Abstract
Background
Pediatric restrictive cardiomyopathy (RCM) has high mortality in historical cohorts, and traditional management often involves early referral for heart transplantation (HTx). This study sought to determine outcomes of pediatric RCM at a center that has favored medical management over early listing for HTx.
Methods
All patients (N = 43) with pure RCM phenotype (RCM, N = 26) and hypertrophic cardiomyopathy with restrictive physiology (RCM/HCM, N = 17) managed at our center over a 15-year period were investigated. Outcomes of those listed for HTx (N = 18) were compared to a benchmark of contemporaneous pediatric RCM patients in the UNOS database (N = 377). Proportional hazards models were used to determine predictors of adverse outcomes.
Results
The mean age was 11 ± 9 years and 49% were male. 14 of 18 patients listed received HTx. Overall mortality (12%) was identical between the phenotypes; however, RCM patients were more likely to be listed (P = 0.001) and receive HTx (P = 0.02) compared to RCM/HCM. Prior to HTx, 60% had documented arrhythmia, 16% had cardiac arrest, and 7% required mechanical circulatory support. 4 of 17 patients with an ICD/PM received device therapies (four of five shocks appropriate for VT/VF, and two effective anti-tachycardia pacing interventions). Outcomes of those listed for HTx at our center were similar to the UNOS benchmark. In multivariate analysis, markers of congestive heart failure were associated with adverse outcomes.
Conclusion
Heart failure and arrhythmia treatments can delay or possibly prevent the need for HTx in some cases of pediatric RCM. Survival post-HTx is not compromised using this approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pediatric restrictive cardiomyopathy (RCM) is a rare disease defined by “normal or decreased volume of both ventricles associated with biatrial enlargement, normal LV wall thickness and AV valves, impaired ventricular filling with restrictive physiology, and normal (or near normal) systolic function” [1]. The annual incidence is approximately 0.04 per 10,000 children [2, 3] making outcomes difficult to study. Historical, single-center cohort studies have indicated exceedingly poor outcomes with mortality as high as 50% within 2 years of diagnosis, as well as devastating events such as thromboembolism, severe pulmonary hypertension precluding heart transplant (HTx), and sudden cardiac death without preceding symptoms [4,5,6,7,8,9,10]. These data have largely driven practice to the point that children with RCM are often referred for HTx at the time of initial diagnosis regardless of heart failure (HF) severity. Despite comprising < 5% of pediatric cardiomyopathies [2, 3, 11], and evidence that waitlist and post-HTx outcomes are comparable to dilated cardiomyopathy (DCM) except in infants [12], a disproportionately higher number of pediatric RCM patients are listed and transplanted [13].
The most robust and contemporary study of pediatric RCM outcomes to date is the North American Pediatric Cardiomyopathy Registry (PCMR) study by Webber et al. [11] which analyzed “pure” RCM cases (N = 101) as well as “mixed” hypertrophic cardiomyopathy with restrictive physiology cases (RCM/HCM, N = 51). Survival at 5 years was 68% and that did not differ between the phenotypic subtypes; however, more than half of pure RCM patients were transplanted within a year after diagnosis. In multivariable analysis, only low LV fractional shortening z-score, a rare feature of the disease, was a risk factor for time to death. At the present time, management of pediatric RCM remains clouded by a lack of knowledge regarding its natural history and risk factors for clinical deterioration.
In this context, our institution has adopted a management approach that aims for early diagnosis prior to the development of HF through family screening and genetic testing, frequent screening for conduction abnormalities and arrhythmia along with implantable cardioverter defibrillator or pacemaker (ICD/PM) use when indicated, medical therapy for treatment of symptoms related to diastolic dysfunction, serial cardiac catheterization to assess for an indolent rise in pulmonary vascular resistance (PVR), and mechanical circulatory support (MCS) tailored to the anatomical constraints of RCM (i.e., atrial cannulation) when it is required as a bridge to HTx. The objectives of this study were (1) to describe the contemporary outcomes of pediatric RCM at our institution, (2) compare these to a benchmark of other pediatric centers participating in the United Network for Organ Sharing [14], and (3) identify risk factors for adverse outcomes including death, HTx, MCS, and cardiac arrest.
Methods
Study Population
This was a single-center, retrospective cohort study of consecutive RCM patients followed at Cincinnati Children’s Hospital Medical Center (CCHMC) from January 1, 2000 to October 1, 2016. Subjects were identified by screening the electronic medical record (EMR) with the following search criteria: (1) diagnosis of RCM (ICD 9 code 425.4, ICD10 code I42.5) or HCM (ICD 9 code 425.1, ICD 10 code I42.2), and (2) date of diagnosis within the study period. This initial pool was reduced by manual chart review excluding subjects without one or more of the following criteria: (1) elevated left ventricular (LV) end-diastolic pressure (LVEDP) > 12 mmHg or right ventricular (RV) end-diastolic pressure (RVEDP) > 8 mmHg; (2) left atrial (LA) diameter z-score > + 2.0 or LA volume > 30 mL/m2, LV end-diastolic diameter (LVEDD) z-score ≤ + 2.0, and shortening fraction normal for age or LV ejection fraction (LVEF) > 50%. Subjects fitting the inclusion criteria along with a diagnosis of HCM were classified as “mixed” RCM/HCM phenotype [11, 15]. Those without the additional HCM diagnosis were classified as “pure” RCM phenotype. Patients with congenital heart disease or significant valvar heart disease were excluded. Echocardiographic images from the time of diagnosis were reviewed by an independent cardiologist to verify the classifications. Echocardiographic z-scores were calculated relative to body-surface area (LVEDD, LV end-diastolic posterior wall and septal thicknesses, and LV mass) or relative to age (LV fractional shortening) [16]. LA volume was calculated using the area-length method [17]. LVEF was calculated using the 5/6 area-length method [14]. The most recent genetic testing results were used to define the presence of disease-causing mutation(s). Baseline demographics and medications were extracted from the EMR by the Biomedical Informatics team at CCHMC. For those transplanted during the study period, the last outpatient encounter prior to HTx was used to define the medication classes prescribed for the cardiomyopathy. For those not transplanted, the most recent outpatient encounter was used. The study was approved by the Institutional Review Board at our institution.
Management Algorithm
The general management strategy at our institution for this cardiomyopathy was as follows: patients were seen in cardiology clinic with a resting transthoracic echocardiogram and 12-lead electrocardiogram every 3–6 months. A 24-h Holter monitor was ordered annually or more frequently for concerning symptoms. A diagnostic right heart catheterization was performed every 6–12 months. Beta blockers were prescribed for depressed LV systolic function or RCM/HCM with LV outflow tract obstruction. Diuretics were prescribed for symptoms of congestive heart failure. Patients with prior cardiac arrest or sustained ventricular tachycardia (VT), non-sustained VT detected on Holter monitor, or other standard HCM criteria for prevention of sudden cardiac death [18] were referred for ICD/PM. At the time of referral or diagnosis, all patients were counseled on the potential need for HTx. The decision to evaluate and ultimately list a patient for HTx at our institution was made by a multidisciplinary selection committee and depended primarily on an assessment of medical necessity including (1) inability to leave the hospital due to advanced heart failure therapies (e.g., inotropic support, mechanical ventilation, MCS), (2) recurrent HF hospitalizations, or (3) life expectancy estimated to be improved with HTx.
Outcomes
Hospitalization count and type, echocardiographic parameters, hemodynamics per cardiac catheterization, endomyocardial biopsy results, arrhythmia, and MCS-related data were manually extracted from clinical reports in the EMR. Heart failure hospitalization was defined as that which was unplanned and cardiac-related and thus excluded surveillance cardiac catheterization. Implantable cardioverter defibrillator/pacemaker (ICD/PM) therapy count and type were manually extracted from device interrogation reports. The classifications of appropriate versus inappropriate device therapies were adjudicated by an independent electrophysiologist. Left heart filling pressure (LVEDP) was recorded as that directly measured when left heart catheterization was performed or, if not available, it was estimated from the pulmonary capillary wedge pressure a-wave. Cardiac Index (CI) was measured by the thermodilution method [19]. Survival outcomes analyzed included time to death, HTx, and a composite endpoint of death, HTx, MCS, or cardiac arrest. For those listed for HTx, waitlist and post-HTx survival were compared to all other contemporaneous RCM patients age < 18 years at time of listing in the UNOS database (N = 377).
Statistical Analysis
Normally distributed continuous variables are summarized as mean with standard deviation and were analyzed using t tests. Non-normally distributed continuous variables are summarized as median with interquartile range (IQR), and were analyzed using Wilcoxon rank-sum tests. Categorical variables are summarized as frequency and proportion, and compared using Chi-square or Fisher exact tests as appropriate. Waitlist and post-HTx survival between CCHMC and the other UNOS centers were compared using Kaplan–Meier log-rank analyses. Multivariable Cox proportional hazards models were constructed to determine predictors of individual and composite adverse outcomes (death, HTx, MCS, or cardiac arrest). Factors considered were age, sex, race, phenotypic subtype, medications, initial echocardiographic and catheterization data, documented clinically significant arrhythmia, ICD/PM, and number of HF hospitalizations. The proportional hazards assumption for Cox models was evaluated using the Supremum Test in PROC PHREG in SAS with no significant violations noted. All tests were two-sided, and a P value of < 0.05 was considered significant. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Patient Characteristics
A total of 43 patients (pure RCM, N = 26; mixed RCM/HCM phenotype, N = 17) were followed at our center during the study period. The baseline demographic and clinical characteristics are detailed in Table 1. The mean age at diagnosis was 11 ± 9 years and 49% were male. The majority (74%) were White or Caucasian and 21% were Black or African American. There were no significant demographic differences between the two phenotypes. The majority (72%) did not have a comorbid systemic disease and would be classified as idiopathic RCM. The most common systemic diagnosis was sickle cell disease affecting 4 (15%) of pure RCM cases. RCM/HCM subjects were more likely to have an identified genetic mutation associated with cardiomyopathy (P < 0.001) with MYH7 mutations (41%) being the most common in that group. Among pure RCM cases, TNNI3 mutations (19%) were the most common. Heart failure medications used in the entire cohort included diuretics (30%), beta blockers (30%), and angiotensin-converting enzyme (ACE) inhibitors (9%) and were similar between the groups. Aspirin was more commonly used in RCM compared to RCM/HCM (54% vs. 18%, P = 0.018). Antiarrhythmic medication (amiodarone, digoxin, and sotalol) (7%) and warfarin anticoagulants (rivaroxaban and warfarin) (16%) use was rare and similar between the groups. Consistent with elevated filling pressures at diagnosis, echocardiography demonstrated a median LA diameter z-score of + 2.7 (Q1 + 1.64, Q3 + 3.75) and median LA volume of 41.3 (Q1 33, Q3 56.5) mL/m2. As expected, the median interventricular septal diameter z-score was significantly higher in the RCM/HCM group compared to RCM group (+ 2.39 vs. + 0.47, P = 0.004); LV posterior wall diameter z-score was similar between the two groups (+ 0.96 vs. + 0.4, P = 0.47). Additionally, LV cavity size and LV systolic function were normal at diagnosis (Table 2).
Pre-transplant Outcomes
Clinical deterioration in this cohort pre-HTx was rare as illustrated in Table 2. Only 40% of subjects were hospitalized for heart failure which reflects the fact that most were diagnosed as outpatients. The majority underwent at least one diagnostic cardiac catheterization at our institution (74% overall; 81% RCM vs. 65% RCM/HCM). Baseline hemodynamic indices were similar between the groups with an overall median RVEDP of 10.5 mmHg, LVEDP 19.5 mmHg, mean pulmonary artery pressure 20 mmHg, cardiac index 3.45 L/min/m2, and indexed pulmonary vascular resistance (PVRi) 1.56 indexed Woods units (iWu). Following a practice of serial catheterizations after cardiomyopathy diagnosis to monitor for elevated PVR, the pure RCM group was tested more frequently (median 6 vs. 2 catheterizations per patient, P = 0.007). There were no significant complications attributable to the diagnostic catheterizations. An elevated PVRi > 2.5 iWu was detected more frequently in the pure RCM group approaching statistical significance (P = 0.06). A single subject with pure RCM was identified with a PVRi > 6 iWu which prompted listing for HTx with successful transplantation 13 months later. For those patients that underwent HTx, the trend in cardiac index and PVRi over time is shown in Fig. 1. As expected, there were improvements in both parameters immediately post-HTx, and these improvements were sustained for several years post-HTx.
Trends in hemodynamic parameters over time. a Cardiac index as a function of time. b Pulmonary vascular resistance as a function of time. Data are displayed for all patients that had at least one diagnostic cardiac catheterization. Each open circle and unique color represent an individual patient. The slopes of the regression lines pre- and post-HTx are significantly different (P < 0.05). HTx heart transplant, iWu indexed Wood units, PVRi pulmonary vascular resistance
Documented arrhythmias were relatively common (60% overall; 54% RCM vs. 71% RCM/HCM, P = 0.27). Tachyarrhythmia was more common than bradyarrhythmia (69% vs. 27% of arrhythmias). Atrial arrhythmias occurred in 35% of the entire cohort, ventricular arrhythmias in 46%, and 19% had both atrial and ventricular arrhythmias. Documented cardiac arrest was less common and occurred at the same rate (16%) between the groups. An ICD/PM was implanted in 17 patients (40% overall; 35% RCM vs. 47% RCM/HCM, P = 0.41). The median number of days with an ICD/PM was 1618 (Q1 778, Q3 2418, minimum 137, maximum 3533) days. The total exposure to ICD/PM in the cohort was 77 patient-years. 4 of 17 (24%) patients received ICD/PM therapies (five defibrillator shocks and two anti-tachycardia pacing interventions). Four of the five defibrillator shocks were appropriate for VT/VF. The single inappropriate shock was delivered while a patient was likely in sinus tachycardia. Since device reprogramming, this patient has not had additional inappropriate shocks. The two anti-tachycardia pacing interventions were effective (Fig. 2).
Mechanical circulatory support was employed in three cases, all of whom had pure RCM phenotype (12%). The Berlin Heart was used in the two cases that were successfully bridged to HTx. Venoarterial ECMO was used in the other patient who died on the waitlist. Cerebrovascular accident (CVA) with significant neurologic deficit occurred in two cases; one on the Berlin Heart and the other on venoarterial ECMO. No thromboembolic events or intra-cardiac thrombus were recorded in patients not on MCS.
Overall survival was 92% at 1 and 2 years, and 86% at 5 and 10 years post-diagnosis. These survival rates were similar between the two phenotypic subtypes as shown in Fig. 3. For those never listed for HTx (N = 25), the overall survival was 88% at 1 and 10 years, consistent with a period of early mortality post-diagnosis followed by relatively low mortality after risk stratification and medical management. The causes for the five pre-HTx deaths were sepsis (N = 2), cardiac arrest with documented ventricular tachycardia (N = 1), and unknown (N = 2).
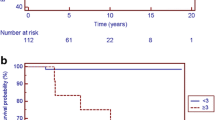
Kaplan–Meier survival curves for all patients (N = 43) and dichotomized by RCM phenotype (RCM, N = 26 vs. RCM/HCM, N = 17; Log-rank P = 0.97). Patients were censored at death. The corresponding number at risk at each time point is included below the curves. Dx diagnosis, RCM restrictive cardiomyopathy, RCM/HCM hypertrophic cardiomyopathy with restrictive physiology
Transplant-Related Outcomes
Eighteen (42% overall) subjects were listed for HTx and 14 (33% overall) were transplanted during the study period (Table 3). Those with pure RCM were more likely to be listed (62 vs. 12%, P = 0.001), and also to be transplanted (46 vs. 12%, P = 0.019). The two RCM/HCM subjects who received HTx had longer median waitlist time (median 623 vs. 134 days) though this difference did not reach statistical significance. Two pure RCM patients were removed from the waitlist due to clinical improvement. Overall median follow-up time since HTx was 2.43 years (887 days).
The majority who were transplanted at our center were alive at the end of the study period (92% actuarial survival at 1 year, and 61% at 5 years post-HTx per analysis of the UNOS database). As shown in Table 4, post-operative arrhythmia occurred in 5 (36%) of HTx patients, predominately ventricular tachycardia (80% of arrhythmias). These were short-lived and no patients required chronic antiarrhythmic medication or ICD/PM. Cellular-mediated rejection detected by endomyocardial biopsy was common; 65% had a maximum cellular rejection grade ≥ 2. Conversely, antibody -mediated rejection was relatively uncommon; 79% patients had a maximum antibody-mediated rejection grade of 0. The single patient with PVRi > 6 iWu prior to HTx was doing well at 479 days post-HTx (most recent PVRi 1.33 iWu).
Compared to all pediatric RCM patients listed for HTx at other UNOS centers during the study period (N = 377), those at CCHMC (N = 18) had longer waitlist times; however, post-HTx outcomes were similar. Median waitlist time, including inactive time, was longer at 164 (Q1 47, Q3 752) days at CCHMC versus 84 (Q1 33, Q3 228) days at other UNOS centers (P = 0.038). The rate of death and deterioration on the waitlist up to 1 year was similar at CCHMC versus other centers (11 vs. 13%; P = 0.92). Once on the waitlist, HTx occurred at a similar rate between the centers (P = 0.48) as shown in Fig. 4a. Post-HTx survival was similar between CCHMC (N = 14) and the other centers (N = 311) at 1 year (92 vs. 89%), 5 years (61% vs. 76%), and 10 years (61 vs. 62%) (P = 0.84) as shown in Fig. 4b. No RCM patients at CCHMC were re-listed for HTx, while 1.74% of those listed at other centers received a second HTx. Two patients (14.3%) at CCHMC were on MCS at time of HTx versus 16 (5.8%) at other UNOS centers (P = 0.20).
a Kaplan–Meier curves of transplant-free survival on the waitlist for pediatric RCM patients at CCHMC (N = 18) and all other centers in the UNOS database (N = 377) (Log-rank P = 0.48). Patients were censored at transplant. b Kaplan–Meier survival curves post-transplant for pediatric RCM patients at CCHMC (N = 14) and all other centers in the UNOS database (N = 311) (Log-rank P = 0.84). Patients were censored at death. CCHMC Cincinnati Children’s Hospital Medical Center
Predictors of Adverse Outcomes
On multivariable Cox proportional hazards analysis, greater than two HF admissions prior to HTx was associated with the composite adverse event (HR 4.08; 95% CI 1.44–11.59; P = 0.008) in our institutional cohort. Diuretic use at last outpatient encounter (HR 11.04; 95% CI 1.19–102.21; P = 0.035) and a higher initial echocardiographic LA diameter z-score (HR 2.32; 95% CI 1.01–5.30; P = 0.047) increased the risk of death specifically. Younger age at diagnosis (HR 0.87; 95% CI 0.8–0.96; P = 0.004) and having pure RCM phenotype (HR 73.19; 95% CI 6.99–766.02; P = 0.0003), as opposed to HCM/RCM, increased the risk of HTx (data not shown).
Discussion
In this retrospective cohort study, we observed an improved overall survival rate (92% at 1 year and 86% at 5 and 10 years post-diagnosis) compared to historical cohort studies [4,5,6,7,8,9,10] which have largely driven clinical decision-making in pediatric RCM. It has been suggested that younger age is a risk factor for poor outcome [5, 7, 8], although in the modern era this has only been demonstrated in infants with RCM and HCM listed for HTx [12]. While the mean age in our cohort was comparatively high (11.2 years), age was not an independent risk factor for adverse events in our multivariable models nor was it significant in the North American Pediatric Cardiomyopathy Registry (PCMR) study by Webber et al. [11]. Freedom from death (81% at 1 year and 71% at 5 years) in that study was closer to our center’s experience. These improved, contemporary outcomes could be due to earlier diagnosis of RCM before the development of HF or elevated PVR. Indeed, a PVRi > 2.5 iWu was measured in only 23% of our patients who underwent cardiac catheterization. Our institution’s practice has evolved based on this evidence such that diagnostic cardiac catheterizations are performed prior to listing for HTx annually instead of every 6 months. Other potential reasons include effective screening for arrhythmia and conduction abnormalities, ICD/PM use, aggressive medical management of HF symptoms when present, and evolving MCS strategies [20, 21] in this population.
We observed no difference in mortality between the pure RCM and mixed RCM/HCM phenotypes, yet pure RCM had lower transplant-free survival. These findings mirror those seen in the PCMR study by Webber et al. [11]. In fact, pure RCM and younger age were independent predictors of time to HTx on multivariable analysis. This pattern of early listing for HTx in these subpopulations likely reflects physician behavior based on the poor pre-HTx outcomes from historical reports as mentioned. It may also be that the technical challenges and morbidity associated with advanced HF therapies in smaller patients (e.g., pocket infection with ICD/PM, CVA with VAD therapy) increase the incremental effectiveness of HTx. Younger patients with pure RCM may indeed be at higher risk for clinical deterioration, but this is not a certainty with the natural history drastically altered by current practice. As the donor supply continues to fall short of demand, the need for sophisticated risk stratification tools and organ allocation schemes, as well as more effective management strategies, becomes paramount.
Investigators have previously published on the types of conduction abnormalities [11, 22] and associated risk of sudden cardiac death in pediatric RCM [22]. The present study is, to our knowledge, the first report of ICD/PM-related outcomes. While 40% received an ICD/PM, device therapies were relatively rare (7 total in 77 patient-years at risk), and inappropriate therapies were exceedingly rare (1 total). These results reinforce the practice of regular arrhythmia monitoring and the judicious implantation of ICD/PMs which can abort potentially lethal arrhythmias. Further research is required to track acute and chronic device complications since this was not a focus of the present study.
Mechanical circulatory support as a bridge to HTx continues to be a challenge in this population [21]. In the present study, the only two cases of CVA occurred while on MCS, so the complication likely cannot be attributed to the cardiomyopathy itself. Similar to the PCMR study [11], no intra-cardiac thrombi were recorded in our cohort. It may be that the use of anticoagulation for pediatric RCM in the contemporary era (56% in our cohort, 43% in the published PCMR population) has decreased the frequency of this complication. Alternatively, early HTx in patients with very low cardiac output at highest risk for thromboembolism might account for the rarity of this complication. Further study is required to understand the risk factors for thromboembolism in this population.
In multivariable analysis, greater than two HF admissions was an independent risk factor for time to the composite adverse outcome while markers of congestion (LA dilation and diuretic use) were predictive of mortality. Interestingly, young age and pure RCM phenotype were significant predictors of HTx only. Surprising to us, a documented arrhythmia was not a significant risk factor in our models; ICD/PMs may have altered the natural history of disease in some cases. Of course, the numbers in each variable category are modest and reflect our own institution’s referral pattern. Nevertheless, these data are hypothesis generating and may shift the focus of pediatric RCM management more toward the assessment and aggressive treatment of common yet dangerous problems such as congestive HF and arrhythmia, and away from the now less common problems of low cardiac output, elevated PVR, and thromboembolism.
Compared to a contemporaneous UNOS benchmark, pediatric RCM patients listed for HTx at our institution had a significantly longer median waitlist time; however, post-HTx survival was non-inferior. The reason for the longer waitlist time is unknown, but it is not related to a difference in UNOS listing strategy; 61% were listed status 2 at our institution and the other UNOS centers. Similarly, in the Pediatric Heart Transplant Study (PHTS) that evaluated waitlist outcomes in 145 pediatric RCM patients from 1993 to 2006 [13], 56% of patients were initially listed status 2. Post-HTx survival in that study was nearly identical to our cohort as well. These data suggest that HTx can be safely delayed in certain cases without worsening waitlist or post-HTx outcomes. Indeed, two pediatric RCM patients in our cohort were removed from the waitlist due to clinical improvement.
Study Limitations
Our method of retrospective chart review limited the ability to capture all outcomes, especially in those patients that transitioned to adult cardiomyopathy/transplant programs. In order to avoid this problem for survival analysis post-HTx, we leveraged the UNOS database that tracks patients regardless of such transition. However, for those not listed for HTx, adverse outcomes including mortality could be underestimated in our analysis.
While this cohort represents one of the largest single institutional experiences published to date, the still modest number of patients limits the power of the study to evaluate potential predictors of outcomes. Multi-institutional databases, such as the PCMR to which our institution contributes, are designed to overcome this limitation. Furthermore, our focus on patient characteristics at presentation might have missed clinically relevant risk factors that would have been identified through analysis of serial data.
Conclusion
Traditional management of pediatric patients with RCM comprises of listing for HTx at diagnosis. In the current, single-center study, we demonstrate the outcomes of an approach that incorporates close monitoring and management of arrhythmias with medical and device therapy to delay eventual listing. Utilizing this approach, our patient cohort was maintained off the transplant list 58% of the time overall (38% RCM vs. 88% RCM/HCM), without negative effects on survival post-transplant when compared to a contemporaneous UNOS benchmark. These data support a treatment pathway other than immediate HTx listing that may be appropriate for select patients, although further research is needed to determine specific clinical markers for patient selection.
References
Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113(14):1807–1816. https://doi.org/10.1161/circulationaha.106.174287
Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, Lurie PR, McCoy KL, McDonald MA, Messere JE, Colan SD (2003) The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med 348(17):1647–1655. https://doi.org/10.1056/NEJMoa021715
Nugent AW, Daubeney PEF, Chondros P, Carlin JB, Cheung M, Wilkinson LC, Davis AM, Kahler SG, Chow CW, Wilkinson JL, Weintraub RG (2003) The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med 348(17):1639–1646. https://doi.org/10.1056/NEJMoa021737
Weller RJ, Weintraub R, Addonizio LJ, Chrisant MRK, Gersony WM, Hsu DT (2002) Outcome of idiopathic restrictive cardiomyopathy in children. Am J Cardiol 90(5):501–506. https://doi.org/10.1016/S0002-9149(02)02522-5
Chen S-C, Balfour IC, Jureidini S (2001) Clinical spectrum of restrictive cardiomyopathy in children. J Heart Lung Transplant 20(1):90–92. https://doi.org/10.1016/S1053-2498(00)00162-5
Lewis AB (1992) Clinical profile and outcome of restrictive cardiomyopathy in children. Am Heart J 123(6):1589–1593. https://doi.org/10.1016/0002-8703(92)90814-C
Cetta F, O’Leary PW, Seward JB, Driscoll DJ (1995) Idiopathic restrictive cardiomyopathy in childhood: diagnostic features and clinical course. Mayo Clin Proc 70(7):634–640. https://doi.org/10.4065/70.7.634
Denfield SW, Rosenthal G, Gajarski RJ, Bricker JT, Schowengerdt KO, Price JK, Towbin JA (1997) Restrictive cardiomyopathies in childhood. Etiologies and natural history. Tex Heart Inst J 24(1):38–44
Russo LM, Webber SA (2005) Idiopathic restrictive cardiomyopathy in children. Heart 91(9):1199–1202. https://doi.org/10.1136/hrt.2004.043869
Rivenes SM, Kearney DL, Smith EOB, Towbin JA, Denfield SW (2000) Sudden death and cardiovascular collapse in children with restrictive cardiomyopathy. Circulation 102(8):876–882. https://doi.org/10.1161/01.cir.102.8.876
Webber SA, Lipshultz SE, Sleeper LA, Lu M, Wilkinson JD, Addonizio LJ, Canter CE, Colan SD, Everitt MD, Jefferies JL, Kantor P, Lamour JM, Margossian R, Pahl E, Rusconi P, Towbin JA (2012) Outcomes of restrictive cardiomyopathy in childhood and the influence of phenotype: a report from the pediatric cardiomyopathy registry. Circulation. https://doi.org/10.1161/circulationaha.112.104638
Dipchand AI, Naftel DC, Feingold B, Spicer R, Yung D, Kaufman B, Kirklin JK, Allain-Rooney T, Hsu D (2009) Outcomes of children with cardiomyopathy listed for transplant: a multi-institutional study. J Heart Lung Transplant 28(12):1312–1321. https://doi.org/10.1016/j.healun.2009.05.019
Zangwill SD, Naftel D, L’Ecuyer T, Rosenthal D, Robinson B, Kirklin JK, Stendahl G, Dipchand AI (2009) Outcomes of children with restrictive cardiomyopathy listed for heart transplant: a multi-institutional study. J Heart Lung Transplant 28(12):1335–1340. https://doi.org/10.1016/j.healun.2009.06.028
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 23(5):465–495. https://doi.org/10.1016/j.echo.2010.03.019
Mogensen J, Kubo T, Duque M, Uribe W, Shaw A, Murphy R, Gimeno JR, Elliott P, McKenna WJ (2003) Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J Clin Investig 111(2):209–216. https://doi.org/10.1172/JCI16336
Sluysmans T, Colan SD (2005) Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol 99(2):445–457. https://doi.org/10.1152/japplphysiol.01144.2004
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28(1):1–39.e14. https://doi.org/10.1016/j.echo.2014.10.003
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H (2011) 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines developed in collaboration with the American Association for Thoracic Surgery, American Society of echocardiography, American Society of nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 58(25):e212–e260
Pauli C, Fakler U, Genz T, Hennig M, Lorenz H-P, Hess J (2002) Cardiac output determination in children: equivalence of the transpulmonary thermodilution method to the direct Fick principle. Intensive Care Med 28(7):947–952. https://doi.org/10.1007/s00134-002-1334-2
Su JA, Menteer J (2017) Outcomes of Berlin Heart EXCOR® pediatric ventricular assist device support in patients with restrictive and hypertrophic cardiomyopathy. Pediatr Transplant 21(8):e13048. https://doi.org/10.1111/petr.13048
Villa C, Broderick J, Rizwan R, Lorts A (2018) Utilization of VADs in children with restrictive and hypertrophic cardiomyopathy: are we there yet? Prog Pediatr Cardiol. https://doi.org/10.1016/j.ppedcard.2018.03.003
Walsh MA, Grenier MA, Jefferies JL, Towbin JA, Lorts A, Czosek RJ (2012) Conduction abnormalities in pediatric patients with restrictive cardiomyopathy. Circulation: Heart Fail. https://doi.org/10.1161/circheartfailure.111.964395
Acknowledgements
We acknowledge Tricia A. Hengehold, BS for her substantial efforts in data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Research Involving Human and Animal Participants
This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Wittekind, S.G., Ryan, T.D., Gao, Z. et al. Contemporary Outcomes of Pediatric Restrictive Cardiomyopathy: A Single-Center Experience. Pediatr Cardiol 40, 694–704 (2019). https://doi.org/10.1007/s00246-018-2043-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-018-2043-0