Abstract
Acute heart failure (AHF) can cause low cardiac output and poor end-organ perfusion. Inotropic agents along with vasodilators can improve organ perfusion. Arginine vasopressin (AVP) and calcium chloride (CaCl) infusions are increasingly being used in low cardiac output states in pediatric AHF. We retrospectively reviewed 77 patients (0–18 years) with AHF admitted between January 2014 and May 2017 who received concurrent AVP and CaCl infusions. Surrogates of cardiac output and organ perfusion included hemodynamic vital signs, laboratory parameters, and urine output (UO). Organ dysfunction and vasopressor inotropic scores were also calculated. Median (IQR) age was 0.88 years (0, 3.75), and median weight was 6.62 kg (3.5, 13.7). Congenital heart disease was present in 70% (46/77) patients. Univentricular physiology was present in 25% (25/77) patients. None of the patients were in the immediate postoperative period. Median durations of AVP and CaCl were 2 days (1, 3) and 3 days (2, 6), respectively. Using Wilcoxon-signed rank test and Bonferroni correction, post hoc comparison showed that at 8 h post infusion, all systolic blood pressure (SBP) and diastolic blood pressure (DBP) results, and UO were greater than those 1 h prior to infusion. Median SBP increased from 79 mm Hg (71, 92) 1 h prior to 97 mm Hg (84, 107) 8 h post. Median DBP increased from 44 mm Hg (35, 52) 1 h prior to 54 mm Hg (44, 62) 8 h post. Heart rate showed a decrease between measurements 1 h prior to infusion and 8 h post, with median scores 146 (127, 162) and 136 (114, 150) beats per minute, respectively. Within first 8 h, median UO continuously increased from 6 mL/h. (0, 25) at 1 h post infusion to 20 mL/h. (2, 62) at 8 h post infusion. Median pediatric logarithmic organ dysfunction scores on days 4 through 7 post infusion were lower compared to day 1; median vasopressor inotropic scores on day 2 through 7 post infusion were lower compared to day 1. Serum lactate level, arterial pH, and base excess all showed favorable trend. Concurrent use of AVP and CaCl infusions may improve surrogates of cardiac output, and intensive care outcomes, and prevent organ dysfunction in children with AHF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute cardiocirculatory failure is a commonly encountered pathophysiologic state in the intensive care unit (ICU). It can be characterized by a constellation of signs and symptoms including hypotension, tachycardia, hypoxemia, vasomotor disturbances, and abnormalities of acid–base equilibrium. This can result in a compromise of end-organ perfusion leading to inadequate oxygen delivery, acidosis, and shock and is associated with significant morbidity and mortality [1,2,3]. The traditional first-tier approach includes the use of catecholaminergic inotropes like epinephrine, dopamine, dobutamine, and/or norepinephrine along with fluid resuscitation, stabilization of respiratory status, and other measures. These inotropic agents increase contractility and improve blood pressure but are also associated with significant side effects like tachycardia, tachyarrhythmia, increase in systemic vascular resistance (SVR), increase in myocardial, and total oxygen consumption [4]. They are less effective in hypoxic and acidotic milieu and are also associated with long-term mortality [5, 6].
Recently, calcium chloride (CaCl) and arginine vasopressin (AVP) infusions are increasingly being used in cardiocirculatory failure [7, 8]. Use of AVP–CaCl combination in children with acute cardiocirculatory failure has not been reported in the literature. The purpose of this study is to report the observed effects of AVP–CaCl on hemodynamic variables, surrogates of end-organ perfusion, and their side-effect profile from our practice in pediatric patients with cardiocirculatory failure.
We hypothesize that concurrent infusions of CaCl and AVP in pediatric patients with cardiocirculatory failure improve surrogates of cardiac output and end-organ dysfunction.
Material and Methods
Patients
This single center retrospective study was conducted at Le Bonheur Children’s Hospital, a free standing, tertiary-care children’s hospital that is affiliated with the University of Tennessee Health Science Center (UTHSC) in Memphis, Tennessee. The Institutional review board at UTHSC approved the study. Children between the ages of 0 to 18 years, who were admitted in Cardiovascular ICU(CVICU) and Pediatric ICU(PICU) at Le Bonheur Children’s Hospital between January 2014 and May 2017 with cardiocirculatory failure and received concurrent infusions of CaCl and AVP were included. Patients who underwent ECMO were excluded. The diagnosis of cardiocirculatory failure was made by the primary ICU team on the basis of clinical examination (poor perfusion/vasomotor abnormalities), hemodynamic parameters (tachycardia, hypotension), shock states (abnormalities of gas exchange and acid–base equilibrium, decrease in urine output, lactic acidosis, etc.), and/or echocardiographic evidence of cardiac dysfunction.
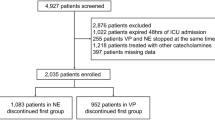
Among the 110 patients who received CaCl and AVP, 77 patients were eligible to be included in this study. Subgroup analysis was performed for those with and without a cardiac diagnosis. Electronic medical records were reviewed to extract demographics, primary admitting diagnosis, hemodynamic and laboratory data, and echocardiographic reports. Day zero (D0) and time zero (T0) were defined as the day and time of initiation of concurrent infusions of CaCl and AVP, respectively. Data collection was started at T0 − 1 h. Hemodynamic vital signs included heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MAP), arterial saturations measured by pulse oximetry (SaO2), and cerebral and renal regional oxygen saturations by near-infrared spectrophotometry (NIRS), and these were collected hourly from T0 − 1 h until T0 + 8 h. Urine output (UO) was measured from T0 until T0 + 8 h, whereas lactic acid level and acid–base analysis were measured daily from D0 to D7. Requirement of inotropic support was assessed by recording all the vasoactive and inotropic medications with their dosages from D0 until D7. A vasoactive-inotropic score (VIS) was then calculated according to a formula described by Wernovsky and Gaies [9, 10]. To assess end-organ dysfunction, a pediatric logarithmic organ dysfunction (PELOD) score, as described by Leteurte et al., was calculated daily from D0 to D7 [11,12,13]. Biochemical markers like serum electrolytes, platelet count, serum creatinine, and ionized calcium (iCal) level were recorded to assess side-effect profile of CaCl and AVP.
Statistics
Continuous variables are reported as medians and interquartile ranges (IQRs), while categorical variables are summarized as frequency counts and percentages. Data were tested across time points (hours and days) to see if the data were normally distributed. The assumption of normality was not met, so a nonparametric test was used. Due to the repeated measures, a Friedman’s Test was used to account for the within-patient comparisons, controlling for each patient and comparing across the time points. In the Friedman’s Test, there are no differences in the time points within each subject. Variables of interest were first compared based on an hourly timeframe: the initial time point being 1 h prior to T0, start time of simultaneous infusion of calcium chloride and arginine vasopressin, and other time points being 1, 2, 4, 6, and 8 h post T0. This was conducted to look for short-term changes due to the continuous simultaneous infusions. A 30-min window on either side of the hour mark was used to capture data within the time frames. For the effect over days, the variables of interest were compared across 8 days, with the day of T0 being used as the beginning point of D0, and days 1 through 7 following. The highest value recorded for each day for the patient was used. A post hoc comparison was conducted for those deemed statistically significant (α < 0.05) using the Wilcoxon Signed Rank Test and a Bonferroni Correction. Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient Demographics
Seventy-seven patients with acute cardiocirculatory failure received concurrent CaCl and AVP infusions during the study period. Out of the 77 patients, 52 patients (67%) had a cardiac diagnosis. Among the patients with a cardiac diagnosis, 46 (88%) patients had underlying congenital heart disease (CHD), and 6 (12%) patients had cardiomyopathy. In the overall cohort, 25 (32%) patients had uni-ventricular physiology. Patient demographics are summarized in Table 1. The median durations of calcium chloride and AVP infusions were 3 days (IQR 2–5) and 2 days (IQR 1–3), respectively. The median starting and median maximum doses of calcium chloride infusion were 10 mg per kg per hour (IQR 5–10) and 10 mg per kg per hour (IQR 10–10), respectively. The median starting and median maximum doses of AVP were 0.3 milliunits per kilogram per minute (IQR 0.3–0.5) and 0.5 milliunits per kg per min (IQR 0.3–0.7), respectively.
Effects of CaCl and AVP Infusions on Hemodynamic Variables (Tables 2, 4; Figs. 1, 3)
In the overall cohort, the median heart rate improved from 145 beats per minute (bpm) (IQR 127–167) at T0 − 1 h (baseline) to 136 bpm (IQR114–150, p < 0.044) at T0 + 8 h. This trend continued over several days with a median heart rate of 165 bpm (IQR 152–184) on D1 compared to a median heart rate of 152 bpm (IQR 127–168, p < 0.001) on D7. Median systolic blood pressure increased from 81 mmHg (IQR 71–93) at baseline to 98 mmHg (IQR 84–107, p < 0.001) at T0 + 8 h. Similarly, the median diastolic blood pressure increased from 44 mm Hg (IQR 35–55) at baseline to 56 mmHg (IQR 46–63, p < 0.001) at T0 + 8 h.
Similar results were obtained in the cardiac subgroup analysis. The median heart rate improved from 145 bpm (IQR 131–162) at baseline to 133 bpm (IQR 115–150, p < 0.079) at T0 + 8 h. This trend continued over the next several days with a median HR of 162 bpm (IQR 146–180) on D1 compared to 150 bpm (IQR 125–166, p < 0.001) on D7. Median systolic blood pressure increased from 81 mm Hg (IQR 72–88) at baseline to 99 mm Hg (IQR 86–106, p < 0.001) at T0 + 8 h. Similarly, median diastolic blood pressure increased from 44 mmHg (IQR 35–54) at baseline to 57 mmHg (IQR 49–63, p < 0.001) at T0 + 8 h.
Effects of CaCl and AVP Infusions on Surrogates of Organ Perfusion (Tables 2, 3, 4; Figs. 1, 2, 3, 4)
In the overall group, the median urine output increased from 0.8 mL per kilogram per hour (IQR 0–3.7) at baseline to 3.45 mL per kilogram per hour (IQR 0.3–8.5, p < 0.0001). Similarly, in the cardiac subgroup, the median urine output increased from 1 mL per kilogram per hour (IQR 0–3.5) baseline to 2.75 mL per kilogram per hour (IQR 0.3–7.2, p < 0.0001) at T0 + 8 h. Median pediatric logistic organ dysfunction (PELOD) score decreased from 11 (IQR 11–20) on D1 to 10 (IQR 10–10, p < 0.001) on D7 in the overall group of patients. In the cardiac subgroup, median PELOD score decreased from 11 (IQR 11–16) on D0 to 10 (IQR 10–10, p < 0.0001) on D7.
Median lactic acid level decreased from 4.5 mmol/L (IQR 3.1–9) on D0 to 1.2 mmol/L (IQR 0.8–1.9) on D7, although it did not reach statistical significance. In the cardiac subgroup, the median lactic acid level decreased from 4 mmol/Liter (IQR 2.5–7.6) on D0 to 1.2 mmol/L (IQR 0.8–1.9) on D7.
Effects of CaCl and AVP on Need for Inotropic Support (Tables 2, 4; Figs. 2, 4)
In the overall group of patients, median VIS score decreased from 10 (IQR 5–17.5) on D0 to 0 (IQR 0–5, p < 0.0001) on D7. In the cardiac subgroup, median vasoactive inotropic score (VIS) decreased from 8 (IQR 5–11.5) on D0 to 0 (IQR 0–5, p < 0.0001) on D7.
Side-Effect Profiles of CaCl and AVP (Tables 2, 4, 5; Figs. 2, 4)
We looked at the most common side effects associated with the use of CaCl and AVP. Overall, median sodium levels decreased from 143 mEq/L (IQR 140–148) on D0 to 139 mEq/L (IQR 135–142), p < 0.001 on D7. Median serum creatinine level decreased from 0.5 mg/dL (IQR 0.4–0.9) on D0 to 0.3 mg/dL (IQR 0.2–0.5, p < 0.0001). Median platelet count showed an initial decrease in the first 48 h followed by a sustained increase through D3–D7 with a count of 1,50,000/µL (IQR 94–189) on D0 to a count of 1,62,000/µL (IQR 83–269) on D7. The median, minimum, and maximum iCal levels were 1.46 mmol/L, IQR (1.23–1.72), 0.9 mmol/L, and 2.47 mmol/L, respectively.
Discussion
In this study, we observed that in children presenting with acute cardiocirculatory failure, use of concurrent infusions of CaCl and AVP improves certain surrogate markers of cardiac output and end-organ perfusion as demonstrated by improvement in hemodynamics, urine output, and lactic acid level. We also observed that this combination was able to provide enough cardiocirculatory support to improve overall organ dysfunction and decrease the need for catecholaminergic inotropic agents. This novel strategy provided an optimal hemodynamic response without any major adverse effects. Although this was a heterogeneous group of patients, all patients had one common characteristic—acute need for pharmacologic vasopressor and/or inotropic support. Per traditional management, most patients received a combination of several catecholaminergic inotropic agents. They were then initiated on this novel combination of CaCl and AVP with a goal of providing positive circulatory support as well as improvement of vasomotor tone. Within the first 8 h, there was a clinical improvement in hemodynamic parameters with a notable reduction in heart rate. A sustained decrease in heart rate from day 1 to day 7 is an indirect evidence of absent chronotropic properties of CaCl and AVP. Improvement in cardiac output results in increase in systemic blood flow and better end-organ perfusion. The significant increase in urine output in the first 8 h, a decrease in lactic acid level from day 1 to 7 and a decrease in PELOD scores suggest that organ dysfunction continued to improve. The combination of CaCl and AVP also allowed rapid weaning of catecholaminergic inotropes as reflected by a decrease in VIS.
Previous studies have shown CaCl and AVP to be useful in a low cardiac output state, immediately following palliative and corrective cardiac surgeries and also in vasodilatory shock [7, 8, 14]. Our study showed that CaCl–AVP combination can be used when these pathophysiologies coexist and can also avoid potential adverse effects of catecholaminergic inotropes. CaCl and AVP are effective “inotropes” with minimal chronotropic effects [7, 14]. The resultant decrease in heart rate while maintaining cardiac output is helpful for two reasons—it decreases myocardial oxygen consumption and improves ventricular filling in patients with diastolic dysfunction [15]. Thus, tachycardia, tachyarrhythmia, myocardial work, and excessive increase in systemic vascular resistance can be avoided. Second, catecholaminergic drugs may not be effective in a hypoxic and acidotic milieu [16, 17]. Patients with chronic heart failure have downregulation and blockade of adrenergic and angiotensin receptors due to being on beta and angiotensin receptor blockers [18, 19]. CaCl–AVP combination can be effective in such situations owing to a different mechanism of action.
Calcium homeostasis is crucial for effective myocardial performance [20]. Neonates and infants have immature myocytes with structurally and functionally underdeveloped sarcoplasmic reticulum [21]. The relative contribution of calcium influx across the sarcolemma is thought to play a major role in contraction [22, 23]. CaCl infusions have previously been shown to improve markers of cardiac output in a heterogeneous group of pediatric patients in a cardiac ICU [7]. Thus, our practice of using CaCl in all age-groups is not an entirely unknown concept.
AVP exerts its hemodynamic effects by acting on V1 receptors located on vascular smooth muscle [24]. Animal studies have identified V1 receptors coupled to increase in myocyte [Ca+2], and this could, at the cellular level, provide a direct role for AVP in the regulation of contractility in neonatal cardiomyocytes [25]. AVP may increase cardiac index by slightly increasing afterload but at the same time, increases coronary perfusion and causes coronary vasodilation [26,27,28]. In the renal vasculature, AVP dilates afferent arterioles and constricts efferent arterioles resulting in increase in glomerular filtration, in addition to improving systemic cardiac output [29, 30]. AVP also decreases the PVR/SVR ratio [31,32,33] and increases cerebral blood flow [34, 35].
Multiorgan dysfunction is associated with increase in morbidity and mortality [36]. We observed from this study that overall end-organ function improved as shown by the decrease in PELOD score. Moreover, in some patients with CHD or cardiomyopathy with refractory heart failure, one of the main goal is to preserve end-organ function so that they can be successfully bridged to long-term mechanical circulatory support and/or be listed for heart transplantation if recovery does not occur. Early introduction of concurrent infusion of calcium and AVP may be helpful in preventing further organ dysfunction.
Use of AVP and CaCl can sometimes be associated with certain metabolic and hematological abnormalities [37, 38]. As shown in previous studies, there was an expected but transient drop in serum sodium levels. Similarly, thrombocytopenia was observed in some patients but it was temporary and reversible. Serum creatinine level remained stable during the study period. Even though hyponatremia and thrombocytopenia were not associated with seizure activity or bleeding diathesis in our study, close monitoring of these laboratory parameters is warranted.
Our study has inherent limitations of a retrospective design. The patient population is heterogeneous and sample size is small. We only studied the short-term effects and few side effects. The patient population was very complex with some confounders. Due to significant amount of missing data all the variables could not be analyzed. Future prospective studies are needed to confirm and validate our findings.
Conclusion
The concurrent infusion of CaCl and AVP in pediatric patients with acute cardiocirculatory failure may improve hemodynamics, improve organ perfusion, decrease catecholamine requirements, and improve overall organ dysfunction without any major adverse events.
Abbreviations
- ICU:
-
Intensive care unit
- HR:
-
Heart rate
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- SVR:
-
Systemic vascular resistance
- NIRS:
-
Near-infrared spectroscopy
- VIS:
-
Vasoactive inotropic score
- PELOD:
-
Pediatric logistic organ dysfunction score
References
Shaddy RE, George AT, Jaecklin T et al (2018) Systematic literature review on the incidence and prevalence of heart failure in children and adolescents. Pediatr Cardiol 39(3):415–436
Hinton RB, Ware SM (2017) Heart failure in pediatric patients with congenital heart disease. Circ Res 120(6):978–994
Rossano JW, Kim JJ, Decker JA et al (2012) Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: a population-based study. J Card Fail 18(6):459–470
Bangash MN, Kong ML, Pearse RM (2012) Use of inotropes and vasopressor agents in critically ill patients. Br J Pharmacol 165(7):2015–2033
Colucci WS, Sawyer DB, Singh K, Communal C (2000) Adrenergic overload and apoptosis in heart failure: implications for therapy. J Card Fail 6(2 Suppl 1):1–7
Singh K, Communal C, Sawyer DB, Colucci WS (2000) Adrenergic regulation of myocardial apoptosis. Cardiovasc Res 45(3):713–719
Averin K, Villa C, Krawczeski CD et al (2016) Initial observations of the effects of calcium chloride infusions in pediatric patients with low cardiac output. Pediatr Cardiol 37(3):610–617
Lechner E, Hofer A, Mair R, Moosbauer W, Sames-Dolzer E, Tulzer G (2007) Arginine-vasopressin in neonates with vasodilatory shock after cardiopulmonary bypass. Eur J Pediatr 166(12):1221–1227
Wernovsky G, Wypij D, Jonas RA et al (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. Circulation 92(8):2226–2235
Gaies MG, Gurney JG, Yen AH et al (2010) Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 11(2):234–238
Leteurtre S, Martinot A, Duhamel A et al (1999) Development of a pediatric multiple organ dysfunction score : use of two strategies. Med Decis Mak 19:399–410
Leteurtre S, Martinot A, Duhamel A et al (2003) Validation of the paediatric logistic organ dysfunction (PELOD) score : prospective, observational, multicentre study. Lancet 362:192–197
Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F (2013) PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med 41(7):1761–1773
Dünser M, Mayr A, Stallinger A et al (2002) Cardiac performance during vasopressin infusion in postcardiotomy shock. Intens Care Med 28(6):746–751
Gordon AC, Wang N, Walley KR, Ashby D, Russell JA (2012) The cardiopulmonary effects of vasopressin compared with norepinephrine in septic shock. Chest 142(3):593–605. https://doi.org/10.1378/chest.11-2604
Mutlu GM, Factor P (2004) Role of vasopressin in the management of septic shock. Intens Care Med 30(7):1276–1291
Morales DLS, Garrido MJ, Madigan JD et al (2003) A double-blind randomized trial: prophylactic vasopressin reduces hypotension after cardiopulmonary bypass. Ann Thorac Surg 75(3):926–930
Lewis A, Chabot M (1993) The effect of treatment of angiotensin-converting enzyme inhibitors on survival of pediatric patients with dilated cardiomyopathy. Pediatr Cardiol 14(1):9–12
Huang M, Zhang X, Chen S et al (2013) The effect of carvedilol treatment on chronic heart failure in pediatric patients with dilated cardiomyopathy: a prospective, randomized-controlled study. Pediatr Cardiol 34(3):680–685
Schwartz SM, Duffy JY, Pearl JM, Nelson DP (2001) Cellular and molecular aspects of myocardial dysfunction. Crit Care Med 29(10 Suppl):S214–S219
Pegg W, Michalak M (1987) Differentiation of sarcoplasmic reticulum during cardiac myogenesis. Am J Physiol 252:H22–H31
Nassar R, Reedy M, Anderson P (1987) Developmental-changes in the ultrastructure and sarcomere shortening of the isolated rabbit ventricular myocyte. Circ Res 61(3):465–483
Seguchi M, Harding JA, Jarmakani JM (1986) Developmental change in the function of sarcoplasmic reticulum. J Mol Cell Cardiol 18(2):189–195
Holmes CL, Landry DW, Granton JT (2003) Science review: vasopressin and the cardiovascular system part 1—receptor physiology. Crit Care 7(6):427–434
Xu YJ, Gopalakrishnan V (1991) Vasopressin increases cytosolic free [Ca2+] in the neonatal rat cardiomyocyte. Evidence for V1 subtype receptors. Circ Res 69:239–245
Okamura T, Ayajiki K, Fujioka H, Toda N (1999) Mechanisms underlying arginine vasopressin-induced relaxation in monkey isolated coronary arteries. J Hypertens 17(5):673–678
Wenzel V, Kern KB, Hilwig RW et al (2005) Effects of intravenous arginine vasopressin on epicardial coronary artery cross sectional area in a swine resuscitation model. Resuscitation 64(2):219–226
Mayr VD, Wenzel V, Wagner-Berger HG et al (2007) Arginine vasopressin during sinus rhythm: effects on haemodynamic variables, left anterior descending coronary artery cross sectional area and cardiac index, before and after inhibition of NO-synthase, in pigs. Resuscitation 74(2):366–371
Rudichenko V, Beierwaltes W (1995) Arginine vasopressin- induced renal vasodilatation mediated by nitric oxide. J Vasc Res 32(2):100–105
Eisenman A, Armali Z, Enat R, Bankir L, Baruch Y (1999) Low-dose vasopressin restores diuresis both in patients with hepatorenal syndrome and in anuric patients with end-stage heart failure. J Intern Med 246(2):183–190
Mohamed A, Nasef N, Shah V, McNamara PJ (2014) Vasopressin as a rescue therapy for refractory pulmonary hypertension in neonates: case series. Pediatr Crit Care Med 15(2):148–154
Evora PR, Pearson PJ, Schaff HV (1993) ARginine vasopressin induces endothelium-dependent vasodilatation of the pulmonary artery. v1-receptor-mediated production of nitric oxide. Chest 103(4):1241–1245. https://doi.org/10.1378/chest.103.4.1241
Tayama E, Ueda T, Shojima T et al (2007) Arginine vasopressin is an ideal drug after cardiac surgery for the management of low systemic vascular resistant hypotension concomitant with pulmonary hypertension. Interact Cardiovasc Thorac Surg. 6(6):715–719
Suzuki Y, Satoh SI, Oyama H, Takayasu M, Shibuya M (1993) Regional differences in the vasodilator response to vasopressin in canine cerebral arteries in vivo. Stroke 24(7):1049–1053
Vanhoutte PM, Katusić ZS, Shepherd JT (1984) Vasopressin induces endothelium-dependent relaxations of cerebral and coronary, but not of systemic arteries. J Hypertens Suppl. 2(3):S421–S422
Tantaleán JA, León RJ, Santos AA, Sánchez E (2003) Multiple organ dysfunction syndrome in children. Pediatr Crit Care Med 4(2):181–185
Davalos MC, Barrett R, Seshadri S et al (2013) Hyponatremia during arginine vasopressin therapy in children following cardiac surgery. Pediatr Crit Care Med 14(3):290–297
Jerath N, Frndova H, McCrindle BW, Gurofsky R, Humpl T (2008) Clinical impact of vasopressin infusion on hemodynamics, liver and renal function in pediatric patients. Intens Care Med 34(7):1274–1280
Acknowledgements
We would like to thank Mark Rayburn, Pharm D. Department of Pharmacy, Le Bonheur Children’s Hospital Tamekia Jones, PhD, Camden Harrell, MS and Oluwaseun J Ajayi (Children Foundation Research Institute (CFRI) at Le Bonheur Children’s Hospital.
Funding
The study was supported by the intramural grant from Division of Pediatric Critical Care at Le Bonheur Children’s Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karki, K.B., Towbin, J.A., Harrell, C. et al. Concurrent Use of Calcium Chloride and Arginine Vasopressin Infusions in Pediatric Patients with Acute Cardiocirculatory Failure. Pediatr Cardiol 40, 1046–1056 (2019). https://doi.org/10.1007/s00246-019-02114-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-019-02114-2