Abstract
Nonsurgical closure of congenital ventricular septal defects (VSD) has become increasingly acceptable with the availability of different occlusion systems. Transcatheter device treatment is used for perimembranous and muscular defects. Atrio-ventricular block remains the most troublesome complication of device closure. The aim of this study was to describe our experience with closure of VSD using the Amplatzer Duct Occluder II (ADO II) as an “off-label” approach in children and adults. Between 2004 and 2012 transcatheter closure of 31 VSD (20 perimembranous, 10 muscular VSD and 1 ruptured sinus valsalva) with ADO II was undertaken in patients between 3 months and 55 years of age and with a body weight ranging from 4 to 105 kg in our institution. In 29 of 31 procedures, the defect was successfully closed (93.5 %) without any significant complications. No increase of aortic or tricuspid valve regurgitation was found in any after procedure. Small residual shunts were observed immediately after the device implantation, but disappeared during a median follow-up period of 38 months (0.4–63) in 27 of 31 patients. There was no incidence of AV block or other conductance abnormalities during implantation or follow-up. The ADO II device is safe and effective for transcatheter VSD closure, but this is still an “off-label” use. After long-term follow-up in a large number of patients this device may be approved for VSD closure in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ventricular septal defect (VSD) is the most common congenital heart defect. These defects are classified according to their location within the septum: perimembranous, muscular and supracristal, with perimembranous VSD being the most common. Percutaneous closure of VSD started in the late 1980s, after devices designed for the closure of atrial septal defects (ASD) or patent ductus arteriosus (PDA) were implanted in the interventricular septum [4, 16, 20, 21].
Transcatheter VSD closure avoids extracorporeal circulatory support and the surgical approach and has the benefit of faster recovery, but both methods carry a potential risk of atrioventricular block (AVB). This occurs at an average rate of 5 % in most series but may be as high as 22 % [8, 14, 25]. AVB may develop intraoperatively or postoperatively; with transcatheter closure it is more unpredictable and may occur late, months or even years after device implantation. The incidence of AVB also depends on the device used, and more flexible devices may reduce this incidence significantly.
The aim of this study was to review data from our VSD closure experience with the Amplatzer® Duct Occluder II (ADO II) at our institution and to evaluate the risk–benefit ratio of the ADO II used for transcutaneous VSD closure (Table 1).
Patients and Methods
We reviewed retrospectively patients with VSD who underwent transcatheter VSD closure with an ADO II between 01/2008 and 12/2013 in our institution. All patients underwent transthoracic echocardiography (TTE) before catheterization. The inclusion criteria were clinical (including failure to thrive, limited capacity with New York Heart Association functional class II or higher and frequent respiratory infections) and/or technical evidence of a significant left to right shunt through the VSD (including signs of left ventricular enlargement in the ECG, cardiomegaly in the chest X-ray, echocardiographic left atrial and/or left ventricular enlargement).
Candidacy for Transcatheter Closure
A TTE examination was performed in all patients to determine the suitability of transcatheter closure based on the size, position, and relevance of the VSD. In perimembranous VSD (pmVSD) images from four-chamber views and parasternal short axis were analyzed for the presence of a VSD aneurysm and separation of the aneurysm from the tricuspid valve. Patients with a pmVSD with small rim (<3 mm) to the aortic valve were excluded. Informed written consent for the procedure was given by the patients or parents of the children before the procedure.
Catheterization Procedure
The catheterization procedure was performed under sedation in all patients. The sedation was initiated with intravenous Midazolam 0.1 mg/kg (maximum 5 mg) and maintained during the procedure with a continuous infusion of Disoprivan in all patients with body weight >6 kg, or with Ketamin (Ketanest S) in patients with body weight <6 kg. Heparin (100 IU/kg, 5000 IU maximum) was administered to all patients at the start of the procedure and continued with 300 IU/kg per day for 24 h after the intervention. Cefazolin was given as prophylaxis at a dosage of 100 mg/kg/d with 3 doses in 24 h.
The femoral approach was used in all patients: After puncture of the femoral vein and artery complete right and left heart catheterization was performed with calculation of flows and resistances. Shunt volume was calculated by oxymetric measurements. Angiograms in the left ventricle were performed at RAO 60°/LAO 30° or RAO 45°/LAO 45°, depending on the VSD location (see Figs. 1, 2). The diameter of the VSD was determined by echocardiography and angiography using the largest diameter (see Figs. 1, 2).
Device and Delivery System
A 4 Fr. Amplatz right catheter was introduced into the left ventricle for better orientation to the defect. The defect was passed from the left ventricle using a hydrophilic guide wire (Road Runner, Boston Scientific, Natick, MA, USA) or a 0.014 in. Hi-Torque Balance Middleweight Universal guide wire (Abbott Vascular, Abbott Park, IL, USA) or 0.018 in. Terumo (Terumo, Ann Arbor, MI, USA). The wire was placed in the pulmonary artery, right atrium, or superior caval vein. A micro-snare catheter was used to catch the wire from the pulmonary artery or equivalent to the femoral vein forming the arteriovenous circuit. A long sheath (4–5 Fr) was introduced into the femoral vein over the arteriovenous circuit wire and advanced through the VSD to the left ventricle and aorta.
After sizing of the defect from the angiogram, an adequately sized ADO II was selected and introduced over a flexible and low profile sheath catheter (4 or 5 French). The left ventricular disk was deployed either in the left ventricular outflow tract or in the middle of the left ventricle. Occasionally sheath kinking was seen and was eliminated by pulling back slightly.
Peri-Interventional Care and Follow-Up
After the procedure all patients underwent clinical examination, monitoring, TTE, Holter ECG monitoring, and chest X-rays before discharge. Platelet anti-aggregation therapy with Aspirin 3–5 mg/kg/day p.o. and endocarditis prophylaxis was prescribed for 6 months.
Statistical Analysis
Data were collected using Microsoft Excel software and were described as medians (ranges). The SPSS software (SPSS, Chicago, IL, USA) was utilized to evaluate data. Wilcoxon rank-sum testing was not applied to normally distributed data and the nonparametric Mann–Whitney U test was used to compare variables according to type of operation. A p value of less than 0.05 was considered to be statistically significant.
Results
Patient Groups
The rationale for VSD closure was a significant left to right shunt with enlargement of the left ventricle without clinical symptoms in 20 patients, reduced physical capacity in seven patients and failure to thrive in four patients. Severe regurgitation of the tricuspid valve was seen in two patients, caused by the VSD jet, leading to an interventional approach. Twenty-five of the 31 patients (80.6 %) were below 18 years of age and 18/31 (58.1 %) had body weight <15 kg. Four patients underwent percutaneous closure of a post-surgical residual VSD: one with status post correction of pulmonary atresia with VSD and one with status post Senning procedure (both with residual muscular VSD). The other two patients had a residual muscular VSD after arterial switch operation. All these patients were treated electively.
The percutaneous femoral venous and arterial approaches were used in all procedures. The size of the VSD measured in the angiogram ranged from 2 to 6 mm (median 5 mm), in the echocardiogram from 3 to 6 mm (median 4 mm). The size of the implanted ADO II device ranged from 3 × 4 mm to 6 × 6 mm (mean 4 mm). Combined procedures were performed in two subjects: stenting of the right pulmonary artery and ASD closure.
Safety and Efficacy
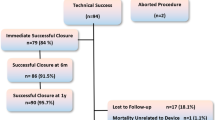
In 29 of 31 procedures (93.5 %), the defect was successfully closed. A stable position of the device was demonstrated by angiography and TTE. Significant shunt reduction was detected in all patients after successful VSD closure and only minor residual shunts were documented (see Table 2). Procedural data are reported in Table 3. There was no increase in aortic or tricuspid valve regurgitation (TR) in any patient after procedure. In one patient with severe tricuspid regurgitation (a 2-year-old girl) the regurgitations decreased to mild immediately after ADO II release (see Figs. 3, 4).
Complications
No deaths occurred. Neither AV block (AVB) nor implantation of a pacemaker because of rhythm abnormalities was reported directly after implantation or during follow-up. No vascular complication such as thrombosis, dissection, or aneurysm occurred.
In one patient the device (VSD 5 mm, ADOII 6/4 mm) had to be explanted before its release from the delivery system due to significant residual shunt and lack of possibility to upsize the device.
One significant complication occurred (1/31 patients, 3.2 %) in a 2.2-year-old girl (12.6 kg) with a perimembranous VSD (4 mm), a 4/4 mm ADO II was used to close the VSD. After release from the delivery system a device embolization in the RV occurred. It was possible to retrieve the device percutaneously: it was recaptured in the right ventricle using a goose-neck snare and an 8-F long sheath. The VSD was closed by surgery.
Hemolysis
No significant hemolysis with the need for retrieval of the device occurred. However, two patients (6.4 %) showed a positive urine dipstick test for erythrocytes during the first 24 h. The blood analysis showed high hemolysis parameters without a relevant decrease of hemoglobin. The hemolysis parameters returned to normal values during the first postprocedural week. There was no need for blood transfusion.
Follow-Up
Follow-up data were obtained from all patients with successful VSD closure with the ADO II device. Echocardiographic and electrocardiographic data incl. holter ECG were analyzed in the majority of patients. Median duration of follow-up was 41 months (range 0.6–67 months). No deaths or endocarditis occurred.
Arrhythmia
Arrhythmic complications (minor) occurred in three patients (9.6 %). A 2-year-old girl (mVSD) developed supraventricular tachycardia with 210 bpm 2 h after device implantation that lasted 5 min and was terminated by adenosine administration. One patient (boy, 7 years, pmVSD) had AV rhythm (80/min) for 24 h postimplantation; this resolved spontaneously. One patient showed in the Holter ECG isolated ventricular and supraventricular extrasystoles without need for treatment. There was no incidence of AVB, left bundle branch block, P-Q prolongation or complete heart block initially or during the follow-up evaluation.
Valve Regurgitation and Residual Shunt
There were four patients with mild aortic valve regurgitation (AR) and 26 with TR before the procedure. No significant increase or new regurgitation of the aortic or tricuspid valves occurred during or after the procedure. Aortic valve regurgitation disappeared in 2/4 patients during follow-up (see Table 3; Fig. 5). A reduction of pre-existing TR by at least 2 grades was seen in two patients after VSD closure; in one with pre-existing TR, the TR disappeared after VSD closure immediately (see Table 3).
Same patient as in Fig. 1: Aortography to obtain the distance between ADO II and aortic valve. RCA right coronary artery, AV aortic valve, Cath angiography catheter
A trivial residual shunt was present in 10 patients (32.2 %) at the end of the procedure. At discharge only three patients (10.3 %) had a tiny residual shunt, and the number was reduced to only two patients at follow-up (see Table 3).
Discussion
Ventricular septal defects are the most common congenital heart defects, with the perimembranous VSD as the most common subtype [22]. Spontaneous closure occurs in 40–60 % of patients, mostly in preschoolers [6]. In patients with signs of LV volume overload only from left to right shunting from a VSD, an increase of right ventricular or pulmonary artery pressure has to be excluded. These patients may require defect closure to prevent ventricular dilatation and dysfunction, arrhythmias, aortic or significant tricuspid regurgitation, or endocarditis [17].
Surgical closure has been the standard method of VSD closure. However, although it is generally a safe procedure, it does have some potential risks: complete AVB in 1–5 % of the patients, significant residual VSD in 1–5 % with the necessity to reoperate in 2 %, and perioperative death in 0.5 %. Furthermore sternotomy, general anesthesia and cardiopulmonary bypass may initiate significant co-morbidities, such as infection, tachyarrhythmias, and neurologic complications [12, 18, 22].
Pediatric cardiologists have been interested in closing VSDs interventionally for several years. Moderate success was achieved with initially large delivery systems and devices, where residual shunting, induction of regurgitation of the valves (tricuspid and aortic valve), as well as arrhythmia and hemorrhage were dangers [15, 27]. A transcatheter approach for the treatment of congenital heart diseases is appreciated by patients and their parents because of its lesser psychological impact (absence of a skin scar), shorter hospital time, less pain and discomfort, and avoidance of admission to an intensive care unit [8]. Closure of ASD, PDA, muscular VSD, and perimembranous VSD using transcatheter devices such as Amplatzer occluders has been greatly improved and widely reported [5, 9, 13, 23]. Therefore, in the current era, transcatheter closure of perimembranous VSD provides a valuable alternative to surgery. In our experience, the results of transcatheter VSD closure with the ADO II device are satisfactory: the procedure was successfully performed in 29 of 31 patients (93.5 %), confirming the results reported in other published studies for interventional VSD closure [1, 3, 10, 24].
One significant early complication occurred in our study with device embolization. This complication was transient and the patient had no sequelae. Retrospectively this event could have been prevented with appropriate sizing because the device may have been too small: 4/4 mm ADO II in a 4 mm sized defect. As a result of that experience we now use the defect size and add 0.5–1 mm in order to determine the appropriate device size. Implantation of the ADO II has been described with the approach from either the LV or RV side due to the high flexibility of the device. Deployment of the LV disk first followed by passage through the defect and release of the RV disk at the end makes an appropriate distance to the aortic valve—in our experience—more realistic.
Statistical analysis showed that no variable predicted the occurrence of early complications. The incidence of major complications reported in the literature ranges between 0 and 8.6 % [3, 11]. No significant valve regurgitation occurred in our series. Three patients showed a significant decrease of pre-existing tricuspid regurgitation.
The complete VSD closure rate was 71.8 % immediately after the procedure, as documented by left ventricular angiography and TTE. It increased to 89.7 % at discharge and 93.6 % at last follow-up. These results confirm good closure rates, as reported by other studies of percutaneous VSD closure using the ADO II [19, 28].
One serious concern of VSD closure is still the occurrence of complete AV block (cAVB). This complication may require pacemaker implantation, and it seems to be more frequent in young patients [7]. The cAVB after surgical closure has been reported to occur in around 5.7 % of interventionally treated patients, but late occurrence 3–5 years after implantation has also been reported [3, 10]. Various mechanisms may be involved: mechanical compression, tissue, or inflammatory reaction followed by scar formation in the conduction tissue [2]. It might be an advantage of the ADO II device that it may keep the incidence of cAVB low, due to either the flexibility of the device or the low profile of the implantation process. There was no incidence of cAVB in our study group with a median follow-up of 41 (range 0.6–67) months, and 2/31 patients also fulfilled a 5-year follow-up.
The ADO II is made of a multi-layered, flexible, Nitinol wire mesh with retention disks on either end. Hemostasis is produced by the multiple layers of wire mesh. Fabric-free technology makes the device low profile, which makes it easier to track through angulations. The delivery system needs only a 4F or 5F Amplatzer® TorqVue® LP Delivery Sheath (St. Jude Medical, Plymouth, MN, USA), which fits through 4F or 5F venous introducer sheaths. Thus, the use of ADO II simplified the procedure, improved deliverability, and lessened vascular access-related complications. The device has a tendency to elongate with tension applied through the delivery cable and the longer central waist of the ADO II might avoid mechanical compression of the perinodal tissue [19, 26].
Ventricular septal defects in selected patients may be closed percutaneously using an ADO II device, as an off-label therapy. It appears that ADO II may be the preferable device for the closure of defects of moderate size (2–5 mm), especially in infants and small children, because of its better profile and trackability. The incidence of complications is acceptably low. Its possible superiority in terms of less AVB development needs to be proven with longer follow-up periods, as late development has been described for other devices [25].
References
Arora R, Trehan V, Kumar A (2003) Transcatheter closure of congenital ventricular septal defects. Experience with various devices. J Interv Cardiol 16:83–91
Bass JL, Gruenstein D (2012) Transcatheter closure of the perimembranous ventricular septal defect-preclinical trial of a new Amplatzer device. Catheter Cardiovasc Interv 79(7):1153–1160. doi:10.1002/ccd.23367 Epub 2011 Dec 12
Bass JL, Kalra GS, Arora R et al (2003) Initial human experience with the Amplatzer perimembranous ventricular septal occluder device. Cathet Cardiovasc Intervent 58:238–245
Benton JP, Barker KS (1992) Transcatheter closure of ventricular septal defect: anonsurgical approach to the care of the patient with acute ventricular septal rupture. Heart Lung 4:356–364
Bol-Raap G, Weerheim J, Kappetein AP, Witsenburg M, Bogers AJ (2003) Follow-up after surgical closure of congenital ventricular septal defects. Eur J Cardiothorac Surg 24:511–515
Butera G, Chessa M, Piazza L, Negura D, Micheletti A, Carminati M (2006) Percutaneous closure of ventricular septal defects. Expert Rev Cardiovasc Ther. 4(5):671–680 Review
Butera G, Carminati M, Chessa M et al (2006) Percutaneous closure of ventricular septal defects in children aged b 12: early and midterm results. Eur Heart J 27(23):2889–2895
Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG, Abella R, Giamberti A, Frigiola A (2007) Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol 50(12):1189–1195
Chessa M, Carminati M, Butera G et al (2002) Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol 39:1061–1065
Hijazi ZM, Hakim F, Al Fadley F, Abdelhamid J, Cao QL (2000) Transcatheter closure of single muscular ventricular septal defects using the Amplatzer muscular VSD occluder: initial results and technical considerations. Catheter Cardiovasc Intervent 49:167–172
Hijazi ZM, Hakim F, Haweleh AA et al (2002) Catheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: initial clinical experience. Cathet Cardiovasc Interv 56:508–515
Hobbins SM, Izukawa T, Radford DJ, Williams WG, Trusler GA (1979) Conduction disturbances after surgical correction of ventricular septal defect by the atrial approach. Br Heart J 41:289–293
Holzer R, Balzer D, Qi-Ling C, Lock K, Hijazi ZM, Amplatzer Muscular Ventricular Septal Defect Investigators (2004) Device closure of muscular ventricular septal defects using the Amplatzer muscular ventricular septal defect occluder. J Am Coll Cardiol 43:1257–1263
Holzer R, de Giovanni J, Walsh KP et al (2006) Transcatheter closure of perimem-branous ventricular septal defects using the amplatzer membranous VSD occluder: immediate and midterm results of an international registry. Catheter Cardiovasc Interv 68:620–628
Janorkar S, Goh T, Wilkinson J (1999) Transcatheter closure of ventricular septal defects using the Rashkind device: initial experience. Catheter Cardiovasc Interv 1:43–48
Kalra GS, Verma PK, Dhall A et al (1999) Transcatheter device closure of ventricular septal defects: Immediate results and intermediate-term follow-up. Am Heart J 2(Pt 1):339–344
Kidd L, Discroll DJ, Gersony WH et al (1993) Second natural history study of congenital heart defects: results of treatment of patients with ventricular septal defects. Circulation 87(Suppl I):138–151
Kirklin JW, Barrat-Boyes BG (1993) Ventricular septal defect. In: Kouchoukos NT, Blacksgowe EH, Doty DB, Hawley FL, Karp RB (eds) Cardiac surgery, 3rd edn. Churchill Livingstone, New York, pp 850–910
Koneti NR, Sreeram N, Penumatsa RR, Arramraj SK, Karunakar V, Trieschmann U (2012) Transcatheter retrograde closure of perimembranous ventricular septal defects in children with the Amplatzer duct occluder II device. J Am Coll Cardiol. 60(23):2421–2422. doi:10.1016/j.jacc.2012.08.1004 Epub 2012 Nov 7
Latiff HA, Alwi M, Kandhavel G et al (1999) Transcatheter closure of multiple muscular ventricular septal defects using Gianturco coils. Ann Thorac Surg 4:1400–1401
Lock JE, Block PC, McKay RG et al (1988) Transcatheter closure of ventricular septal defects. Circulation 2:361–368
Mavroudis C, Backer CL, Jacobs JP (2003) Ventricular septal defect. Pediatric cardiac surgery, 3rd edn. Mosby, Philadelphia, pp 298–320
Pass RH, Hijazi Z, Hsu DT, Lewis V, Hellenbrand WE (2004) Multicenter USA Amplatzer patent ductus arteriosus occlusion device trial. J Am Coll Cardiol 44:513–519
Pedra AC, Pedra SRF, Esteves CA et al (2004) Percutaneous closure of peri-membranous ventricular septal defects with the Amplatzer device: technical and morphological considerations. Cathet Cardiovasc Interv 61:403–410
Predescu D, Chaturvedi RR, Friedberg MK, Benson LN, Ozawa A, Lee KJ (2008) Complete heart block associated with device closure of perimembranous ventricular septal defects. J Thorac Cardiovasc Surg 136:1223–1228
Ramakrishnan S, Saxena A, Choudhary SK (2011) Residual VSD closure with an ADO II device in an infant. Congenit Heart Dis 6(1):60–63. doi:10.1111/j.1747-0803.2010.00468
Rigby ML, Redington AN (1994) Primary transcatheter umbrella closure of peri-membranous ventricular septal defect. Br Heart J 4:368–371
Zhao PJ, Yu ZQ, Gao W, Li F, Fu LJ, Liu TL, Li Y, Zhang YQ, Huang MR, Guo Y (2012) Efficacy of the transcatheter closure of perimembranous and muscular ventricular septal defects with the Amplatzer duct occluder II. Zhonghua Xin Xue Guan Bing Za Zhi 40(10):817–820
Conflict of interest
Peter Ewert and Felix Berger are consultants for St. Jude Medical. All other authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanaan, M., Ewert, P., Berger, F. et al. Follow-Up of Patients with Interventional Closure of Ventricular Septal Defects with Amplatzer Duct Occluder II. Pediatr Cardiol 36, 379–385 (2015). https://doi.org/10.1007/s00246-014-1017-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-014-1017-0