six had follow-up imaging (CTA, cardiac MRI, or cardiac catheterization) where one could try to correlate the findings with the previous CTA knowing in these cases these follow-up imaging studies were performed at later dates which introduces a number of other complicating factors into the analysis. Finally, the biphasic CTA requires a second acquisition which involves doubling the radiation exposure to the patient, which should be considered.
Overall our results suggest that the single-site biphasic CTA protocol has greater diagnostic capability to detect the presence of Fontan thromboembolic disease when compared to the more traditional monophasic CTA protocol. Future prospective studies are needed to confirm these findings.
References
Fontan F, Baudet E (1971) Surgical repair of tricuspid atresia. Thorax 26(3):240–248
Puga FJ, Chiavarelli M, Hagler DJ (1987) Modifications of the Fontan operation applicable to patients with left atrioventricular valve atresia or single atrioventricular valve. Circulation 76(3):53–60
DeLeval MR, Kilner P, Gewillig M, Bull C (1988) Total cavopulmonary connection: a logical alternative to atriopulmonary connections for complex Fontan operations. J Thorac Cardiovasc Surg 96:682–695
Jonas RA, Castaneda AR (1988) Modified Fontan procedure: atrial baffle and systemic venous to pulmonary artery anastomotic techniques. J Card Surg 3(2):91–96
Laschinger JC, Ringel RE, Brenner JI, McLaughlin JS (1992) Extracardiac total cavopulmonary connection. Ann Thorac Surg 54(2):371–373
de Leval MR, Dubini G, Migliavacca F (1996) Use of computational fluid dynamics in the design of surgical procedures: application to the study of competitive flows in cavo-pulmonary connections. J Thorac Cardiovasc Surg 111:502–513
DeGroff CG (2008) Modeling the Fontan circulation: where we are and where we need to go. Pediatr Cardiol 29:3–12
Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, Spray TL (2001) Thrombus formation after the Fontan operation. Ann Thorac Surg 71(6):1990–1994
Jacobs ML (2005) The Fontan operation, thromboembolism, and anticoagulation: a reappraisal of the single bullet theory. J Thorac Cardiovasc Surg 129(3):491–495
Allen K, Downing TE, Glatz AC, Rogers LS, Ravishankar C, Rychik J, Fuller S, Montenegro LM, Steven JM, Spray TL, Nicolson SC, Gaynor JW, Goldberg DJ (2017) Effect of Fontan-associated morbidities on survival with intact Fontan circulation. Am J Cardiol 119(11):1866–1871
Khairy P, Fernandes SM, Mayer JE Jr, Triedman JK, Walsh EP, Lock JE, Landzberg MJ (2008) Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 117(1):85–92
Alsaied T, Bokma JP, Engel ME, Kuijpers JM, Hanke SP, Zuhlke L, Zhang B, Veldtman GR (2017) Factors associated with long-term mortality after Fontan procedures: a systematic review. Heart 103(2):104–110
Goldberg DJ, Dodds K, Rychlik J (2010) Rare problems associated with the Fontan circulation. Cardiol Young 20(3):113–119
Tomkiewicz-Pajak L, Hoffman P, Trojnarska O, Lipczyńska M, Podolec P, Undas A (2014) Abnormalities in blood coagulation, fibrinolysis, and platelet activation in adult patients after the Fontan procedure. J Thorac Cardiovasc Surg 147:1284–1290
Sandler KL, Markham LW, Mah ML, Byrum EP, Williams JR (2014) Optimizing CT angiography in patients with Fontan physiology: single-center experience of dual-site power injection. Clin Radiol 69(12):562–567
Mahani M, Agarwal P, Rigsby C, Lu J, Dehkordy S, Wright R, Dorfman A, Krishnamurthy R (2016) CT for assessment of thrombosis and pulmonary embolism in multiple stages of single-ventricle palliation: challenges and suggested protocols. Radiographics 36(5):1273–1284
Funding
There are no industry relationships. There were no funding sources for the study.
Author information
Authors and Affiliations
Contributions
RB: Contributed to the development of the design of the study, participated in the chart review, data analysis, data interpretation, and statistical analysis, drafted the original manuscript, and participated in the revision process. TB: Contributed to the development of the design of the study, participated in the chart review, data analysis, data interpretation, and statistical analysis, and participated in the revision process. JC-V: Contributed to the development of the design of the study, participated in the chart review, data analysis, data interpretation, and statistical analysis, and participated in the revision process. CD: Contributed to data analysis, data interpretation, and statistical analysis, and participated in the revision process. NQ: Contributed to the development of the design of the study and critical revision of the manuscript. AC: Contributed to the development of the design of the study, participated in the chart review, data analysis, data interpretation, and statistical analysis, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boggs, R., Dibert, T., Co-Vu, J. et al. Optimized Computed Tomography Angiography Protocol for the Evaluation of Thrombus in Patients with Fontan Anatomy. Pediatr Cardiol 41, 1601–1607 (2020). https://doi.org/10.1007/s00246-020-02417-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-020-02417-9