Abstract
The growing population of childhood cancer survivors—currently estimated at 1 in 900 young adults aged 15–45 years—underscores the importance of studying long-term complications of oncotherapy. While these patients are returning to the mainstream of life, they carry with them toxicities from prior therapy that may compound or potentiate changes typically seen with the normal aging process. Skeletal toxicities such as scoliosis, craniofacial dysplasia, and limb-length discrepancy are readily apparent. However, others such as osteoporosis and osteonecrosis are silent until they reach advanced stages when attempts at amelioration may be unsuccessful. This review addresses bone-mineral density deficits that may predispose childhood cancer survivors to earlier onset and more severe osteopenia and osteoporosis than the normal population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The evolution of multiagent chemotherapy, improved surgical techniques, refined radiation therapy, and the development of intricate imaging technologies for earlier staging and monitoring of disease response have led to an ever growing population of childhood cancer survivors with an overall estimated survival of 60–77% [1, 2, 3]. It is estimated that 1 in every 250 young adults aged 15–45 years will be a childhood cancer survivor by the year 2010 [4]. Thus, we are now faced with the task of identifying and ameliorating long-term adverse effects of cancer and its therapy. Many of these skeletal toxicities, such as scoliosis, craniofacial dysplasia, and limb-length discrepancy, are readily apparent. However, other toxicities such as osteoporosis and osteonecrosis are silent until they reach advanced stages when they may compromise quality and duration of survival and be more difficult to ameliorate.
Inasmuch as children and young adults are at the most rapidly developing stages of their lives when childhood cancer is diagnosed, the disease and its therapy can severely compromise normal growth and development and present deformities and handicaps that can last a lifetime.
As pediatric radiologists, we need to understand the mechanisms of skeletal development and monitor skeletal health. We are often the first line of assessment of the pediatric skeleton, which is included on virtually every image we review. We need to expand our expertise to indentifying signs of bone-mineral loss and direct the clinicians to proper assessment of bone density—quantitative computed tomography (QCT), dual-energy X-ray absorptiometry (DXA), ultrasound, and radiographs. This review will address bone-mineral density (BMD) deficits in survivors of childhood cancer.
Bone-mineral density deficits
In the general population, osteoporosis can be included in the growing list of adult diseases that originate during childhood and adolescent development [5, 6, 7, 8]. Worldwide, inadequate dietary intake of calcium is prevalent in today’s adolescents [6, 9, 10]. Further, the majority of the normal adolescent population gets minimal to no exercise and does not approach the current recommendation of at least 30 min of weight-bearing exercise 3–5 times per week [11, 12, 13, 14]. Unfortunately, the presence of osteoporosis is not typically detected until fractures occur and is seldom considered as a health hazard in pediatric patients. Once osteoporosis is established in adulthood, improvement in bone-mineral deficits is very difficult to achieve.
It is well proven that children receiving chronic steroid therapy are at risk for bone-mineral deficits [15, 16]. However, until recently, little attention has been paid to childhood cancer survivors who are at risk for bone-mineral deficits related to disease and treatment. There is convincing evidence that these children and young adults are at risk for BMD deficits over and above that seen in the general age-comparable population (see below). When lifestyle factors that compromise bone-mineral accretion in the general population—smoking, carbonated beverages, low levels of activity—are compounded by the altered lifestyle that typically occurs during treatment for childhood cancer—nutritional deficits, prolonged sedentary lifestyle, secondary physiologic effects created by the disease itself and by the onco-therapy—bone-mineral accretion is severely compromised [5, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25].
Bone-mineral accretion is typically most rapid during adolescence when growth hormones and sex hormones are most active and when calcium absorption is greatest [5, 6, 9, 13, 22, 26, 27]. However, the myriad endocrinopathies from which many childhood cancer survivors suffer compound the effects of any nutritional deficits they may have incurred during therapy, and their relatively sedentary lifestyle during and even after therapy [2, 22, 23, 25]. Further, many chemotherapeutic agents (e.g., glucocorticoids, methotrexate, cyclophosphamide) interfere with bone-mineral accretion and skeletal development [16, 17, 22, 28, 29, 30, 31, 32].
Available literature is inconclusive as to the time of onset and the degree of recovery of BMD relative to oncotherapy. Further, the significance of marginal BMD deficits in children and young adult cancer survivors that may compound the BMD loss seen in adulthood has yet to be studied. We know that in adults, a deficit of 1 SD in bone mass is associated with a 1.5- to 3-fold increased incidence of fracture [33]. However, few longitudinal data are available regarding bone-mineral accretion and skeletal development in healthy, normally developing children, much less in those who have survived childhood cancer. So far, no correlation between BMD and fracture risk in children has been determined.
Seventy-five to 85% of the variance in bone strength is accounted for by BMD, which can be determined many years in advance of fracture. Its early identification allows intervention to improve bone health at a time when preventive measures may be effective [33, 34]. However until recently, little attention has been paid to skeletal development in children and adolescents.
Trabecular bone is more metabolically active than cortical bone and more chemosensitive [32]. Furthermore, the BMD determination varies with the skeletal site examined. For example, the distal radius is composed mostly of trabecular bone, whereas the tibia is primarily cortical bone [35, 36]. Normative values must be established for any site of interest, taking into account age, gender, and race variances. The size and shape of the skeleton change continuously during development and maturation. These two factors add additional complexity to BMD determination and the establishment of “normal values” for children and adolescents [35]. The final size and configuration of the individual bones result from complex interaction between genetics, physical activity and muscular tension, nutritional status, gender, race, etc. [5, 35].
At-risk patient groups
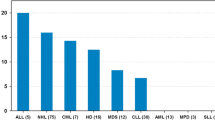
Prospective evaluations of childhood cancer survivors are warranted both for guiding clinical care and for determining the incidence, onset, and risk factors for the development of BMD deficits. Best studied have been children treated for acute lymphoblastic leukemia (ALL) [3, 17, 18, 19, 20, 22, 37, 38, 39, 40, 41, 42, 43, 44, 45]. Children treated for ALL are at particular risk for BMD deficits if they are male (P=0.038), Caucasian (P<0.0001), and have received cranial irradiation (P=.0087) as part of their therapy. BMD has been found to inversely correlate with cranial irradiation dose. BMD Z-scores of patients who received higher doses of antimetabolites were lower than those of patients who received lower doses of the same drugs, though in the series reported, did not reach statistical significance [17]. Among 141 survivors of ALL treated at our institution, (median age, 15.9 years; median time after diagnosis, 11.5 years), the median BMD Z-score was −0.78 SD (range, −3.23 to 3.61 SDs). Thirty participants (21%; 95% confidence interval, 15–29%) had abnormally low BMD, a proportion significantly (P<0.0001) greater than the expected 5% in normal populations [17].
BMD deficits have been reported in children who have undergone treatment for brain tumors [3, 46, 47, 48, 49]. Of 19 children whose BMD was evaluated using DXA on average of 7 years from diagnosis, Barr et al. reported a median decrease in lumbar spine BMD Z-scores of −1.05 (range, −2.91 to +1.15) and of the femoral neck, a median BMD deficit of −0.84 (range, −2.38 to +2.38) was reported [48]. Further, assessments of global quality of life tended to be inversely associated with BMD Z-scores, i.e., patients with low BMD Z-scores (osteopenia), had more pain and more compromise in health-related quality of life than those who were not osteopenic, though statistical significance was not reached. Further, pain associated with osteopenia significantly limited physical activity of the brain-tumor survivors [48].
More recently, attention has also been turned to children and adolescents who have undergone allogeneic bone-marrow transplantation (alloBMT) [50, 51, 52]. Our group recently reported on 48 pediatric patients who had undergone allogeneic bone marrow transplant for a variety of diagnoses. The median age at the time of transplant for this cohort was 10.3 years (range, 1.6–20.4 years), and the median time between alloBMT and the time of BMD assessment was 5.1 years (1.0–10.2 years). BMD Z-score determined by QCT demonstrated a median deficit of −0.89 (range, −4.06–3.05) and tended to be associated with the female sex (P=0.0559) but not with age at BMT, race, primary diagnosis, time from alloBMT, T-cell depletion of graft, TBI, or acute/chronic GVHD [50]. Our study corroborates the work of Bhatia et al. [53] who found that the DXA-determined BMD of 10 pediatric patients aged 3–8 years, most of whom underwent allogeneic BMT for acute myeloid leukemia, was significantly decreased with a median Z-score of −0.5 and was lower than that of 13 adults in the same study (median Z-score of 0.0, P=0.03).
Although few large series are available that address BMD in survivors of childhood solid tumors, preliminary results indicate that these children may be at risk for BMD deficits as well [19, 47, 54, 55]. Those with possibly the greatest risk are children treated with ifosfamide or methotrexate [19, 55]. Holzer et al. recently reported a series of 48 adults who had been treated for osteosarcoma between the ages of 6 and 20 years. These patients then underwent BMD determination using DXA of the lumbar spine and femoral neck as adults (mean age at BMD determination was 31±4.2 years; mean follow-up from diagnosis of 16±2.2 years). Of this patient cohort, only 17 had BMD above the mean; 65% had BMD deficits [55].
Methods of BMD determination
The criterion for diagnosing osteopenia and osteoporosis was established by the World Health Organization (WHO) for postmenopausal women [56] and reflects the risk of fracture for a given BMD. For clinical use, most facilities report Z- and/or T-scores for the anatomic sites examined (usually hip and spine). The Z-score is a comparison of the individual’s BMD against age-, gender-, and sometimes race-matched controls. The T-score, on the other hand, is a comparison of the patient’s BMD with maximum young adult BMD [5, 27, 35, 57]. Thus, the T-score is nearly meaningless in children as it compares the child’s BMD to that of a mature adult [57]. Contrary to established fracture risk in adults, the association of fracture risk in children and adolescents for any given BMD has not been determined, further emphasizing the need for prospective pediatric studies on skeletal development as well as mandating comparison of a patient’s BMD to age- and gender-adjusted norms (i.e., Z-score).
Low bone mass (or bone-mineral density) can be determined only by direct measurement [58]. Methods for BMD determination are numerous and have evolved over decades of use and technological evolution. Common contemporary methods include DXA, US, QCT, and radiographs. Most methods are based on absorptiometric techniques where beam attenuation reflects the tissues interrogated. As such, the accuracy of most of the modalities in determing BMD is affected by the degree of fatty marrow and surrounding tissues [59].
Radiographic absorptiometry
In general, radiographic determination of BMD deficits is insensitive; BMD deficits are typically not radiographically apparent until about 40% of BMD has been lost [31]. However, radiographic absorptiometry (RA) of the phalanges provides good BMD assessment and has an excellent correlation with phalangeal ashed weight (r=0.983; accuracy 4.8%). This method requires placement of an aluminum alloy reference wedge, provided by the manufacturer and placed parallel to the middle phalanx of the index finger prior to imaging. Two radiographs of the left hand are then obtained, each obtained with slightly different exposures. The settings are adjusted to match a sample case provided by the RA manufacturer. Using electronic image capture, BMD is estimated and the average density of the middle phalanges of the second, third, and fourth digits are reported [59].
Dual-energy X-ray absorptiometry
Dual-energy X-ray absorptiometry (DXA) quantifies the degree of attenuation of an X-ray beam that passes through bone and soft tissues. BMD estimates and quantification of soft tissue result from comparison of attenuation between two distinct photoelectric peaks that optimize attenuation from soft tissues as compared with bone [59].
Areal measurements are obtained from DXA as bone-mineral content/bone area, expressed as grams per square centimeter; three-dimensional or volumetric measurements are calculated [5, 29, 34, 58]. DXA is available worldwide and associated with a very low level of ionizing radiation [59, 60, 61, 62, 63, 64]. It has the advantage of being able to assess BMD and composition of the whole body or individual portions of the skeleton. Though historically accepted as “the” method for BMD assessment, DXA is hampered by its inability to differentiate cortical from trabecular bone, its sensitivity to overlying soft tissues and fat distribution, which impact BMD determinations, its sensitivity to artifacts and scoliosis, and the need to calculate as opposed to directly measure volumetric BMD [5, 29, 33, 35]. Additionally, cross-calibration is needed for accurate comparison of values obtained on individual machines not only of different manufacturers, but also different machines of the same manufacturer [59, 65].
Quantitative computed tomography
As with the previously described methods, quantitative computed techonology (QCT) is based on photon absorptiometry. It is unique in that it provides direct densitometric assessment of bone in a three-dimensional (volumetric) manner [59]. Peripheral QCT units are available, but in the United States, use of commercial software to adapt standard clinical CT scanners to assessment of BMD is more widespread [59].
The cost of QCT software is affordable, but an initial investment of a CT scanner and support staff is required. Thus, the total cost has contributed to limiting the availability and acceptance of QCT as a screening tool for BMD. Radiation dose is very low, though higher than DXA [60, 61, 62, 63, 64]. Advantages of QCT include its ability to differentiate cortical from trabecular bone, its ability to directly obtain volumetric (as opposed to calculated) BMD values, and the ability to exclude potential artifacts. Disadvantages include the inability to currently obtain whole-body BMD and the exposure dose from ionizing radiation, though it is considerably lower than natural background radiation [34, 56, 60, 61, 62, 63, 64]. Particularly limiting in growing children, is DXA’s inability to account for marked changes in skeletal and body size and configuration during the rapid growth of adolescence [66].
The interest in peripheral QCT (pQCT) is growing as an independent method of determining BMD. This method is less costly to install and by imaging the extremities (typically the forearm), avoids significant radiation exposure to the body [56, 67]. Establishment of normative values and further technologic development are ongoing.
Quantitative ultrasound
Bone-density determination by broadband ultrasound [5, 33, 34, 37, 58, 68, 69, 70, 71, 72, 73] and quantitative magnetic resonance imaging and magnetic resonance microscopy techniques [36, 58] show promise, but are not yet fully developed for clinical use, especially for pediatric imaging. Quantitative ultrasound (QUS) techniques have the advantage of portability and lack of ionizing radiation, two factors that are particularly enticing for use in pediatric patients. Similarly, the lack of ionizing radiation exposure with MR is attractive for pediatric imaging, but the high cost is restrictive. Experience to date with US or MR determination of BMD is limited in children and adolescents.
Ultrasound bone densitometry is based on soft-tissue attenuation of the interrogated tissue (typically calcaneus, proximal tibia, or phalanges) using a frequency range of 0.1–1 MHz [73]. The term broadband ultrasound attenuation (BUA) has arisen from the slope of attenuation being a function of frequency. Evolution and refinement of technology are ongoing. There are several commercially available units. Reproducibility of results, while improved over earlier models, remains a limiting factor. The currently available systems target different anatomic sites and information obtained from one system cannot be translated to other systems. Prospective studies have shown the ability of QUS to discriminate between normal and osteoporotic patient cohorts in adults [73] as well as predict fracture risk. Validation of ultrasound parameters and research into their clinical significance is ongoing [68, 72, 73].
In conclusion, once cured of their primary disease, childhood cancer survivors then face a lifetime of potential toxicities that may compromise their quality of life; BMD deficits may be masked until late in their evolution. As pediatric radiologists, we need to be cognizant of late toxicities, encourage appropriate screening of at-risk cohorts, and guide our clinical colleagues to the most appropriate modality to identify and monitor these disease- and treatment-related sequelae.
References
Parisi MT, Fahmy JL, Kaminsky CK, et al (1999) Complications of cancer therapy in children: a radiologist’s guide. Radiographics19:283–297
Meadows AT, Hobbie WL (1986) The medical consequences of cure. Cancer 58[Suppl 2]:524–528
Green DM (2002) Paediatric update. Eur J Cancer 38:1251–1253
Bleyer WA (1990) The impact of childhood cancer on the United States and the world. CA Cancer J Clin 40:355–367
Hartman C, Hochberg Z, Shamir R (2003) Osteoporosis in pediatrics. Isr Med Assoc J 5:509–515
Baker SS, Cochran WJ, Floes CA, et al (1999) American Academy of Pediatrics. Committee on Nutrition: calcium requirements of infants, children, and adolescents. Pediatrics 104:1152–1157
Matkovic V (1992) Calcium and peak bone mass. J Intern Med 231:151–160
Matkovic V (1992) Osteoporosis as a pediatric disease: role of calcium and heredity. J Rheumatol 19[Suppl 33]:54–59
Horwath C, Parnell WR, Wilson NC, et al (2001) Attaining optimal bone status:lessons learned from the 1997 National Nutrition Survey. N Z Med J 114:138–141
Rousseau ME (1997) Dietary prevention of osteoporosis. Lippincott’s Primary Care Pract 1:307–319
Weyer C, Linkeschowa R, Heise T, et al (1998) Implications of the traditional and the new ACS physical activity recommendations on weight reduction in dietary treated obese subjects. Int J Obes Relat Metab Disord 22:1071–1078
Camaione DN, Burns KJ, Chatterton CT (1997) Counseling for physical activity: what primary-care physicians should know. Conn Med 61:391–395
Ulrich CM, Georgiou CC, Gillis DE, et al (1999) Lifetime physical activity is associated with bone mineral density in premonopausal women. J Womens Health 8:365–375
The Surgeon General’s Report on Nutrition and Health (1988) U.S. Department of Health and Human Services, Public Health Service, Washington, D.C.
Bianchi ML (2002) Glucocorticoids and bone: some general remarks and some special observations in pediatric patients. Calcif Tissue Int 70:384–390
Kaste SC, Chesney RW, Hudson MM, et al (1999) Bone mineral status during and after therapy of childhood cancer: an increasing population with multiple risk factors for impaired bone health. J Bone Miner Res 14:2010–2014
Kaste SC, Jones-Wallace, D, Rose SR, et al (2001) Bone mineral decrements in survivors of childhood acute lymphoblastic leukemia: frequency of occurrence and risk factors for their development. Leukemia 15:728–734
Arikoski P, Komulainen J, Riikonen P, et al (1999) Impaired development of bone mineral density during chemotherapy—a prospective analysis of 46 newly diagnosed children with cancer. J Bone Miner Res 14:2002–2009
Arikoski P, Komulainen J, Riikonen P, et al (1999) Alterations in bone turnover and impaired development of bone mineral density in newly diagnosed children with cancer: a one year propsective study. J Clin Endocrinol Metab 84:3174–3181
Arikoski P, Komulainen J, Voutilainen R, et al (1998) Reduced bone mineral density in long-term survivors of childhood acute lymphoblastic leukaemia J Pediatr Hematol Oncol 20:234–240
Gilsanz V, Carlson ME, Roe TF, et al (1990) Osteoporosis after cranial irradiation for acute lymphoblastic leukemia. J Pediatr 117:238–244
Tillmann V, Darlington AS, Eiser C, et al (2002) Male sex and low physical activity are associated with reduced spine bone mineral density in survivors of childhood acute lymphoblastic leukemia. J Bone Miner Res 17:1073–1080
Warner JT, Bell W, Webb DKH, et al (1998) Daily energy expenditure and physical activity in survivors of childhood malignancy Pediatr Res 43:607–613
Jenney MEM, Levitt GA (2002) The quality of survival after childhood cancer. Eur J Cancer 38:1241–1250
Jenney MEM, Faragher EB, Morris-Jones PH, et al (1995) Lung function and exercise tolerance in survivors of childhood malignancy. Med Pediatr Oncol 24:222–230
Eastell R, Lambert H (2002) Diet and healthy bones. Calcif Tissue Int 70:400–404
Wang MC, Crawford PB, Hudes M, et al (2003) Diet in midpuberty and sedentary activity in prepuberty predict peak bone mass. Am J Clin Nutr 77:495–503
Byrd R (1985) Late effects of treatment of cancer in children. Pediatr Clin N Am 32:835–857
Leonard MB (2003) Assessment of bone health in children and adolescents with cancer: promises and pitfalls of current techniques. Med Pediatr Oncol 41:198–207
Robson H, Anderson E, Eden OB, et al (1998) Chemotherapeutic agents used in the treatment of childhood malignancies have direct effects on the growth plate chondrocyte proliferation. J Endocrinol 157:225–235
Neglia JP, Nesbit ME Jr (1993) Care and treatment of long-term survivors of childhood cancer. Cancer 71:3386–3391
Davies JH, Evans BAJ, Jenney MEM, et al (2002) In vitro effects of chemotherapeutic agents on human osteoblast-like cell numbers. Calcif Tissue Int 70:408–415
Melton LJ, Chrischilles EA, Cooper C, et al (1992) Perspective: how many women have osteoporosis? J Bone Miner Res 7:1005–1010
Gilsanz V (1998) Bone density in children: a review of the available techniques and indications. Eur J Radiol 26:177–182
Leonard M (2002) Dual energy x-ray absorptiometry: shortcomings in the assessment of bone health in children. Calcif Tissue Int 70:355–383 (abstract)
McKay H (2002) Beyond DXA: is MRI a useful tool for the assessment of bone in children? Calcif Tissue Int 70:355–383 (abstract)
Lequin MH, van der Sluis M, van Rijn RR, et al (2002) Bone mineral assessment with tibial ultrasonometry and dual-energy x-ray absorptiometry in long-term survivors of acute lymphoblastic leukemia in childhood. J Clin Densitom 5:167–173
Nysom K, Holm K, Michaelsen KF, et al (1998) Bone mass after treatment for acute lymphoblastic leukemia in childhood. J Clin Oncol 16:3752–3760
Warner JT, Evans WD, Webb DKH, et al (1999) Relative osteopenia after treatment for acute lymphoblastic leukemia Pediatr Res 45:544–551
Henderson RC, Madsen CD, Davies C, et al (1996) Bone density in survivors of childhood malignancies. J Pediatr Hematol Oncol 18:367–371
Atkinson SA, Halton JM, Bradley C, et al (1998) Bone and mineral abnormalities in childhood acute lymphoblastic leukemia: influence of disease, drugs, and nutrition. Int J Cancer [Suppl] 11:35–39
Strauss AJ, Su JT, Kimball Dalton VM, et al (2001) Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol 19:3066–3072
Brennan BM, Rahim A, Adams JA, et al (1999) Reduced bone mineral density in young adults following cure of acute lymphoblastic leukaemia in childhood. Br J Cancer 79:1859–1863
Kadan-Lottick N, Marshall JA, Baron AE, et al (2001) Normal bone mineral density after treatment for childhood acute lymphoblastic leukemia diagnosed between 1991 and 1998. J Pediatr 138:898–904
Swiatkiewicz V, Wysocki M, Odrowaz-Sypniewska G, et al (2003) Bone mass and bone mineral metabolism at diagnosis and after intensive treatment in children with acute lymphoblastic leukemia (DOI 10.1002/mpo.10415). Med Pediatr Oncol 41:578–580
Gurney JG, Kadan-Lottick NS, Packer RJ, et al (2003) Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer. 97:663–673
Hesseling PB, Hough SF, Nel ED, et al (1998) Bone mineral density in long-term survivors of childhood cancer. Int J Cancer [Suppl] 11:44–47
Barr RD, Simpson T, Webber CE, et al (1998) Osteopenia in children surviving brain tumors. Eur J Cancer 34:873–877
Mithal NP, Almond MK, Evans K, et al (1993) Br J Radiol 66:814–816
Kaste SC, Shidler TJ, Tong X, et al (2004) Bone mineral density and osteonecrosis in survivors of childhood allogeneic bone marrow transplantation. Bone Marrow Transplant. (in press)
Daniels MW, Wilson DM, Pagauntalan HG, et al (2003) Bone mineral density in pediatric transplant recipients. Transplantation 76:673–678
Kashyap A, Kandeel F, Yamauchi D, et al (2000) Effects of allogeneic bone marrow transplantation on recipient bone mineral density: a prospective study. Biol Blood Marrow Transplant 6:344–351
Bhatia S, Ramsay NK, Weisdorf D, et al (1998) Bone mineral density in patients undergoing bone marrow transplantation for myeloid malignancies. Bone Marrow Transplant 22:87–90
Henderson RC, Madsen CD, Davis C, et al (1996) Bone density in survivors of childhood malignancies. J Pediatr Hematol Oncol 18: 367–371
Holzer G, Krepler MA, Grampp S, et al (2003) Bone mineral density in long-term survivors of highly malignant osteosarcoma. J Bone Joint Surg Br 85:231–237
National Osteoporosis Foundation. (1998) Physician’s guide to prevention and treatment of osteoporosis. Exerpta, Belle Mead
Plotkin H (2003) Mind your T’s and Z’s. J Bone Joint Surg Am 85:1390–1391
Genant HK, Engelke K, Fuerst T, et al (1996) Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res 11:707–730
Bonnick SL (1998) Densitometry techniques in medicine today. In: Bonnick SL (ed) Bone densitometry in clinical practice, application and interpretation. Humana Press, Totwa, pp 1–30
Cann CE (1987) A rational approach to radiation exposure in bone densitometry. Radiology 165:184
ICRP Publication 60 (1991) 1990 Recommendations of the International Commission on Radiological Protection. Ann ICRP 21:1–3
Kalendar WA (1992) Effective dose values in bone mineral measurements by photon absorptiometry and computed tomography. Osteoporosis Int 2:82–87
Lewis MK, Blake GM, Fogelman I (1994) Patient dose in dual x-ray absorptiometry. Osteoporosis Int 4:11–15
Mindways Software (1998) Bone densitometry and radiation exposure. Informational flyer
Lantz H, Samuelson G, Bratteby LE, et al (1999) Differences in whole body measurements by DXA scanning using two Lunar DPX-L machines. Int J Obes Relat Metab Disord 23:764–770
Gilsanz V (1998) Bone density in children: a review of the available techniques and indications Eur J Radiol 26:177–182
Schoenau E (2002) QCT and peripheral QCT. Calcif Tissue Int 70:355–383 (abstract)
Hoffmeister BK, Whitten SA, Kaste SC, et al (2002) Effect of collagen and mineral content on the high frequency ultrasonic properties of human cancellous bone. Osteoporosis Int 13:26–32
Lappe JM, Recker RR, Malleck MK, et al (1995) Patellar ultrasound transmission velocity in healthy children and adolescents. Bone 16[Suppl 4]:251S–256S
Zewekh JE, Antich PP, Sakhaee K, et al (1991) Assessment by reflection ultrasound method of the effect of intermittent slow-release sodium fluoride calcium citrate therapy on material strength of bone. J Bone Mineral Res 6:239–244
Brandenberger GH (1993) Clinical determination of bone quality: is ultrasound an answer? Calcif Tissue Int 53[Suppl1]:S151–156
Brukx LJ, Waelkens JJ (2003) Evaluation of the usefulness of a quantitative ultrasound device screening of bone mineral density in children. Ann Hum Biol 30:304–315
Hand D, Gluer CC, Njeh CF (1998) Ultrasonic evaluation of osteoporosis. In: Meunier PJ (ed) Osteoporosis: diagnosis and management. Mosby, St Louis, pp 59–78
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported in part by grants P30 CA-21765 and P01 CA-20180 from the National Cancer Institute, a Center of Excellence grant from the state of Tennessee from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC)
Rights and permissions
About this article
Cite this article
Kaste, S.C. Bone-mineral density deficits from childhood cancer and its therapy. Pediatr Radiol 34, 373–378 (2004). https://doi.org/10.1007/s00247-003-1132-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-003-1132-1