Abstract
Autism is a pervasive neurodevelopmental disorder. Because of the deficits associated with the condition, sedation of children with autism has been considered more challenging than sedation of other children. Objective: To test this hypothesis, we compared children with autism against clinical controls to determine differences in requirements for moderate sedation for MRI. Materials and methods: Children ages 18–36 months with autism (group 1, n = 41) and children with no autistic behavior (group 2, n = 42) were sedated with a combination of pentobarbital and fentanyl per sedation service protocol. The sedation nurse was consistent for all patients, and all were sedated to achieve a Modified Ramsay Score of 4. Demographics and doses of sedatives were recorded and compared. Results: There were no sedation failures in either group. Children in group 1 (autism) were significantly older than group 2 (32.02±3.6 months vs 28.16±6.7 months) and weighed significantly more (14.87±2.1 kg vs 13.42±2.2 kg). When compared on a per-kilogram basis, however, group 1 had a significantly lower fentanyl requirement than group 2 (1.25±0.55 mcg/kg vs 1.57±0.81 mcg/kg), but no significant difference was found in pentobarbital dosing between groups 1 and 2, respectively (4.92±0.92 mg/kg vs 5.21±1.6 mg/kg). Conclusion: Autistic children in this age range are not more difficult to sedate and do not require higher doses of sedative agents for noninvasive imaging studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism is a complex neurodevelopmental disorder defined by the presence of social deficits, abnormalities in communication, the presence of stereotyped, repetitive behaviors, and a characteristic course [1]. In addition, up to 75% of autistic children also have comorbid mental retardation. As a result of their difficulty adjusting to changes in routine and the environment, children with autism have often been described as difficult to sedate or anesthetize, which creates a challenge in obtaining diagnostic studies that require the child to be motionless [2–4]. A variety of sedation regimens have been used in children; however, no study has specifically addressed moderate sedation for children with autism. The more common sedatives such as chloral hydrate might not adequately sedate a child with autism, and intravenous agents remain mostly untested in this population [2, 5]. We reviewed the sedation records of 41 autistic children between the ages of 18 and 36 months who received sedation for MRI and compared this group with 43 clinical controls in the same age range to determine any differences in the sedation requirements between children with and without autism. Our findings will help to guide sedation protocols for autistic children in the age group tested.
Materials and methods
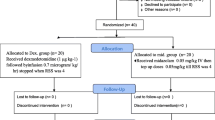
After IRB approval and appropriate HIPAA compliance measures, a retrospective chart review was performed on two groups of children ages 18–36 months who underwent moderate sedation for MRI. All children were sedated for their MRI per the Duke University Medical Center Department of Radiology Sedation Protocol. The parents filled out a questionnaire regarding medical history of the child, and nil per os (NPO) status was confirmed. A complete history was obtained and a physical was performed by the physician responsible for ordering the sedation medications, and the sedative medications were delivered by a trained radiology sedation nurse. For this age range of patients, the sedation protocol required the placement of an intravenous line and the delivery of alternating doses of fentanyl and pentobarbital until sleep was achieved, consistent with a Modified Ramsay Score of 4 (Table 1). Oral premedication is not a part of the Radiology Sedation Protocol. Fentanyl 1 μg/kg followed by pentobarbital 1–3 mg/kg was delivered and observed for effect. Any additional doses were at the discretion of the sedation nurse (CW) and would not exceed either 1 μg/kg fentanyl or 1 mg/kg pentobarbital per dose. The total doses could not exceed the protocol’s maximum limits of fentanyl 4 μg/kg and pentobarbital 8 mg/kg. Vital signs were collected at baseline and recorded every 5 min during the sedation and MRI scan. Pulse oximetry, respiratory rate, and EKG were monitored continuously, and blow-by oxygen was delivered to all children throughout the scanning period. After the completion of the scan, discharge from the recovery area occurred when discharge criteria were met. Discharge criteria included the ability to sit up unaided and a stable cardiovascular and respiratory status. Parents were given an instruction sheet with an emergency contact number for questions or concerns.
Group 1 (41 children) had a diagnosis of autism and were participants in a separate study funded by the National Institutes of Health conducted by our research team that included diagnostic imaging with MRI of the brain. The children were initially identified as having a diagnosis of autistic spectrum disorder by one of nine specialty clinics for pervasive developmental disorders in North Carolina and referred for the imaging study. Children were excluded from this group if they had a medical condition that might have been associated with autism, such as fragile X syndrome, cerebral palsy, seizures, or significant motor or sensory impairments. After being identified as having autistic spectrum disorder, the children were tested and included in the imaging study if they met the DSM-IV criteria for diagnosis of autism, autism diagnostic interview-revised (ADI-R) algorithm criteria, and if they obtained autism diagnostic observation schedule-G (ADOS-G) scores consistent with autism [6, 7]. If the diagnosis of autism was confirmed, the children were enrolled for the imaging study. An MRI of the brain was performed with the child under moderate sedation using the protocol of alternating pentobarbital and fentanyl at the discretion of the radiology sedation nurse. For this investigation, one consistent radiology sedation nurse (CW) administered the sedative medication, and one pediatric anesthesiologist (AKR) was present to perform the history and physical examination and to supervise the sedation procedure. All medications and monitoring were performed under the guidelines of the Duke Sedation Policy as previously described.
Data from group 2 (43 children) were acquired through review of the radiology sedation suite database and served as the control group. These children required sedation for an MRI of the brain as part of a workup of their various disease states. Information on each child at Duke who has sedation for an MRI is recorded in a database that includes diagnosis, medications used, sedation nurse, and any adverse events. This database was searched for children who would be suitable controls to compare with our population of children with autism. Suitable controls included children who met the following criteria: child was 18–36 months, was sedated by the same sedation nurse (CW) from group 1, and received the fentanyl/pentobarbital regimen.
The data collected included age, weight, sex, race, total fentanyl dose (μg/kg), and total pentobarbital dose (mg/kg). Groups were compared using SPSS Version 11.0 with respect to demographics and total doses required to achieve a Modified Ramsay Score of 4.
Results
There were no sedation failures or adverse events recorded for either group. Group demographics are presented in Table 2. Initial group comparisons on group characteristics were conducted using an independent samples t-test. There were statistically significant group differences between group 1 (autism) and group 2 (controls) with respect to age and weight, with the autistic group being of greater age and weight. In group 1 there were 28 white children, 7 Afro-American, 1 Asian, 1 Hispanic and 4 other ethnic groups represented. Group 2 included 30 white, 10 Afro-American, 1 Asian and 2 Hispanic children.
Comparisons of total doses of fentanyl and pentobarbital for the two groups are presented in Table 3. There was a statistically significant difference between the groups with respect to total dose of fentanyl administered on a per-kilogram basis, as group 2 (controls) had higher required doses for effect.
Discussion
This paper demonstrates two important points. The first point is that children with autism who are 18–36 months of age are not more difficult to sedate than clinical controls. The second point is that an established sedation service that is successful for the average child might be an essential component of successful sedation in children who present with behavioral challenges.
Children with autism have historically been considered difficult to manage for medical procedures [3, 8, 9]. The dental literature in particular addressed early the management of the autistic child who was uncooperative, yet required extensive dental care [4, 10, 11]. Although some of these children might be managed in the dental office for straightforward dental work, up to 37% of autistic children require a general anesthetic in an operating room for comprehensive dental care or difficult procedures [12].
Our review is the first to describe the efficacy of a regimen of sedative agents in the young autistic child undergoing a noninvasive radiological procedure. Oral clonidine in doses of 2–7 μg/kg has been used in autistic children to perform electroencephalograms (EEGs) with a sedation rate of 85% [2]. For preoperative sedation, oral ketamine has been used successfully in autistic children; however, ketamine also comes with undesirable side effects such as emergence delirium [3, 13, 14]. It is unknown whether these agents would be successful for an autistic child receiving an MRI.
At Duke, a combination of pentobarbital and fentanyl has been the pharmacological regimen for MRIs in the pediatric sedation program for children who are older than 1 year. Pentobarbital has been used successfully for sedating pediatric patients in radiology suites either alone or in combination with other sedatives or narcotics for many years [15–18]. In fact, the use of pentobarbital either alone or with fentanyl should provide a failure rate of less than 1% [17–20]. Although serious events are extremely rare, paradoxical reactions that are defined as extreme, inconsolable irritability for more than 30 min after administration of pentobarbital can occur in 1.2–14% of children [19, 20]. Midazolam has been used in an attempt to control paradoxical reactions to pentobarbital, but is not routinely used for sedation, as there is no evidence of a beneficial effect from its use, including the reduction of paradoxical reactions. The addition of a second drug to pentobarbital sedation must only be done with strict adherence to maximum allowable dosing and vigilant monitoring. Fentanyl has been used as the second agent with success and safety. When end-tidal carbon dioxide was measured in 165 children who received either pentobarbital 2–6 mg/kg alone or in combination with fentanyl 1–3 μg/kg for MRI sedation, the end-tidal remained between 37 and 42 mmHg during sedation in both groups, whether fentanyl was used or not [21]. Although some practitioners remain committed to using pentobarbital alone, Duke adopted the combination approach and has used this successfully for pediatric patients receiving clinical scans as well as study scans.
Rather than focus on specific sedative regimens, previous reports have presented the advantages of outlining a process and system to minimize the stress and successfully manage children with autism who present for medical procedures [8]. A successful periprocedure process for autistic children should include the following: early notification to practitioners, family education prior to the procedure date, a quiet room near the procedure suite, the option of oral premedication after discussion with the family, and rapid return to normal home environment [8, 9]. The child’s routine should be kept as constant as possible, and there should be minimal separation from the attachment figure (usually the mother) [9]. It is also recommended that distraction techniques such as computer games or music be used or be made available [9].
A prospective audit of the experience of 59 autistic children requiring a general anesthetic was reported to describe a system to optimize the overall perioperative management [3]. This process included a telephone interview and questionnaire focusing on the patient’s required information as well as a checklist that was specific to the child’s autistic needs such as developmental level and likes or dislikes. The anesthetic plan is developed at that point and shared with all members of the care team. One component of the anesthetic plan was the preoperative sedation that included either midazolam 0.5 mg/kg for the mildly autistic children or ketamine 7 mg/kg for the moderate and severe autistic children. By incorporating the perioperative experience into an “Autistic Register,” the information can be used for subsequent visits by the child. Out of the 59 children who were in the register, 21 of them required two or three procedures. The authors’ conclusion was that good results are possible when this type of program with coordinated efforts is in place.
These papers validate the second point of our report, the advantages of a sedation routine that provides familiarity for the practitioners as well as a consistent environment for the mentally challenged patient. With the published guidelines for sedation and monitoring of children by the American Academy of Pediatrics (AAP) and American Society of Anesthesiology (ASA), there has been more consistency with types of sedation, personnel, and basic requirements for safe practice [22]. Additionally, presedation assessment and repeated assessment of sedation score as recommended by the guidelines of the ASA and AAP reduce the risk of deep sedation [23]. The practice of safe sedation with familiar protocols and consistent guidelines is not only advantageous for sedating a child without cognitive impairment, but is imperative in sedating a child who presents challenges, such as the autistic child. Reviews that address pediatric sedation have confirmed that an established sedation service with appropriately trained personnel is essential to the safety and success of a program [24, 25].
An additional layer of safety that was evident in our report was the presence of a pediatric anesthesiologist to perform the history and physical examination and supervise the sedation regimen. Although a pediatric anesthesiologist might not be a necessary component of an established sedation service for routine clinical scans, when children are sedated for investigational purposes, the addition of a physician who is trained in advanced airway management adds an invaluable service of protecting the safety of a research subject. Although parental satisfaction was not measured, there were no complaints regarding the overall sedation or MRI experience, and no parents have turned down the opportunity to have their child scanned again at later ages (presently ongoing Time 2 scanning study through NIH-J. Piven). This cooperation leads one to believe that the parents of autistic children appreciate the gains that are being made in the field of autism through research.
One issue that might be raised with this review is the difference between groups with respect to demographics. The preponderance of boys to girls in group 1 is a function of the demographics of autism, as this condition has a male-to-female ratio of 4:1. There was also a statistically significant difference between the groups when age and weight were compared. Clinically, the difference between the sedation requirements of a 28 month old and a 32 month old should be insignificant, as the pharmacokinetic principles would be nearly identical for these ages. The fact that the control group required significantly more fentanyl per kg to achieve a Modified Ramsay Score of 4 than the autism group only further supports the suggestion that autistic children in this age group are not more difficult to sedate.
In summary, autistic children are a unique population of children that present practitioners with additional challenges. There should be processes in place to make the child and parent comfortable when presented with a change in routine such as an imaging study in a hospital setting. In our study we have shown that in autistic children between the ages of 18 and 36 months there are no increased requirements in the doses of sedative medications needed for safe and effective sedation for MRI scans. This information should help practitioners in managing this young age group of children with autism and spark interest in testing the same hypothesis in the older, perhaps more challenging, autistic child. Additional study of the sedation of autistic children using greater numbers of patients is warranted to provide a true idea of success and failure in the various ages of this unique pediatric population.
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. DSM-IV, Washington, DC
Mehta UC, Patel I, Castello FV (1994) EEG sedation for children with autism. Dev Behav Ped 25:102–104
Van der Walt JH, Moran C (2001) An audit of perioperative management of autistic children. Paediatr Anaesth 11:401–408
Davila JM, Jensen OE (1988) Behavioral and pharmacological dental management of a patient with autism. Spec Care Dent 8:58–60
Rumm PD, Takato RT, Fox DJ, et al (1990) Efficacy of sedation of children with chloral hydrate. South Med J 83:1040–1043
Lord C, Rutter M, LeCouteur A (1994) Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685
Lord C, Rutter M, Goude S, et al (1989) Autism diagnostic observation scale: a standardized observation of communicative and social behavior. J Autism Dev Disord 19:185–212
Rainey L, van der Walt JH (1998) The anaesthetic management of autistic children. Anaesth Intensive Care 26:682–686
Seid M, Sherman M, Seid AB (1997) Perioperative psychosocial interventions for autistic children undergoing ENT surgery. Int J Pediatr Otorhinolaryngol 40:107–113
Kopel HM (1977) The autistic child in dental practice. J Dent Child 44:302–309
Braff MH, Nealon L (1979) Sedation of the autistic patient for dental procedures. J Dent Child 46:404–407
Klein U, Nowak AJ (1999) Characteristics of patients with autistic disorder (AD) presenting for dental treatment: a survey and chart review. Spec Care Dent 19:200–207
Gutstein HB, Johnson KL, Heard MB, et al (1992) Oral ketamine preanesthetic medication in children. Anesthesiology 76:28–33
Donahue PJ, Dineen PS (1992) Emergence delirium following oral ketamine. Anesthesiology 77:604–605
Strain JD, Campbell JB, Harvey LA, et al (1988) IV Nembutal: safe sedation for children undergoing CT. AJR 151:975–979
Frush D, Bissett G, Hall S (1996) Pediatric sedation in radiology: the practice of safe sleep. AJR 167:1381–1387
Mason KP, Zurakowski D, Karian VE, et al (2001) Sedatives used in pediatric imaging: comparison of IV pentobarbital with IV pentobarbital with midazolam added. AJR 177:427–430
Karian VE, Burrows PE, Zurakowski D, et al (1999) Sedation for pediatric radiologic procedures: analysis of potential causes of sedation failure and paradoxical reactions. Pediatr Radiol 29:869–873
Karian VE, Burrows PE, Zukarowski D, et al (2002) The development of a pediatric radiology sedation program. Pediatr Radiol 32:348–353
Malviya S, Voepel-Lewis T, Tait AR, et al (2004) Pentobarbital versus chloral hydrate for sedation of children undergoing MRI: efficacy and recovery characteristics. Paediatr Anaesth 14:589–595
Connor L, Burrows PE, Zurakowski D, et al (2003) Effects of IV pentobarbital with and without fentanyl on end-tidal carbon dioxide levels during deep sedation of pediatric patients undergoing MRI. AJR 181:1601–1694
American Academy of Pediatric Committee on Drugs (1992) Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics 89:1110–1115
Hoffman GM, Nowakowski R, Troshynski TJ, et al (2002) Risk reduction in pediatric procedural sedation by application of an American Academy of Pediatrics/American Society of Anesthesiologists process model. Pediatrics 109:236–243
Cote CJ, Notterman DA, Karl HW, et al (2000) Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics 105:805–814
Cravero JP, Blike GT (2004) Review of pediatric sedation. Anesth Analg 99:1355–1364
Acknowledgements
The authors would like to recognize Michele Poe, PhD, for her statistical support on this project, as well as all members of the Autism Research Group. Research supported by NIH Grant MH61696 (J. Piven) and NIH MRDDRC Grant 5 P30 HD03110 (J. Piven).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ross, A.K., Hazlett, H.C., Garrett, N.T. et al. Moderate sedation for MRI in young children with autism. Pediatr Radiol 35, 867–871 (2005). https://doi.org/10.1007/s00247-005-1499-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-005-1499-2