Abstract
Background
Tuberous sclerosis complex (TSC) is an autosomal dominant phakomatosis associated with intracardiac rhabdomyomas.
Objective
The aim of our study was to examine the value of cerebral MRI in diagnosing TSC in fetuses with intracardiac rhabdomyomas, applying the TSC Consensus Conference (TSCCC) criteria.
Materials and methods
In a prospective manner six consecutive fetuses with cardiac rhabdomyomas (21–34 weeks’ gestation) underwent cerebral MRI. The MRI results were correlated with clinical follow-up at 10–34 months after birth, histology, and genetic data.
Results
In five of the six fetuses the diagnosis of TSC was established. In two of five fetuses MRI demonstrated cerebral manifestations of TSC that correlated well with severe epilepsy manifesting during the follow-up period. In another two of five fetuses MRI as well as clinical follow-up were normal. One of five pregnancies was terminated and histology demonstrated microscopically small subependymal nodules not demonstrated by MRI.
Conclusion
The results of our study agree with the available literature that fetal MRI is sufficient for the detection of cerebral lesions in TSC and should be better promoted. The TSCCC criteria can also be applied to fetal MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant phakomatosis with variable penetrance, a spontaneous mutation rate of 50–80%, and an incidence of 1 in 6–12,000 births. The classic triad of symptoms comprises seizures, mental retardation, and adenoma sebaceum [1–5]. Other organs that may be affected are the kidneys (angiomyolipoma, polycystic kidneys), eyes (hamartoma), lungs (lymphangioleiomyomatosis), skin (hypomelanotic macules), skeleton (desmoplastic fibroma), teeth (dental pitting) and liver (angiomyelolipoma) [2, 6–12]. Subungual or periungual fibromas are a regular finding [13]. Café-au-lait spots, which are a typical finding in other forms of phakomatosis, have the same incidence in TSC as in the normal population and cannot, therefore, be used as a diagnostic criterion [2, 14].
TSC is caused by gene mutations and deletions at two loci—TSC1 (9q34) and TSC2 (16p13-3) [15–21]. Two gene products, hamartin (TSC1) and tuberin (TSC2), form a complex in vivo that instigates a negative feedback loop in the translation of proteins, cellular proliferation, and cell growth, and acts as a tumour suppressor [16, 17, 22–26].
Besides cases due to spontaneous mutation, there are cases in which unaffected parents have one or more affected children. Since nonpenetrance in segregating families is rare, these cases must be attributed to germ-line mosaicism [11, 23, 27]. The recurrence risk of unaffected parents having an affected child is reported to be 2–3% in the literature [11, 27]. Due to genetic heterogeneity, genetic testing has a 15% false-negative rate [28], which explains the significance of imaging modalities in prenatal diagnostic evaluation.
The pathomorphological correlate of TSC in cerebral imaging is the demonstration of supratentorial tubers [29], which combine properties of a migratory disorder and a space-occupying lesion, and are found in 85% of patients [4, 15]. This descriptive term is used to refer to both subependymal nodules (SENs) and cortical hamartomas (dysplasia) and should no longer be used [11]. Both lesions have the same signal intensity on MRI, but differ histologically. Histologically, SENs are characterized by the presence of giant cells (balloon cells), which are also found in lymphangioleiomyomatosis and hemimegalencephaly [7, 15, 17]. About 6–14% of SENs develop into subependymal giant-cell astrocytomas (SGCAs), which may be complicated by internal hydrocephalus [30, 31]. SGCA differs from SEN only in a slightly increased mitosis rate and polymorphism, and the associated growth tendency [11, 32].
Fetal US is not very sensitive in diagnosing cerebral lesions, except for large subependymal tumours. Moreover, it is difficult to differentiate SENs from subependymal haemorrhage or heterotopia on the basis of the US findings [1, 14, 15]. In contrast, fetal MRI depicts cerebral lesions as high signal intensity on T1-weighted (T1-W) images and low signal intensity on T2-weighted (T2-W) images relative to nonmyelinated white matter [1, 3, 14, 15, 33–36].
The incidence of TSC in fetuses in which US demonstrates intracardiac rhabdomyomas, which account for 78–89% of all cardiac tumours in the fetus and child [37, 38], is reported to range from 51% to 87% [5, 39]. Conversely, about 80% of fetuses with TSC have cardiac rhabdomyomas [40]. Therefore the demonstration of cardiac rhabdomyomas by fetal US is highly predictive of TSC [1, 9, 15, 41–43].
The aim of the study presented here was to investigate the clinical role of supplementary fetal cerebral MRI in establishing the diagnosis of TSC after demonstration of intracardiac rhabdomyomas by US. The MR images were analyzed using the TSC Consensus Conference criteria [11, 30] and the findings were correlated with the genetic, clinical, and histological findings.
Materials and methods
The study was approved by the local ethics committee and written informed consent was obtained from all patients. The pregnant women were informed about the risk of vena cava compression syndrome and instructed to immediately press the emergency bell in the event of nausea or cold sweat. Six consecutive pregnant women (21–34 weeks’ gestation) with fetal intracardiac rhabdomyomas demonstrated by antenatal US were examined prospectively. Ultrasonography was performed using high-resolution (4–8 MHz) probes. Doppler examination of the heart was obligatory. The women were examined by MRI to identify possible fetal cerebral lesions. The number of intracardiac rhabdomyomas ranged from one to over ten, with a diameter of up to 40 mm.
The MRI examinations were performed on a Magnetom Vision scanner (cases 1 and 2) or a Sonata scanner (cases 3–6) (Siemens, Erlangen, Germany) using a body phased-array coil. Images were acquired using a T2-W HASTE (half-acquisition single-shot turbo-spin-echo) sequence (TR/TE 1,100/90 ms and matrix 256 for the Vision scanner; TR/TE 1,260/74 ms and matrix 256 for the Sonata scanner) and a T1-W gradient-echo sequence (TR/TE 134/4 ms, matrix 128 and α70 for the Vision scanner; TR/TE 130–160 ms/5–10 ms, matrix 128 and α70 for the Sonata scanner) in the axial plane with a slice thickness of 4 mm. The women were imaged in the supine position without sedation. Breath-hold imaging was performed optionally.
The images were read by two radiologists experienced in fetal imaging. MR imaging results were correlated with clinical follow-up, genetic data and/or histology. The latter were used as the gold standard to establish the diagnosis of TSC. Clinical follow-up data were obtained from the infants’ medical records and from interviews with the treating paediatricians. The cases were evaluated using the classification system established by the Tuberous Sclerosis Complex Consensus Conference (TSCCC) [11, 28, 30, 44].
No routine postnatal imaging was performed. In one case (case 1), a paediatrician who doubted the prenatal diagnosis requested postnatal MRI for confirmation of the findings. In this case and two others (cases 2 and 6), postnatal cerebral US was performed. The data relating to all cases are summarized in Table 1, which also shows supplementary findings from postnatal imaging not taken into account for the evaluation.
Results
Table 1 presents the results of prenatal US, postnatal imaging, fetal MRI, genetic testing, and histological examination including postnatal clinical follow-up (range 10–34 months).
In four of the six cases (cases 1–4) included in our study, the diagnosis was confirmed by genetic testing. In another case (case 6) the family history and clinical course were highly indicative of TSC. Thus, we had five cases with a diagnosis of TSC in our population.
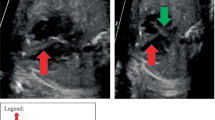
In two cases (cases 1 and 6), fetal MRI demonstrated typical characteristics of TSC. In both cases the fetal MRI findings were confirmed by postnatal cranial US although fetal MRI was more convincing in one case. Both infants developed epilepsy later during the period of follow-up. In one of these cases (case 1), the diagnosis was additionally confirmed by repeat MRI 5 days after birth (Fig. 1).
Case 1. a, b Prenatal axial HASTE (a) and gradient-echo (b) MR images at 32 + 4 weeks’ gestation. c, d Postnatal (day 5) axial T2-W (c) and T1-W (d) MR images. Both the prenatal and the postnatal images show SENs. Additionally there is cortical dysplasia and a white-matter lesion in the left frontal area that are more clearly depicted on the postnatal images; however, prenatal and postnatal slice position do differ
In another two cases (cases 2 and 4), fetal MRI was negative and the infants had no neurological symptoms during follow-up. In one of the cases (case 2) postnatal US was performed confirming the fetal MRI findings. In another case (case 3), autopsy after termination of pregnancy identified small SENs that escaped detection by MRI.
Case 5 was referred for fetal MRI due to manifestations of TSC in the mother and fetal cardiac rhabdomyomas. In this case cerebral MRI of the mother was additionally performed. Both examinations failed to demonstrate typical changes of TSC. Genetic testing of the mother also failed to prove TSC; thus we assume an incorrect diagnosis or faulty history in this case. The mother showed mild mental retardation and café-au-lait spots, which are not specific for TSC and do not allow establishment of the diagnosis. We concluded that TSC was not present in either the mother or the child. The mother was not very compliant, so that further examinations (e.g. genetic testing of the child) or clinical follow-up data were not available. We were also unable to locate the mother’s general practitioner or the paediatrician treating the child.
Discussion
The aim of our study was to investigate the role of cerebral MRI in fetuses with US evidence of intracardiac rhabdomyomas. TSC was diagnosed in the fetuses using the TSCCC criteria [11]. These criteria are general in nature and can, therefore, also be applied to fetal MRI. A distinction is made between major and minor criteria, depending on how specific the clinical and radiological findings are for TSC [11]. Some criteria such as calcification of SENs are not applicable to fetal imaging. Fetal CT is not undertaken because of the radiation exposure involved. Moreover, SENs typically do not calcify until 1 year of age. Menor et al. [45] have suggested that cerebral CT be undertaken to search for calcified SENs when TSC is suspected, but MRI is negative.
The TSCCC has assigned an important role to radiological diagnosis. Cardiac rhabdomyomas and SENs demonstrated by radiological methods are major criteria [11]. The presence of two major criteria constitutes a definite diagnosis of TSC [11]. Radiologically proven cortical dysplasia [14] and white-matter lesions are minor criteria because they are less specific for TSC than SENs. The coincidence of white-matter lesions and cortical dysplasia counts as one minor criterion because the disease is classified as a migratory disorder [11]. The combination of a major criterion (e.g. cardiac rhabdomyoma) and a minor criterion (e.g. cortical dysplasia) constitutes a probable diagnosis [11].
There is a known positive correlation between the number of tubers, seizures, and mental retardation [2, 14, 22, 31, 46]. In our study, the fetuses with cerebral manifestations showed the most severe neurological symptoms (epilepsy). Two fetuses with normal MRI findings had an established diagnosis of TSC. This is important to bear in mind when counselling parents, since a negative MRI does not exclude TSC.
Our results are in agreement with those of Sonigo et al. [5] who performed MRI in eight fetuses with proven cardiac rhabdomyoma (one MRI examination was nondiagnostic due to motion artefacts). Therapeutic abortion was performed in five of the pregnancies. The pathomorphological findings showed a good correlation with the MR findings. In two further cases, there was good agreement between imaging and the cliniconeurological findings. Note that in one of these cases a single SEN was detected and the child showed only mild neurological symptoms during follow-up. There was no such case in our study population. Both our cases with demonstration of SENs by MRI had multiple nodules associated with epilepsy. These observations raise the question as to whether the demonstration of a solitary SEN, just like the absence of SEN, is an indication of a mild course. This question cannot be answered on the basis of currently available data.
Both the study of Sonigo et al. [5] and our study were limited by the small number of cases included. This is due to the rarity of the condition. Moreover, clinical follow-up should be performed for several years, ideally to the end of puberty. Such studies are very difficult to conduct and analysis of data would be problematic due to the development of radiological techniques and possible changes in therapeutic regimens over such a long period. Nevertheless, available experience suggests that fetal MRI should be used more widely and might replace postnatal MRI, which is more difficult to perform, time-consuming and exposes the newborn or infant to side effects from sedation or anaesthesia [47–51].
Our examples (Figs. 1 and 2) show that clinically relevant imaging findings can already be obtained in the fetus. We therefore suggest that fetal MRI should become a component of early interdisciplinary fetal diagnostic work-up in suspected TSC. MRI is a sensitive imaging tool for evaluation of the fetal brain and provides valuable diagnostic information for parental counselling and documents the baseline situation for comparison with postnatal follow-up. An adequate MR examination to diagnose TSC requires a T1-W gradient-echo sequence as well as the work-horse HASTE sequence. Our experience has shown that the cerebral lesions are easier to delineate on gradient-echo imaging because the lesion/tissue contrast is higher. Once the diagnosis has been established, regular imaging follow-up is not necessary, and only children with progression of neurological symptoms need to undergo repeat MRI [3, 45].
The following is offered as recommendation for timing of fetal MRI rather than a strict guideline. Before 28 weeks of gestation fetal cerebral MRI is difficult to interpret since the field of view (FOV) is relative large compared to the size of the fetal head, preventing visualization of small detail [52, 53]. Reducing the FOV to just cover the fetal brain would case aliasing artefacts. The conditions for performing fetal cerebral MRI improve with every week of gestation since the fetal brain grows rapidly (50th centile for cerebral biparietal diameter increases from 50 mm at 23 weeks to 70 mm at 31 weeks [54]). Nevertheless, Levine et al. [1] reported the MR diagnosis of SENs at 21 weeks’ gestation.
Scanner technology is also an important factor. Fetal MRI is limited by the small size of the structure being imaged and the large distance between the fetus and the receiver coil [55]. We perform all fetal MRI examinations with a 1.5-T magnet using a body phased-array coil. This configuration provides a good compromise between imaging speed, anatomic resolution, and signal-to-noise ratio. Glenn and Barkovich [55] recently reported higher image quality because of increased signal-to-noise ratio using an eight-channel torso phased-array coil compared with the standard pelvic phase-array coil [55]. Coils located closer to the region of interest (e.g. intravaginal or endorectal coils) are currently not used owing to the small FOV.
Conclusion
Our results suggest that fetal cerebral MRI should be applied more often and must be promoted to obstetricians. Our study confirms that fetal MRI is a reliable diagnostic tool for demonstrating brain lesions in TSC and that the MRI findings seem to correlate well with the expected neurological symptoms. The TSCCC criteria can and should also be applied to fetal MRI.
References
Levine D, Barnes P, Korf B et al (2000) Tuberous sclerosis in the fetus: second-trimester diagnosis of subependymal tubers with ultrafast MR imaging. AJR 175:1067–1069
Hay WW, Groothuis JR, Hayward AR et al (1995) Tuberous sclerosis (Bourneville’s disease). In: Hay WW, Groothuis JR, Hayward AR et al (eds) Current pediatric diagnosis and treatment. Appleton & Lange, Norwalk, pp 753–754
Baron Y, Barkovich AJ (1999) MR imaging of tuberous sclerosis in neonates and young infants. AJNR 20:907–916
Griffiths PD, Martland TR (1997) Tuberous sclerosis complex: the role of neuroradiology. Neuropediatrics 28:244–252
Sonigo P, Elmaleh A, Fermont L et al (1996) Prenatal MRI diagnosis of fetal cerebral tuberous sclerosis. Pediatr Radiol 26:1–4
Yeo W, Leong A, Ward SC et al (1996) Hepatic angiomyelolipoma and tuberous sclerosis. J Gastroenterol Hepatol 11:196–198
Severien C, Kasten S, Kirchner T et al (2003) Pulmonale Manifestation bei tuberöser Sklerose. Monatsschr Kinderheilkd 151:1306–1310
Vargas-Gonzalez R, San Martin-Brieke W, Gil-Orduna C et al (2004) Desmoplastic fibroma-like tumor of maxillofacial region associated with tuberous sclerosis. Pathol Oncol Res 10:237–239
Nyberg DA, Jeanty P, Glass I (2003) Tuberous sclerosis. In: Nyberg DA, McGahan JP, Pretorius DH et al (eds) Diagnostic imaging of fetal anomalies. Lippincott Williams & Wilkins, Philadelphia, pp 176–178
Wagner M, Fitz H, Schindler C (1996) Early manifestation of polycystic kidney changes in tuberous sclerosis. Pathologe 17:462–466
Roach ES, Gomez MR, Northrup H (1998) Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol 13:624–628
Carette MF, Antoine M, Bazelly B et al (2006) Primary pulmonary artery aneurysm in tuberous sclerosis: CT, angiography and pathological study. Eur Radiol 16:2369–2370
Borelli S (1999) Koenen, Kothe and peri-ungal fibroma in tuberous sclerosis. Hautarzt 50:368–369
Barkovich AJ (2000) Tuberous sclerosis (Bourneville’s disease). In: Barkovich AJ (ed) Pediatric neuroimaging, 3rd edn. Lippincott Williams & Wilkins, Philadelphia, pp 404–415
Garel C (2004) Tuberous sclerosis complex. In: Garel C (ed) MRI of the fetal brain. Springer, Berlin, pp 172–173
Nellist M, Sancak O, Goedbloed MA et al (2005) Distinct effects of single amino-acid changes to tuberin on the function of the tuberin-hamartin complex. Eur J Hum Genet 13:59–68
Tee AR, Fingar DC, Manning BD et al (2002) Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA 99:13571–13576
van Slegtenhorst M, Nellist M, Nagelkerken B et al (1998) Interaction between hamartin and tuberin, the TSC1 and TSC2 gene products. Hum Mol Genet 7:1053–1057
van Slegtenhorst M, Verhoef S, Tempelaars A et al (1999) Mutational spectrum of the TSC1 gene in a cohort of 225 tuberous sclerosis complex patients: no evidence for genotype-phenotype correlation. J Med Genet 36:285–289
van Slegtenhorst M, de Hoogt R, Hermans C et al (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277:805–808
European Chromosome 16 Tuberous Sclerosis Consortium (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75:1305–1315
Jones AC, Shyamsundar MM, Thomas MW et al (1999) Comprehensive mutation analysis of TSC1 and TSC2-and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet 64:1305–1315
Yates JR, van Bakel I, Sepp T et al (1997) Female germline mosaicism in tuberous sclerosis confirmed by molecular genetic analysis. Hum Mol Genet 6:2265–2269
Jentarra G, Snyder SL, Narayanan V (2006) Genetic aspects of neurocutaneous disorders. Semin Pediatr Neurol 13:43–47
Narayanan V (2003) Tuberous sclerosis complex: genetics to pathogenesis. Pediatr Neurol 29:404–409
Jozwiak J (2006) Hamartin and tuberin: working together for tumour suppression. Int J Cancer 118:1–5
Rose VM, Au KS, Pollom G et al (1999) Germ-line mosaicism in tuberous sclerosis: how common? Am J Hum Genet 64:986–992
Roach ES, Sparagana SP (2004) Diagnosis of tuberous sclerosis complex. J Child Neurol 19:643–649
Kothur K, Ray M (2006) Antenatal MRI in the diagnosis of tuberous sclerosis. Indian Pediatr 43:1007–1008
Roach ES, DiMario FJ, Kandt RS et al (1999) Tuberous Sclerosis Consensus Conference: recommendations for diagnostic evaluation. National Tuberous Sclerosis Association. J Child Neurol 14:401–407
Shepherd CW, Houser OW, Gomez MR (1995) MR findings in tuberous sclerosis complex and correlation with seizure development and mental impairment. AJNR 16:149–155
Goh S, Butler W, Thiele EA (2004) Subependymal giant cell tumors in tuberous sclerosis complex. Neurology 63:1457–1461
Christophe C, Bartholome J, Blum D et al (1989) Neonatal tuberous sclerosis. US, CT, and MR diagnosis of brain and cardiac lesions. Pediatr Radiol 19:446–448
Sgro M, Barozzino T, Toi A et al (1999) Prenatal detection of cerebral lesions in a fetus with tuberous sclerosis. Ultrasound Obstet Gynecol 14:356–359
Kim BS, LaMarca V, Jallo GI (2002) Tuberous sclerosis. Pediatr Neurosurg 37:276–277
Brackley KJ, Farndon PA, Weaver JB et al (1999) Prenatal diagnosis of tuberous sclerosis with intracerebral signs at 14 weeks’ gestation. Prenat Diagn 19:575–579
Beghetti M, Gow RM, Haney I et al (1997) Pediatric primary benign cardiac tumors: a 15-year review. Am Heart J 134:1107–1114
Holley DG, Martin GR, Brenner JI et al (1995) Diagnosis and management of fetal cardiac tumors: a multicenter experience and review of published reports. J Am Coll Cardiol 26:516–520
Harding CO, Pagon RA (1990) Incidence of tuberous sclerosis in patients with cardiac rhabdomyoma. Am J Med Genet 37:443–446
Axt-Fliedner R, Qush H, Hendrik HJ et al (2001) Prenatal diagnosis of cerebral lesions and multiple intracardiac rhabdomyomas in a fetus with tuberous sclerosis. J Ultrasound Med 20:63–67
Crawford DC, Garrett C, Tynan M et al (1983) Cardiac rhabdomyomata as a marker for the antenatal detection of tuberous sclerosis. J Med Genet 20:303–304
Wallace G, Smith HC, Watson GH et al (1990) Tuberous sclerosis presenting with fetal and neonatal cardiac tumours. Arch Dis Child 65:377–379
Gushiken BJ, Callen PW, Silverman NH (1999) Prenatal diagnosis of tuberous sclerosis in monozygotic twins with cardiac masses. J Ultrasound Med 18:165–168
Roach ES, Smith M, Huttenlocher P et al (1992) Diagnostic criteria: tuberous sclerosis complex. Report of the Diagnostic Criteria Committee of the National Tuberous Sclerosis Association. J Child Neurol 7:221–224
Menor F, Marti-Bonmati L, Mulas F et al (1992) Neuroimaging in tuberous sclerosis: a clinicoradiological evaluation in pediatric patients. Pediatr Radiol 22:485–489
Chou PC, Chang YJ (2004) Prognostic factors for mental retardation in patients with tuberous sclerosis complex. Acta Neurol Taiwan 13:10–13
Cengiz M, Baysal Z, Ganidagli S (2006) Oral sedation with midazolam and diphenhydramine compared with midazolam alone in children undergoing magnetic resonance imaging. Paediatr Anaesth 16:621–626
de Amorim ES, Mackenzie A, Hallowell L et al (2006) Practice MRI: reducing the need for sedation and general anaesthesia in children undergoing MRI. Australas Radiol 50:319–323
Gottschling S, Larsen R, Meyer S et al (2005) Acute pancreatitis induced by short-term propofol administration. Paediatr Anaesth 15:1006–1008
Kitsa P, Andronikou S, Cardoso JF (2004) Sedation of children undergoing MRI – a risky business! S Afr Med J 94:625–626
Sury MR, Harker H, Begent J et al (2005) The management of infants and children for painless imaging. Clin Radiol 60:731–741
Brugger PC, Stuhr F, Lindner C et al (2006) Methods of fetal MR: beyond T2-weighted imaging. Eur J Radiol 57:172–181
Garel C (2005) Fetal cerebral biometry: normal parenchymal findings and ventricular size. Eur Radiol 15:809–813
Garel C, Chantrel E, Elmaleh M et al (2003) Fetal MRI: normal gestational landmarks for cerebral biometry, gyration and myelination. Childs Nerv Syst 19:422–425
Glenn OA, Barkovich AJ (2006) Magnetic resonance imaging of the fetal brain and spine: an increasingly important tool in prenatal diagnosis, part 1. AJNR 27:1604–1611
Acknowledgement
We are grateful to our colleague Dr. Marc Dewey for reading the manuscript and making many valuable suggestions for improvement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mühler, M.R., Rake, A., Schwabe, M. et al. Value of fetal cerebral MRI in sonographically proven cardiac rhabdomyoma. Pediatr Radiol 37, 467–474 (2007). https://doi.org/10.1007/s00247-007-0436-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-007-0436-y