Abstract
Background: Image-guided core needle biopsy is widely used in paediatric oncology, but many protocols continue to discourage this practice. No published randomized studies compare image-guided needle biopsy with surgical techniques. Objective: To perform a systematic review of the literature on image-guided core needle biopsy in paediatric oncology. Materials and methods: Several computerized databases were searched using the terms [(needle OR core) AND (biops*[ti]) AND (paediatric OR pediatric OR child OR children OR childhood OR boy OR girl)[ti]] to identify series of more than five cases of needle core biopsy for tumour diagnosis in children. Data from included studies were combined to calculate pooled estimates of adequacy, accuracy and complication rates. Results: Thirteen studies fulfilled the inclusion criteria. Overall biopsy adequacy rate (defined as sufficient to make a diagnosis) was 94% (95% CI 92–96%). The diagnostic accuracy rate in cases with adequate material (defined as achieving the correct specific diagnosis) was 94% (95% CI 92–96%). Complications requiring treatment occurred in 1%. Conclusions: Available pooled data suggest that about 95% of image-guided needle core biopsies provide an adequate sample for diagnosis of malignant disease in childhood. In such cases, the pathological diagnosis is correct in about 95%. Complications are rare.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of needle core biopsies for primary histopathological diagnosis of paediatric tumours is now routine practice in many centres, while others continue to rely on open surgical biopsies or primary surgical resection to obtain a tissue diagnosis. This is exemplified by the differences in management of primary renal masses in childhood between the protocols of the United Kingdom Children’s Cancer Study Group (UKCCSG) and the Children’s Oncology Group (COG). UKCCSG recommends primary needle core biopsy diagnosis followed by preoperative chemotherapy, whereas COG requires primary nephrectomy for most patients. The argument against the use of needle core biopsies for diagnosis is based on the assumption that there will be inadequate tissue to obtain a histopathological diagnosis and appropriate molecular studies, and concerns over needle tract seeding of tumour. The aim of this review is to examine the use of needle core biopsies for primary diagnosis of paediatric tumours from the published literature in the form of a systematic review.
Materials and methods
A search of several computerized databases (PUBMED, Science Citation Index (ISI), The Cochrane Collaboration, CINAHL and GOOGLE) was carried out across the full year ranges to identify publications of potential interest using the search terms [(needle OR core) AND (biops*[ti]) AND (paediatric OR pediatric OR child OR children OR childhood OR boy OR girl)[ti]], with no language restrictions. The titles and abstracts of identified citations were then examined in order to determine whether they potentially met the study inclusion criteria, being series reporting on the use of needle core biopsy for tumour diagnosis in more than five patients aged less than 18 years. Studies reporting only fine-needle aspiration biopsies, rather than core or cutting needle biopsies, were excluded. The full text was obtained of studies potentially fulfilling the criteria and the manuscripts were further examined in detail for inclusion and exclusion criteria. The included studies were then scored for quality according to a checklist and their reference lists manually checked for additional studies. The results of the final included citations were then tabulated and are presented. Where possible, data were combined to allow calculation of pooled estimates of adequacy, accuracy and complication rates.
Results
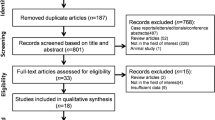
The initial computerized search identified 134 possible relevant citations. A total of 116 were excluded following screening of the titles and abstracts, primarily because they were either case reports only, reported on fine-needle aspiration cytology rather than core biopsy, or were not limited to paediatric tumour diagnosis. This resulted in 18 potentially includable studies, for which the full text was obtained. Following review of the full text a further five studies were excluded because they provided incomplete assessment of malignant disease or other inadequate details. This left 13 studies that fulfilled the inclusion criteria. Quality assessment of the studies was carried out according to whether the study population was clearly defined, whether the technique of needle biopsy was defined, and whether clear outcome criteria were provided including methods of determining diagnostic accuracy and complications. The results of the studies with their quality assessments are provided in Tables 1 and 2 and Figs. 1 and 2 [1–13].
The 13 studies included data on 698 needle core biopsies performed for the diagnosis of paediatric tumours. The pooled data demonstrate that the overall adequacy rate (defined as the biopsy sample being deemed sufficient by the reporting pathologist to make a diagnosis) was 94% (95% CI 92–96%; Table 1, Fig. 1). The diagnostic accuracy rate (defined as correct specific diagnosis following further investigations such as repeat biopsy, resection or clinical follow-up) in the cases with adequate material was 94% (95% CI 92–96%). The diagnostic ‘error’ rate varied between studies from 0% to 16%. Complications requiring treatment other than simple analgesia, most usually haemorrhage requiring transfusion, were reported in 1% of cases. Needle tract tumour recurrence was not reported in any case in these series.
Discussion
The findings of this study, which systematically reviews data from all studies reporting on the use of needle core biopsies for the diagnosis of paediatric tumours, have demonstrated that tissue adequate for diagnosis is obtained in almost 95% of cases. The assessment of ‘adequacy’ is, however, somewhat subjective, since a small amount of tissue demonstrating characteristic and diagnostic features may be ‘adequate’ whereas a larger biopsy demonstrating part of a fibrous or stromal area, for example, may be inadequate for the pathologist to make definitive diagnostic comment. In this regard, it is unclear from many of the publications how many cores were taken, or deemed adequate. Furthermore, it is also uncertain whether adequacy, and ‘diagnosis’ in terms of accuracy, was considered to be confirmation of a malignant tumour consistent with the clinical diagnosis, or a definitive diagnosis of the tumour type or subtype. Nevertheless, according to the criteria described in these publications, it is clear that the sample obtained for histological analysis is adequate for clinically meaningful pathological examination in the vast majority of cases. Furthermore, in the studies that provided such information, almost all cases in which the initial biopsy was deemed inadequate yielded adequate samples on repeat biopsy [5].
Secondly, diagnostic accuracy is high, being 94% from pooled data. The method of ascertainment of ‘accuracy’ in this setting is also prone to variations of definition, since in some studies this was assessed by comparing the diagnosis from needle core biopsy with that following examination of the resected specimen. In other cases, however, especially those in which the clinical, imaging and core biopsy diagnoses were all suggestive of non-malignant disease, further resection was not carried out and the needle biopsy diagnosis was deemed correct if the clinical behaviour of the patient was as expected on the basis of the biopsy diagnosis, and no treatment for possible malignant disease was required. Given these caveats, the pooled data indicate that in cases in which adequate samples were obtained, the accuracy of the core biopsy diagnosis is high, being about 95% (range 84–100%).
Classical histopathological texts usually concentrate on detailed descriptions of the architectural and cytological features of tumours demonstrated on the basis of resection specimens. The features apparent in a small needle core biopsy specimen will by definition, be more limited, and it is possible that some of the cases deemed inadequate or inaccurate in these series may have been due to lack of experience by the reporting pathologist in reporting such specimens. Increasing experience with needle biopsy specimens may lead to recognition of more subtle diagnostic clues on microscopy. Furthermore, and much more importantly, many malignant tumours of childhood show characteristic findings with a limited panel of immunohistochemical markers, enabling a specific diagnosis to be made in many cases in which the light microscopy may simply demonstrate a tumour with a ‘small round cell’ phenotype. Examples are expression of strong, uniform membranous CD99 positivity in primitive neuroectodermal tumours (PNET), expression of CD56 and nuclear WT1 in the blastema of Wilms tumour and expression of desmin and nuclear myogenin in cases of rhabdomyosarcoma. Such immunohistochemical panels require only small amounts of tissue, which can be obtained from the paraffin block, and therefore do not necessitate splitting of the specimen. Unstained sections can be readily obtained for immunohistochemistry as part of routine handling of all core biopsies in specialist units, where the interpretation of all these paediatric biopsies should be carried out.
In addition, an increasing number of paediatric soft tissue malignancies now demonstrate characteristic and diagnostic gene fusions that can be readily demonstrated using fluorescence in-situ hybridisation (FISH) with specific probes on touch preparations, or reverse transcriptase polymerase chain reaction (RT-PCR), either using traditional gel-based methods or via real-time TAQMAN-based analyses. The amount of tissue required for such molecular analysis is very small: approximately 2 mm of a standard core biopsy is sufficient if received fresh and immediately placed in RNA-protection medium (RNAlater, Sigma-Aldrich, St. Louis, Mo.). This will allow determination for the presence of a panel of molecular fusion transcripts, covering most paediatric sarcomas, such as PNET (EWS-FLI and EWS-ERG fusions), alveolar rhabdomyosarcoma (PAX3-FKHR and PAX7-FKHR), desmoplastic small round cell tumour (EWS-WT1) and synovial sarcoma (SSX-SYT). With the more routine use of such diagnostic techniques, definitive diagnosis will be increasingly possible on more limited samples [14].
Finally, a potential argument against the use of needle core biopsies for paediatric tumour diagnosis is that the availability of such limited material does not allow adequate prognostic markers to be evaluated. In neuroblastic tumours, for example, the presence of calcification and a high mitosis-karyorrhexis index have been suggested as adverse prognostic markers. However, these data were obtained prior to the widespread routine determination of biological markers in neuroblastoma such as MYCN copy number and 1p and 17q status, and there is now strong evidence that there is an association between MYCN copy number and the previously described histopathological findings [15]. Indeed, recent publications suggest no additional prognostic significance of such histopathological features once stage and biological marker status has been accounted for [16, 17]. It is routinely possible to assess such molecular markers using FISH and other molecular diagnostic techniques on touch or needle biopsy preparations [1], and it is highly likely that molecular profiling of tumours will play an increasingly important clinical role in the diagnosis, prognostication and determination of further management of malignant tumours.
Needle core biopsies are usually performed with some form of imaging guidance, even when the tumour is easily palpable. Most operators prefer US guidance [3, 7, 9] and reserve CT for lesions that are surrounded by air-filled structures, such as non-peripheral lung lesions [3, 8]. Image guidance allows the operator to sample viable tumour and avoid obviously necrotic areas, as well as selecting a biopsy tract that avoids other organs and major blood vessels and minimises the consequences of needle tract seeding of tumour [1]. It is possible that functional imaging techniques (such as MRI and PET) will further refine the detection of areas of viable tumour for biopsy.
It is widely accepted that needle core biopsy is superior to fine-needle aspiration cytology in paediatric oncology [9]. The size of the needle used varied widely in the studies included in this review. In general, larger needle sizes (15–18 gauge, 1.8–1.2 mm) are used for renal, hepatic and soft-tissue lesions, whereas lung lesions are usually biopsied with smaller needles (18–21 gauge, 0.8–1.3 mm). Complications were absent or rare in the studies in this report. The main immediate risk of needle core biopsy appears to be clinically significant bleeding, although pneumothorax may occur after biopsy of lesions in the lungs or mediastinum [1, 3, 4, 9]. Coaxial biopsy techniques may reduce the incidence of early complications such as haemorrhage, as well as the risk of needle tract recurrence. Although it is sometimes stated that surgical biopsy is safer than image-guided needle core biopsy [18], this is not supported by the limited literature on this subject [19].
Although not stated in all series, several authors comment that an inadequate biopsy is not simply associated with biopsy size, number of cores or operator experience [3, 4]. These issues will be difficult to resolve without a randomized trial, as there are obvious biases in retrospective studies. For example, the more difficult cases were probably performed by more experienced operators, and more cores may well have been taken from necrotic tumours.
It is likely that the accuracy of needle core biopsy in paediatric oncology depends strongly on the type of biopsy performed and the enthusiasm of the radiologists and pathologists involved. Biopsy of very small lesions, particularly in the lung, is difficult, whereas large soft-tissue tumours are easy to biopsy and the accuracy in this context is probably very high. Prospective studies should therefore concentrate on specific clinical situations, for example suspected neuroblastoma or lung metastases, and the results should be analysed in terms of the size and location of the tumours.
In summary, the available pooled data suggest that image-guided needle core biopsies provide an adequate sample for the diagnosis of malignant disease in childhood in around 95% of cases at the first attempt, and in such cases the pathological diagnosis is correct in about 95%. Complications are rare and usually of minor clinical consequence. With increasing specialization of paediatric tumour diagnosis (and future developments in image-guided sampling and molecular profiling of tumours for diagnosis and prognosis) it is likely that these results will improve significantly.
References
Garrett KM, Fuller CE, Santana VM, et al (2005) Percutaneous biopsy of pediatric solid tumors. Cancer 104:644–652
Guimaraes AC, Chapchap P, de Camargo B, et al (2003) Computed tomography-guided needle biopsies in pediatric oncology. J Pediatr Surg 38:1066–1068
Skoldenberg EG, Jakobson AA, Elvin A, et al (2002) Diagnosing childhood tumors: a review of 147 cutting needle biopsies in 110 children. J Pediatr Surg 37:50–56
Hussain HK, Kingston JE, Domizio P, et al (2001) Imaging-guided core biopsy for the diagnosis of malignant tumors in pediatric patients. AJR 176:43–47
Sklair-Levy M, Lebensart PD, Applbaum YH, et al (2001) Percutaneous image-guided needle biopsy in children–summary of our experience with 57 children. Pediatr Radiol 31:732–736
Willman JH, White K, Coffin CM (2001) Pediatric core needle biopsy: strengths and limitations in evaluation of masses. Pediatr Dev Pathol 4:46–52
Bain G, Bearcroft PW, Berman LH, et al (2000) The use of ultrasound-guided cutting-needle biopsy in paediatric neck masses. Eur Radiol 10:512–515
Connolly BL, Chait PG, Duncan DS, et al (1999) CT-guided percutaneous needle biopsy of small lung nodules in children. Pediatr Radiol 29:342–346
Hugosson CO, Nyman RS, Cappelen-Smith JM, et al (1999) Ultrasound-guided biopsy of abdominal and pelvic lesions in children. A comparison between fine-needle aspiration and 1.2-mm needle core biopsy. Pediatr Radiol 29:31–36
Somers JM, Lomas DJ, Hacking JC, et al (1993) Radiologically-guided cutting needle biopsy for suspected malignancy in childhood. Clin Radiol 48:236–240
Saarinen UM, Wikstrom S, Koskimies O, et al (1991) Percutaneous needle biopsy preceding preoperative chemotherapy in the management of massive renal tumors in children. J Clin Oncol 9:406–415
Klose KC, Mertens R, Alzen G, et al (1991) CT-guided percutaneous large-bore biopsies in benign and malignant pediatric lesions. Cardiovasc Intervent Radiol 14:78–83
Sabbah R, Ghandour M, Ali A, et al (1981) Tru-cut needle biopsy of abdominal tumors in children: a safe and diagnostic procedure. Cancer 47:2533–2535
Chang CC, Shidham VB (2003) Molecular genetics of pediatric soft tissue tumors: clinical application. J Mol Diagn 5:143–154
Shimada H, Stram DO, Chatten J, et al (1995) Identification of subsets of neuroblastomas by combined histopathologic and N-myc analysis. J Natl Cancer Inst 87:1470–1476
Simon T, Spitz R, Faldum A, et al (2004) New definition of low-risk neuroblastoma using stage, age, and 1p and MYCN status. J Pediatr Hematol Oncol 26:791–796
Mora J, Cheung NK, Chen L, et al (2001) Survival analysis of clinical, pathologic, and genetic features in neuroblastoma presenting as locoregional disease. Cancer 91:435–442
Pinarli FG, Danaci M, Tander B, et al (2004) Bilateral adrenal cystic neuroblastoma with superior vena cava syndrome and massive intracystic haemorrhage. Pediatr Radiol 34:746–749
Squire R, Willetts I (1999) Problems with biopsying solid tumours in children (abstract). Med Pediatr Oncol 34:344
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sebire, N.J., Roebuck, D.J. Pathological diagnosis of paediatric tumours from image-guided needle core biopsies: a systematic review. Pediatr Radiol 36, 426–431 (2006). https://doi.org/10.1007/s00247-006-0123-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-006-0123-4