Abstract
In August 2010, the Nit-Occlud® Lê (EUREVECO) became available for transcatheter coil occlusion of ventricular septal defects (VSDs). Retrospective European Registry for VSD Closure using the Nit-Occlud® Lê-VSD-Coil; analysis of the feasibility, results, safety and follow-up of VSD-closure over a 3-year period in 18 European centers. In 102 of 111 patients (female 66), successful VSD closure was performed (mean age 8.2 years, mean weight 28.82 kg), 81 perimembranous VSDs (48 with aneurysm), 30 muscular VSDs, mean procedure time was 121.1 min, and mean fluoroscopy time was 26.3 min. Short- and midterm term follow-up was possible in 100/102 patients, there was 1 embolization and 1 explantation after 24 months. Immediate complete closure occurred in 49 of 101 patients (48.5%), trivial residual shunt was present in 51 (50.0%), closure rate was 95% after 6 months and 97% after 1 year. Out of the 102 patients, there were 2 severe complications (1.8%) (1 severe hemolysis, 1 embolization) and 8 moderate/transient (=7.2%) including 1 transient AV block. During a mean follow-up period of 31.3 months (range 24–48) and a total follow-up time of 224.75 patient years, no further problems occurred. VSD closure with the Nit-Occlud® Lê VSD coil is feasible and safe with a minimal risk of severe side effects. The long-term effects and safety require further clinical follow-up studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interventional VSD closure was first described in 1988 by Lock et al. [1] who used a Rashkind double-umbrella device. The Amplatzer devices in the second half of the 1990s increased safety, and the results for interventional VSD closure with suitable devices became increasingly successful and even comparable to surgery [2–5]. In 2007, a retrospective multicenter European study in 430 patients showed acceptable results regarding closure rates but an unacceptable high rate of permanent AV block [6]. Thereafter, the widespread use of the Amplatzer membranous VSD device was abandoned in many institutions.
A new device, the pfm Nit-Occlud® Lê VSD coil (PFM: Produkte für die Medizin AG, Cologne, Germany), was developed for dedicated VSD closure and gained CE mark in August 2010. Very few case reports and case series with this device have been published, and observational midterm or long-term data are missing [7, 8]. Interestingly, there were no cases of permanent complete AV block reported so far. In this study, we report the short- and midterm results of a relatively large cohort of patients where the pfm Nit-Occlud® Lê VSD coil was used for transcatheter VSD closure on an intention-to-treat basis. The data collection was made possible by a close collaboration between many European centers and named as European Registry for Ventricular Septal Defect Coil Occlusion (EUREVECO).
Methods
Patients
In the participating institutions, all successive patients referred for VSD closure from August 2010 until August 2013 were included on an intention-to-treat basis based on clinical signs or echocardiographic parameters. Many of the investigations were performed in the presence of a proctor as recommended by the company training protocol. Patient data were obtained retrospectively with a defined questionnaire. A standardized follow-up protocol was used which includes ECHOs and ECGs on day 1 and/or 2 after VSD closure and during the 3-, 6-, 9-, 12-, 24-, 36-, and 48-month follow-up. Indication for closure was defined by the referring physicians and included left ventricular volume load assessed by echocardiography.
Written and informed consent were obtained from the patients or their legal guardians. The ethical committee of the supervising center (HDZ NRW, Bad Oeynhausen, Germany) approved this retrospective analysis (Ref-Nr. 46/2013). Additional approval was obtained wherever necessary. There was no funding or support of the device manufacturer regarding the conception of the study, data collection, data analysis, or funding of the participating investigators.
Patient Selection
Patients with muscular or perimembranous VSDs seem suitable for interventional closure with the device after echocardiographic assessment if there was a distance between the rim of the VSD and the aortic annulus of minimal 2 mms, or if the final estimated position of the coil in an aneurysmatic perimembranous VSD would allow complete coil placement without touching the aortic valve. In perimembranous VSDs, the patient should be older than 12 months or have a body weight of more than 9 kg.
Device Description
The Nit-Occlud® Lê VSD coil is made of nitinol wires with polyester fibers attached to the left-sided parts of the loops. The device configures as larger left-sided cone with reinforced distal coil loops and a smaller right-sided cone that configures over the left cone. The device comes in the sizes: 8/6, 10/6, 12/6, 12/8, 14/8, and 16/8 mm, whereas the first figure describes the diameter of the device at the left ventricular side and the second number describes the diameter of the device at the right ventricular end (see Fig. 1f). The 8/6, 10/6, and 12/6 coils are premounted on a 6F catheter, and the 14/8 and 16/8 devices are premounted on a 7F catheter and have an increased axial stiffness. Implantation is performed via suitable 6- and 7-F-long sheaths. The coil is fixed by a patented mechanical friction mechanism on a guiding wire and released by pushing it off this wire by using a secured slide control mechanism. The underlying concept how this device achieves VSD closure is more a cuddly filling of the left ventricular entry of the defect with a single right ventricular securing coil spring and not a stenting or clamping of the defect (see Fig. 1). In aneurysmatic defects, a larger device is easily placed and pulled into the aneurysm, underscoring the concept of defect filling rather than closing the minimal orifices only.
a Nit-Occlud® Lê VSD coil. b Bench testing. The coil is partially configured distally. c By further deployment, the proximal loop reverses around the first loops. d The coil is then pulled into the defect. e The coil is fixed from the right side. f Schematic drawing of the coil diameters. P proximal, right side; D distal, left side
This device has an European registration for VSD closure (CE mark) and is available in Asia, Russia, Latin America, and Africa. It is currently under clinical investigation by the FDA and therefore not generally available in the USA yet.
Determination of Coil Size
The distal diameter (D—left ventricular side) of the coil should be selected as at least twice the minimal diameter of the VSD (on the right ventricular side), and equal to or 1–2 mm greater than the diameter of the VSD at the left ventricular opening. Assessment of the size of the VSD becomes more difficult where a septal aneurysm is also involved, particularly in the case of a complex VSD with more than one opening on the right side. In this situation, coil selection has to be carried out solely on the basis of the left ventricular diameter of the aneurysm. The closure of a perimembranous VSD with a pronounced aneurysm does not require the observation of any specific distance from the aortic valve. The coil selected should fill the aneurysm without projecting into the left ventricular outflow tract.
Procedure
The procedures were carried out under general anesthesia or deep conscious sedation according to the institutional protocols. Prophylactic antibiotics were administered, the patients were heparinized using a heparin bolus of 100 IU/kg body weight. An intraprocedural transthoracic (TTE) or rarely transesophageal echocardiography (TOE) was performed for detailed evaluation of the defect and the cardiac structures. Hemodynamic data such as shunt measurement (QP/QS) or pulmonary artery pressures were not routinely measured as by institutional standards. Hence, these values are not reported here. In perimembranous/outlet defects, a 6F right-sided femoral venous access and a 4F left-sided femoral arterial access were obtained, and in apical or mid-muscular defects, a right-sided transjugular approach was used. A LV angiogram was conducted to define the location and size of the defect (see Fig. 2a). A snare was placed in the main pulmonary artery and a complete arteriovenous loop created with a coronary catheter; its tip was advanced to the inferior (or superior) caval vein. The close contact between the catheter and the introducer of the sheath was obtained and secured by two clamps at each end of the noodle wire (i.e., kissing technique). The delivery sheath was advanced into the distal aortic arch, and the delivery catheter was advanced into the aorta and pushed outside the sheath by about 1–2 cm. The loops of the complete left disc and the beginning of the right loops of the coil were configured in the ascending aorta (see Fig. 2b), and then the whole ensemble was slowly retracted until the device jumped across the aortic valve into the LV. There was no entangling or unlooping of the coil by this maneuver. A LV angiography was repeated, and if necessary, the next loops were configured on the left side, and then the device was slowly pulled back into the defect. Thereafter (see Fig. 3c), the final loops were deployed at the right side of the defect.
Perimembranous VSD with an aneurysm. a Note the aneurysmatic appearance of the VSD. b The sheath together with the delivery catheter is placed in the ascending aorta, and the VSD coil is configured there. c The coil is then pulled into the VSD to fill the defect. d After complete release, the VSD is closed
After implantation, a repeated echocardiography was performed to assess the location of the device, potential aortic incompetence as well as tricuspid regurgitation. A trivial to moderate residual shunt was accepted. If the position was judged adequate, the device was released. Thereafter, a repeat assessment was performed to check the location of the device and the residual shunt (see Figs. 1d, 2). If device retrieval was necessary, it could easily be pulled back into the delivery catheter at any stage of the procedure before release.
Intraprocedural Abortion of the Procedure
The procedure was aborted when there was an acute second- or third-degree AV block during the procedure, an impairment of the aortic valve after device placement or a significant residual shunt. Severe tricuspid incompetence or entangling of the tricuspid chordae by the right-sided loops of the device would require reconfiguration of the right-sided loops or device removal.
Follow-Up
The patients were assessed clinically for murmurs and hemolysis and by ECG and ECHO on day 1 (and/or day 2) after the procedure and followed up after 1, 3, 6, 9, 12 months and then yearly after the VSD closure.
Results
Patient Data and VSD Characteristics
One hundred and eleven patients (45 males, 66 females) underwent an attempt of VSD closure with the pfm device between October 2010 and October 2013. The mean age was 8.4 years (8 months 67 years, median 5.1 years), 4 patients were less than 1 year of age, 55 between 1 and 6 years, 38 between 6 and 14 years, and 14 older than 14 years. The mean body weight was 28.82 kg (7.18–109 kg, median 17.0 kg), 9 patients were less than 10 kg, 44 between 10 and 20 kg, 31 between 20 and 40 kg, and 27 above 40 kg. The mean height was 122.2 cm (61–194 cm, median 113 cm). There were 81 perimembranous/outlet VSDs (including 48 with aneurysm, 20 showed multiple exits), 30 muscular VSDs (including 6 apical VSDs) (see Table 1, Fig. 4).
Procedural Data
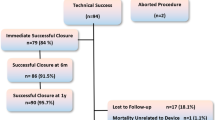
In 110 of 111 patients (98%), it was possible to successfully place the VSD coil in the VSD, the mean procedure time (sheath into sheath out times) was 121.1 min (SD 55.64 min, range 15–278 min, median 110 min), and the mean fluoroscopy time was 26.3 min (SD 14.9, range 7.5–86.3 min, median 21.7 min). A transjugular approach was used in 17/111 patients. Devices used were 8 × 6 (n = 26), 10 × 6 (n = 37), 12 × 6 (n = 30), 14 × 8 (n = 13), and 16 × 8 (n = 4). Additional implantation data are available in Table 2 and in flowchart Fig. 5.
Implantation Success
Definitive device implantation was possible and successful in 102/111 patients (91.9%) and failed in 9 patients (8.1%) (see Table 3; flowchart Fig. 5). The reasons for failure were the inability to advance the delivery sheath through the defect, and one defect was too large to be closed with the devices available; this defect was closed with an 18 PDA Amplatzer Duct Occluder I. In one patient, a transient III° AV block occurred when the sheath was advanced through the VSD and the patient was operated thereafter. Two patients showed an impairment of the aortic valve caused by contact of the implanted coils with the valve. In two other patients, there were severe residual shunts after coil placement so the coils were removed. In two remaining patients, it was impossible to position the coils correctly. Two devices were explanted and the defects closed surgically, one device after embolization 1 day after the procedure, one device after 24 months due to severe residual shunt and late secondary device displacement (see Table 3).
Hemolysis
Hemolysis was assessed clinically (i.e., hematuria) and occurred in four patients. In two patients, it resolved spontaneously after 1 and 2 days, respectively, after cessation of peri-interventional anticoagulation with heparin. In another patient, clinically apparent hemolysis persisted for 14 days requiring transfusion. A second device was implanted and the patient recovered immediately. One patient showed mild hemolysis even after 2 years, and due to device displacement, the decision was made to explant the device and close the VSD surgically.
Complications
Severe complications were defined as those requiring surgical interventions or death. Other complications that were transient, self-limiting or could be managed by medical treatment were classified as moderate or transient. There were two severe complications (1.9%) (see Table 3). In one patient, the device was explanted surgically due to clinically important hemolysis. In another patient, the device was explanted surgically after embolization. Four patients had moderate and transient complications (=3.8%). One patient suffered from an acute complete AV block about 5 days after the implantation. Initially, there was a right bundle branch block (RBBB). After diagnosis of AV block III°, the patient was treated with steroids for 1 week and sinus rhythm recovered completely after 3 days. The patient is still—4 years after the procedure—in stable sinus rhythm without further problems. Three patients developed hemolysis (see above). Mild complications occurred in 15 patients (14.7%). Six patients developed new-onset RBBB without any signs of AV conduction delay, five patients showed a mild tricuspid regurgitation on ECHO without any hemodynamic consequence, and three patients showed a persistent aortic regurgitation that was present before the procedure without contact between the device and the aortic valve. One patient showed a potential device fracture on a chest X-ray without any impairment of device function or cardiac structures.
Closure Rate
Immediate closure during implantation was achieved in 44/102 patients (43.1%); in five additional patients, the defect was completely closed at the time of discharge; and in 51 patients, there was trivial flow detectable across the coil. These results remained stable with higher complete closure rates over the follow-up period, and complete closure was achieved in 90/101 at 3 months, 93/98 at 6 months, 94/97 at 12 months, 82/83 at 18 months, 67/68 at 24 months 31/31 at 36 months, and 16/16 at 48 months, revealing a closure rate of about 99% (see Table 4). The mean follow-up period was 31.3 months (range 24–48, median 29.3 months), and the total follow-up time can be summarized to 224.75 patient years.
Discussion
Interventional VSD closure was first described by Lock et al. who used a Rashkind device [1, 9]. Today, interventional VSD closure can be performed in many defects in various anatomic localizations and different age ranges [10, 11]. The ideal device for transcatheter closure of VSDs should be easy to apply preferably via a small sheath, suitable for all forms of VSDs, be reconfigurable and retrievable and it should close the defect completely within a reasonable time frame. It should not change the anatomy of the surrounding tissue and avoid or minimize the risk of conduction abnormalities especially cAVB. Many devices have been developed, and devices originally designed for other indications are used, thus increasing the options for interventional treatment. These devices are however off label and without CE mark for VSD closure in Europe [12–14]. The device presented was specifically designed for interventional VSD closure, has European CE registration, and is licensed in many countries worldwide.
With the introduction of the Amplatzer VSD devices, safety increased and the results with the original Amplatzer and comparable devices became comparable to a surgical approach [2–5, 12–14]. These occluders consist of a double-disc design with a stent in the middle part and gain stability by stenting the defects and clamping with the discs from both sides [15, 16]. These properties may be the reason for subsequent alterations of the surrounding tissue. In 2007, a multicenter European study with 430 patients showed acceptable closure results but an unacceptable high rate of permanent AV block in 12 of 250 perimembranous VSDs (4.8%) [6]. Similar results were reported by others [7, 17]. It has been postulated that this risk may be minimized by avoiding oversized devices [18]. The pfm device presents with a different mode of action for VSD closure avoiding these mechanical forces; it is noteworthy that no permanent AV block is reported in the literature as well in the study presented.
The newly designed Amplatzer perimembranous VSD occluder device 2 (pmVSD2) should reduce the risk of AV block by decreasing radial and clamping forces [19]. In 3/18 patients, there was mild residual shunting (17%); no new AV block was reported during the follow-up of 14 ± 3 months [20]. The commercial release of the device was stopped to allow for improvements of a new device design.
The Amplatzer Duct Occluder (ADO) was used in selected cases for closure of aneurysmatic pmVSDs due to the anatomic resemblance to a PDA [13, 21, 22]. More recently, the off-label use of the Amplatzer Duct-Occluder II (ADO II) was reported [23]. Kanaan presented a case series of 28/30 (93.5%) successful VSD closures over 9 years by using the ADO II [24]. There was a rate of 2/29 (6.9%) residual shunt across the meshwork of the device. Commonly available PDA coils were used for closing of selected smaller VSDs, the main problem remaining is residual shunting [12, 13, 25].
The recently developed Nit-Occlud® Lê VSD coil (pfm, Cologne, Germany) that was used in this study can be used for many different VSDs [7]. It is implanted transvenously, completely retrievable, allows repositioning and a fine adjustment to the given anatomy at any time during implantation. The underlying concept of this flexible device is more a “cuddly filling of the (mostly funnel shaped) defect” (see Fig. 1), rather than occluding the defect by a “stent-and-clamp” mechanism. Effective closure and adequate stability is achieved by its distal loop structure and the polyester fibers. The implantation success is comparable with the results of Amplatzer-like devices [26], thereby offering equal success rate at a higher safety level.
Odemis et al. [8] presented 20 patients, the mean age was 7.3 years (±4.0 years), the mean weight was 25.7 kg (±11.8 kg), QP/QS was 1.7:1 (±0.4), the total procedure time was 88.5 min, and the fluoroscopy time was 29.4 min (±14.8 min, range 13.3–67.4 min). All patients had perimembranous defects, and in 19/20 patients, there was an aneurysm of the septum, and all defects could be closed successfully. Three patients developed hemolysis that led to surgical device removal in one patient. Two patients had residual minor shunts persisting for 90 days; there was no transient or permanent AV block.
Chungsomprasong et al. [7] retrospectively compared the results of 116 patients, 76 treated with an Amplatzer VSD occluder and 33 with the Nit Occlud® Lê VSD device. There was a significant difference in the complication rate; in five of the 76 patients with an Amplatzer device, cAVB developed; in the Nit-Occlud® group, one patient developed a transient cAVB but did not require pacemaker implantation.
In the study presented, there was a high success rate combined with a relatively low rate of complications or side effects, even in patients with perimembranous defects. These results support the existing publications and make this device an attractive alternative for this patient group.
Complications and Points for Attention
AV Block
We did not encounter any permanent complete AV Block in our patient cohort. The occurrence of cAVB is explained by direct compression trauma, the pressure of radial forces, the clamping forces (i.e., oversized devices), and an inflammatory process: This led to the development of softer devices or the use of muscular VSD devices or PDA devices [18, 19, 21, 27]. Holzer reported two patients out of 100 with permanent complete AV block requiring pacemaker implantation [28], similar to the results reported in the European registry [6]. The studies by Odemis and Chungsomprasong reported no permanent complete AV blocks in the patients treated [7, 8]. In our cohort, we had one patient with a transient complete AV block that could be successfully treated by steroids. The overall risk of permanent AV block was recently analyzed by Yang et al. [26]. The pooled estimate for cAVB was 121/4406 = 2.7% (95% CI 1.6–3.2) with higher rates in smaller children. These results may underscore the potential benefit of this device design as well as the VSD closure mechanism without clamping forces that may alter the conduction system.
Other Conduction Abnormalities and Arrhythmias
The rate of arrhythmias is generally small, with a pooled estimate of 10.6% (95% CI 8.4–12.7) [26]. The international perimembranous VSD registry reported 11/100 patients with new-onset arrhythmias or conduction abnormalities other than permanent AV block including 5/100 with permanent RBBB [28]. Our results compare quite well with these results as we detected 6/102 patients with new-onset RBBB but no left bundle branch block (LBBB) or other arrhythmias (see Table 3).
Aortic and Tricuspid Valve Impairment
Yang et al. reported that secondary impairments of the tricuspid and aortic valve seem common with a pooled estimate of a rate of 4.9% (95% CI 3.4–6.4) [26]. The rate of permanent valvular defect was 2.3% (95% CI 1.3–3.3 with tricuspid regurgitation in 1.7% (95% CI 0.8–2.5) and aortic regurgitation in 2.0% (95% CI 1.0–2.9). Our results show only trivial aortic or tricuspid incompetence. This again may be an indication that the Nit Occlud® Lê VSD device is more flexible and thereby prevent damage of the surrounding tissue.
Residual Shunt and Hemolysis
The most commonly reported complication in VSD device closure is residual shunt; this was documented in 1134 subjects by 35 papers involving 4138 patients, the pooled estimate of transient shunt was 25.5% (95% CI 18.9–32.1), the pooled estimate of permanent shunt was 3.1% (95% CI 2.0–4.1) [26]. Chungsomprasong et al. reported that the residual shunt tended to decrease in the Amplatzer group, while the residual shunt tended to stay the same in the pfm group. In the study of Odemis et al., two of the 20 patients had medium residual shunts and one had a mild residual shunt. Additionally, hemolysis emerged hours after the procedure in three patients.
Zuo et al. [29] reported that 2/301 patients had intravascular hemolysis (0.7%) after VSD closure with the Amplatzer pmVSD device, and Li et al. [30] reported that three (1.3%) of the 223 patients with perimembranous VSDs developed hemolysis. Intravascular hemolysis developed in three (15%) of the patients reported by Odemis et al. [8], two recovered, and in one patient, the device had to be removed surgically. Hemolysis caused by the pfm coil may be due to the device design and softness. In our case series, the rate of residual shunt and hemolysis is comparable to the studies discussed here. Two devices required explantation, and in two other patients, hemolysis resolved. This side effect is of concern as two of the devices had to be removed surgically during follow-up.
Fluoroscopy Time
The reported fluoroscopy time varies between 32, 41, and 43 min (range 11–191 min) [10, 11, 17, 31]. The international perimembranous VSD registry using the Amplatzer membraneous VSD device in 100 patients reported of a mean fluoroscopy time of 22.1 min (range 8.9–96 min) [28]. In the single-center study of Ewert et al. [12], a mean fluoroscopy time of 26.2 min (range 8.3–56.5 min) was reported in 26 patients using Amplatzer Occluders and Nit-Occlud PDA coils. Some single-center series report shorter fluoroscopy times [24]. Many of the procedures analyzed here were however performed in the institutions for the first time as proctoring procedures which lengthen the procedure and fluoroscopy time. Based on this, it may well be explained that the procedure time may seem somewhat long (median 110 min, mean 121.1 min). Despite this, the fluoroscopy times in our series do indicate that the feasibility of device implantation is similar to other series reported.
Study Limitations
Although the data were obtained in a prospective manner, on an intention-to-treat basis and most of the patients were treated in a training surrounding with the attendance of proctors, the inclusion of the patients was not standardized prospectively. Prospective study trials may offer valuable additional information such as shunt size, LV diameters, or other hemodynamic data, all of which were not requested for our data analysis. Therefore, the study suffers the theoretical biases of such investigations. In addition, the sample size and the follow-up period may be judged to be only moderate. Therefore, long-term performance and utility for late device function remains to be investigated. In addition, only VSDs selected and deemed suitable for closure with the device were included and so closure of very large VSDs was not attempted with the devices available.
Conclusion
The Nit-Occlud®-Lê VSD coil device can be used for a large variety of selected cases of VSDs. It is easy to use as reflected by fluoroscopy and procedural time and has a high rate of success. The device proved to be safe with regard to potential effects on the aortic and tricuspid valve, and there was no permanent AV block especially in patients with perimembranous VSDs. Patients with a residual shunt should be followed closely for the development of clinically important hemolysis. Larger and perhaps prospective series may be needed to evaluate the long-term safety and potential future side effects or complications. We believe that this new device offers the possibility for a safe approach in VSD closure especially in patients with perimembranous VSDs.
Impact on Daily Practice
The Nit-Occlud®-Lê VSD coil device can be used for a large variety of selected cases of VSDs and offers the possibility for a safe approach in VSD closure especially in patients with perimembranous VSDs.
The device proved to be safe with regard to potential effects on the aortic and tricuspid valve, and there was no permanent AV block especially in patients with perimembranous VSDs.
Larger and perhaps prospective series may be needed to evaluate the long-term safety and potential future side effects or complications as patients with a residual shunt require close follow-up to assess for the development of clinically important hemolysis.
Abbreviations
- VSD:
-
Ventricular septum defect
- Nit-Occlud® Lê-VSD-Coil:
-
Coil used in this study
- EUREVECO:
-
European Registry for Ventricular Septal Defect Coil Occlusion
- LA:
-
Left atrium
- LV:
-
Left ventricle
- PA:
-
Pulmonary artery
- RV:
-
Right ventricle
- ECHO:
-
Echocardiography
- ECG:
-
Electrocardiogram
- TTE:
-
Transthoracic echocardiography
- TOE:
-
Transesophageal echocardiography
- RBBB:
-
Right bundle branch block
- AVBlock III°:
-
Third-degree complete atrioventricular block
References
Lock JE, Block PC, McKay RG, Baim DS, Keane JF (1988) Transcatheter closure of VSDs. Circulation 78:361–368
Bass JL, Kalra GS, Arora R, Masura J, Gavora P, Thanopoulos BD, Torres W, Sievert H, Carminati M, Fischer G, Ewert P (2003) Initial human experience with the Amplatzer perimembranous ventricular septal occluder device. Catheter Cardiovasc Interv 58:238–245
Yang J, Yang L, Yu S, Liu J, Zuo J, Chen W, Duan W, Zheng Q, Xu X, Li J, Zhang J, Xu J, Sun L, Yang X, Xiong L, Yi D, Wang L, Liu Q, Ge S, Ren J (2014) Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: a randomized controlled trial. J Am Coll Cardiol 63(12):1159–1168
Oses P, Hugues N, Dahdah N, Vobecky SJ, Miro J, Pellerin M, Poirier NC (2010) Treatment of isolated ventricular septal defects in children: Amplatzer versus surgical closure. Ann Thorac Surg 90(5):1593–1598
Masura J, Gao W, Gavora P, Sun K, Zhou AQ, Jiang S, Ting-Liang L, Wang Y (2005) Percutaneous closure of perimembranous ventricular septal defects with the eccentric Amplatzer device: multicenter follow-up study. Pediatr Cardiol 26(3):216–219
Carminati M, Butera G, Chessa M, De Giovanni J, Fisher G, Gewillig M, Peuster M, Piechaud JF, Santoro G, Sievert H, Spadoni I, Walsh K, Investigators of the European VSD Registry (2007) Transcatheter closure of congenital VSDs: results of the European Registry. Eur Heart J 28:2361–2368
Chungsomprasong P, Durongpisitkul K, Vijarnsorn C, Soongswang J, Lê TP (2011) The results of transcatheter closure of VSD using Amplatzer® device and Nit Occlud® Lê coil. Catheter Cardiovasc Interv 78:1032–1040
Odemis E, Saygi M, Guzeltas A, Tanidir IC, Ergul Y, Ozyilmaz I, Bakir I (2014) Transcatheter closure of perimembranous VSDs using Nit-Occlud(®) Lê VSD coil: early and mid-term results. Pediatr Cardiol 35:817–823
Bridges ND, Perry SB, Keane JF, Goldstein SA, Mandell V, Mayer JE Jr, Jonas RA, Casteneda AR, Lock JE (1991) Preoperative transcatheter closure of congenital muscular VSDs. N Engl J Med 324(19):1312–1317
Diab KA, Cao Q-L, Mora B-N, Hijazi ZM (2007) Device closure of muscular VSDs in infants less than one year of age using the Amplatzer devices: feasibility and outcome. Catheter Cardiovasc Interv 70:90–97
Wang J, Zuo J, Yu S, Yi D, Yang X, Zhu X, Li J, Yang L, Xiong L, Ge S, Ren J, Yang J (2016) Effectiveness and safety of transcatheter closure of perimembranous ventricular septal defects in adults. Am J Cardiol 117(6):980–987
Ewert P, Kretschmar O, Peters B, Abdul-Khaliq H, Nagdyman N, Schulze-Neick I, Bass J, Lê TP, Lange PE (2004) Transcatheter closure of congenital septal defects. Z Kardiol 93:147–155
Michel- Behnke I, Le T-P, Waldecker B, Akintuerk H, Valeske K, Schranz D (2005) Percutaneous closure of congenital and acquired VSDs. J Interv Cardiol 18:89–99
Zartner P, Christians C, Stelter JC, Hraška V, Schneider MB (2014) Transvascular closure of single and multiple muscular VSDs in neonates and infants <20 kg. Catheter Cardiovasc Interv 83:564–570
Zhou D, Pan W, Guan L, Ge J (2012) Transcatheter closure of perimembranous VSDs and intracristal ventricular defects with the SHSMA Occluder. Catheter Cardiovasc Interv 79:666–674
Yang J, Yang L, Wan Y, Zuo J, Zhang J, Chen W, Li J, Sun L, Yu S, Liu J, Chen T, Duan W, Xiong L, Yi D (2010) Transcatheter device closure of perimembranous VSDs. Eur Heart J 31(18):2238–2245
Bjørnstad PG, Smevik B, Fischer G (2010) Catheter based closure of VSDs. Scand Cardiovasc J 44:9–14
Bentham JR, Gujral A, Adwani S, Archer N, Wilson N (2011) Does the technique of interventional closure of perimembranous VSD reduce the incidence of heart block? Cardiol Young 21:271–280
Bass JL, Gruenstein D (2012) Transcatheter closure of the perimembranous VSD-preclinical trial of a new Amplatzer device. Catheter Cardiovasc Interv 79:1153–1160
Tzikas A, Ibrahim R, Velasco-Sanchez D, Freixa X, Alburquenque M, Khairy P, Bass JL, Ramirez J, Aguirre D, Miro J (2014) Transcatheter closure of perimembranous VSD with the Amplatzer(®) membranous VSD occluder 2. Catheter Cardiovasc Interv 83:571–580
El Said HG, Bratincsak A, Gordon BM, Moore JW (2012) Closure of perimembranous VSDs with aneurysmal tissue using the Amplazter Duct Occluder I. Catheter Cardiovasc Interv 80:895–903
Lee SM, Song JY, Choi JY, Lee SY, Paik JS, Chang SI, Shim WS, Kim SH (2013) Transcatheter closure of perimembranous VSD using Amplatzer ductal occluder. Catheter Cardiovasc Interv 82:1141–1146
Koneti NR, Verma S, Bakhru S, Vadlamudi K, Kahtare P, Penumatsa RR, Qureshi S (2013) Transcatheter trans-septal antegrade closure of muscular VSD in young children. Cath Cardiovasc Interv 82(4):E500–E506
Kanaan M, Ewert P, Berger F, Assa S, Schubert S (2015) Follow-up of patients with interventional closure of VSDs with Amplatzer Duct Occluder II. Pediatr Cardiol 36:379–385
Nogi S, Haneda N, Tomita H, Yasuda K (2008) Trancatheter coil occlusion of perimembranous VSDs. Catheter Cardiovasc Interv 72:683–690
Yang L, Tai BC, Khin LW, Quek SC (2014) A systematic review on the efficacy and safety of transcatheter device closure of VSDs. J Interv Cardiol 27:260–272
Erdem S, Kizlltaş A, Küçükosmanoğlu O, Ozbarlas N (2012) Temporary atrioventricular complete block that develops following the transcatheter closure of VSD. Turk J Pediatr 54:80–82
Holzer R, de Giovanni J, Walsh KP, Tometzki A, Goh T, Hakim F, Zabal C, de Lezo JS, Cao QL, Hijazi ZM (2006) Transcatheter closure of perimembranous VSD using the Amplatzer membranous VSD occluder. Catheter Cardiovasc Interv 68:620–628
Zuo J, Xie J, Yi W, Yang J, Zhang J, Li J, Yi D (2010) Results of transcatheter closure of perimembranous VSD. Am J Cardiol 106:1034–1037
Li X, Li L, Wang X, Zhao H-B, Zhang S-Y (2011) Clinical analysis of transcatheter closure of perimembranous VSD with occluders made in China. Chin Med J 2011(124):2117–2122
Fu YC, Bass J, Amin Z, Radtke W, Cheatham JP, Hellenbrand WE, Balzer D, Cao QL, Hijazi ZM (2006) Transcatheter closure of perimembranous VSDs using the new Amplatzer membranous VSD occluder. J Am Coll Cardiol 47:319–325
Funding
The funding is by hospital funding only.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest by any of the authors regarding this report.
Additional information
Nikolaus A. Haas and Laura Kock have equally shared the authorship in this publication.
Rights and permissions
About this article
Cite this article
Haas, N.A., Kock, L., Bertram, H. et al. Interventional VSD-Closure with the Nit-Occlud® Lê VSD-Coil in 110 Patients: Early and Midterm Results of the EUREVECO-Registry. Pediatr Cardiol 38, 215–227 (2017). https://doi.org/10.1007/s00246-016-1502-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-016-1502-8