Abstract
Kawasaki disease (KD) is characterized by myocarditis and left ventricular dysfunction during the acute phase of the illness. Despite treatment with intravenous immunoglobulin (IVIG), a significant number of patients are IVIG resistant. We evaluated KD patients in the acute phase of illness using tissue Doppler imaging (TDI) to assess whether myocardial dysfunction may predict IVIG resistance. All patients with acute KD presenting to Children’s Hospital Colorado from February 2007 through March 2014 were included in this study and underwent echocardiograms with TDI evaluation at diagnosis. Patients were divided into two groups: IVIG resistant and IVIG responder. Group differences were assessed using Wilcoxon–Mann–Whitney and Chi-square testing. Receiver operating characteristic (ROC) curve analysis was utilized to determine threshold values of TDI measurements associated with IVIG resistance. Fifty-one age-matched IVIG resistant patients were compared to 51 IVIG responder patients [median age, IQR 44.57 (20.13–77.07) vs. 33.49 (17.30–62.89) months, p < 0.44]. There were significant differences in the septal and mitral early diastolic velocities (E′) (p < 0.001 and p < 0.01), respectively. ROC analysis demonstrated that tricuspid E′ <0.15 cm/s, septal E′ <0.12 cm/s, and mitral E′ <0.16 cm/s were good predictors of IVIG unresponsiveness (AUC = 0.66, 0.66, and 0.70, respectively). There were no differences between the systolic velocities and late diastolic velocities (A′). IVIG resistant KD patients present with significantly greater diastolic dysfunction compared to responders in patients with KD. TDI may be a useful tool to differentiate KD patients at higher risk of IVIG resistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Kawasaki’s disease (KD) is an acute vasculitis of unknown etiology that presents in children of all ages, but primarily occurs in children under the age of 5 years [20]. The primary blood vessels affected are the medium-sized arteries, most notably the coronary arteries, resulting in coronary artery lesions (CAL) in 25 % of untreated patients [20]. KD is now the leading source of acquired heart disease in children [17, 28]. Prior studies have clearly identified the presence of myocardial involvement in the acute phase of KD, specifically myocarditis that has been shown to be associated with subclinical and clinical left ventricular dysfunction [2, 9, 11, 12, 14, 16, 18, 27]. Standard therapy for patients with acute KD is treatment with intravenous immune globulin (IVIG) which reduces the risk of CAL from 25 to 5 % [19]. However, 10–15 % of patients fail to respond or develop recrudescent fever; these patients are characterized as IVIG resistant and ultimately require repeat IVIG dosing or a second line therapy [13]. Prior studies from Japan have used scoring systems such as Egami, Sano, and Kobayashi scores to predict IVIG resistance; however, these scoring systems had low sensitivity in the heterogeneous population and were unable to identify IVIG resistant patients in the North American cohort [6–8, 15, 22, 24, 26]. Currently, we lack tools to predict IVIG resistant among KD patients.
Tissue Doppler imaging (TDI) allows for direct measurement of myocardial velocities and is now considered a standard technique in many echocardiographic laboratories for further assessment of systolic and diastolic ventricular function. TDI has been shown to be abnormal in patients with KD in the acute and convalescent phases [1, 25]. However, differences in myocardial velocities from TDI between IVIG responders and IVIG resistant children with KD have not been well investigated. This study evaluated myocardial function in KD patients during the acute phase of the illness using TDI to determine whether the presence of ventricular dysfunction prior to treatment may help predict IVIG resistance.
Methods
Patients
This retrospective case control study was conducted at Children’s Hospital Colorado in patients diagnosed with acute KD at Children’s Hospital Colorado from February 2007 through March 2014. All patients were diagnosed by pediatric infectious disease physicians; patients met the standard American Heart Association criteria [21] case definition for acute KD. All patients received standard treatment with IVIG (2 g/kg IV over 12 h) and high-dose aspirin (80 mg/kg/day divided every 6 h). IVIG resistance was defined as a fever ≥38.3 °C occurring beyond 36 h after the end of IVIG infusion up to 7 days after completion of the infusion. An IVIG responder was defined as a KD patient who had a good clinical response to IVIG and did not have persistent or recrudescent fever. Age-matched (±6 months) controls were IVIG responders who presented within 6 months of the matched IVIG resistant case patients. Demographic data, treatment with IVIG, and echocardiographic data were included. Coronary artery lesions (CAL), defined as coronary arteries with a Boston z score >2.5 or aneurysms demonstrated on echocardiography, were compared within the resistant and responder KD groups. This study received approval from the Colorado Multiple Institutional Review Board.
Echocardiograms
Complete echocardiograms were performed at diagnosis. Echocardiograms were performed using the GE Vivid 7 cardiac ultrasound system (GE, Horten, Norway) and the appropriate size transducers for pediatric patients. Left ventricular ejection fraction was obtained from M-mode. TDI was obtained with a sample volume placed at the tricuspid, septal, and mitral annuli in the apical four chamber view (Fig. 1). The TDI systolic (S′), early diastolic (E′), and late diastolic (A′) velocities were averaged over three cardiac cycles and recorded from each patient. Nasal versed was used in children for anxiolytics in children who were too inconsolable to lay still for the entire study. Fused TDI E′ and A′ velocities from tachycardia were excluded from analysis.
Statistical Analysis
The data were tested for normality. Non-normally distributed continuous data are presented as median (interquartile range), and categorical data are presented as frequency (percentage). Group differences were assessed using Wilcoxon–Mann–Whitney and Chi-square analyses. Receiver operating characteristic (ROC) curve analysis was utilized to determine the threshold values of TDI measurements associated with unresponsiveness to IVIG. CALs were compared within IVIG resistant and responder groups. A sub analysis of IVIG resistant patients presenting with significant CAL compared to those without was similarly assessed using Wilcoxon–Mann–Whitney and Chi-square analyses. All analyses were performed using Statistical Analysis System (version 9.3; SAS Corporation, Cary, NC). A p value <0.05 was considered significant.
Results
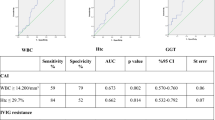
Three hundred and fifty patients with acute KD were identified, and 51/350 (15 %) were identified as IVIG resistant. There were 43 % of IVIG resistant and 33 % of IVIG responder patients who had incomplete KD at diagnosis. Table 1 shows the characteristics of the patients. Fifty-one IVIG resistant patients were age matched with 51 IVIG responder patients [median age, IQR 44.57 (20.13–77.07) vs. 33.49 (17.30–62.89) months, p < 0.44]. There were 34 males in responder group versus 33 males in the resistant group. No significant differences were identified in terms of age or gender. Analysis of the echocardiographic data demonstrated significant differences in the septal and mitral early diastolic velocities (E′) (p < 0.001 and p < 0.01) between the two groups (Table 1). ROC analysis identified tricuspid E′ <0.15 cm/s, septal E′ <0.12 cm/s, and mitral E′ <0.16 cm/s were reasonable predictors of IVIG unresponsiveness (AUC = 0.66, 0.66, and 0.70, respectively) (Fig. 2). There were no differences between the systolic velocities (S′), tricuspid E′, or late diastolic velocities (A′). Although there were significant differences in the incidence of CAL (p < 0.001) between the resistant and responder groups, there were no significant differences between the TDI velocities in patients with or without CAL compared in the two groups (Tables 1, 2). Within the resistant group, there were no significant differences in the TDI velocities between patients with and without CAL (Table 2). Within the responder group, statistical analysis could not be performed because of the small number of CAL patients in this group. The ejection fraction was normal in both groups; however, there was a statistically significant difference between the two groups (p < 0.02) with the left ventricular ejection fraction lower in the resistant group compared to the responders (Table 1).
Discussion
Our study demonstrated that IVIG resistant KD patients present with significantly greater diastolic dysfunction compared with IVIG responders. A well-documented clinical manifestation of KD is the onset of myocarditis. Myocarditis manifests itself in the form of tachycardia and depressed ventricular function and occurs in approximately 50 % of patients diagnosed with KD during the acute phase [14]. The changes in ventricular function generally improve post-IVIG therapy as systemic inflammation subsides [9, 14]. In our study cohort, there was a significantly depressed early diastolic (E′) velocity at the septal and mitral annuli in the resistant group, indicating more impaired left ventricular relaxation compared to the responder group. Our findings are consistent with a previous study by Amoozgar et al. [1] that during the acute phase of KD, the early diastolic velocities (E′) at the septal and mitral annuli were significantly diminished compared to diastolic function 4 weeks after IVIG treatment. Another study by Kurotobi et al. [10] demonstrated that children with KD had left ventricular diastolic dysfunction that was associated with elevated brain natriuretic peptide. Takeuchi et al. also found that left ventricular dysfunction was associated with elevated brain natriuretic peptide and that the left ventricular dysfunction improved during the convalescent phase; however, they did not find any difference in the diastolic parameters between nonresponders and IVIG responders [25]. Our study differed from Takeuchi et al. study because we had larger sample size and were able to demonstrate the difference in diastolic parameters between the resistant and responder groups. Early diastolic E′ at the tricuspid valve approached significance (p value of 0.05) but was not statistically significant; however, if the sample size of our IVIG resistant patients were larger, then perhaps there would have been significance as the AUC of the tricuspid valve E′ demonstrated reasonable predictor of IVIG resistance.
A second finding in our study demonstrated that the diastolic dysfunction found in KD patients may be a consequence of inflammation of the myocardium rather than the effects of macrovascular changes in CAL. In our cohort of patients, there were more CAL in the resistant group, but there were no significant differences in the TDI velocities between patients with CAL and patients with normal coronary arteries within the resistant group. Our study is similar to the previous study reported by Selamet et al. [23] in that patients with KD had impaired relaxation during the acute phase. Myocardial biopsy done by Yutani et al. [29] demonstrated myocardial abnormalities, including fibrosis and cellular disarrangement that were detected at all time periods after onset of KD and that their severity was not related to the presence of CAL. We were not able to demonstrate that the presence of CAL was the cause of diastolic dysfunction in patients with KD but rather a result of inflammation of the entire myocardium and microvascular changes in the coronary arteries.
Lastly, our study showed that TDI may be a useful tool in the identification of IVIG resistant patients. Because long-term consequences of CAL include thrombosis, stenosis, and myocardial infarction, early identification of IVIG resistant patients might allow adjunctive therapy in these high-risk patients to decrease the risk of CALs [4, 5]. Risk scoring system in Japan has been used to identify IVIG resistance with good sensitivity and specificity [6, 7, 15, 22]. However, Tremoulet et al. [26] demonstrated that the Egami score used in 362 children in San Diego County had low sensitivity (38 %) and good specificity (84 %) to detect IVIG resistance. Sleeper et al. [24] also showed that the use of Japanese risk scoring system in the North American population with mixed ethnicity would exclude most patients who were at low risk of IVIG resistance but would not capture most patients who may benefit from more intensive monitoring of their condition and requiring additional therapy to interrupt their disease process. In their conclusion, risk scoring systems for IVIG resistance developed in Japan have a low sensitivity (<45 %) and a good specificity (85–87 %) when applied to the North American cohort [24]. Interestingly, their study found that male sex and albumin were two independent risk factors for IVIG resistance and these factors were not included in the Japanese risk scoring system [24]. Their study highlighted the need for better identification of high-risk patients who may benefit from IVIG re-treatment. In a retrospective analysis of KD children diagnosed from 2002 to 2006, Ashouri et al. [3] found low albumin level, higher band counts, and higher number of abnormal coronaries on echocardiography independently identified high-risk patients for IVIG resistance. Two-third of our IVIG resistant patients were male, and our ROC analysis demonstrated that echocardiographic findings of tricuspid, septal, and mitral TDI velocities were associated with IVIG resistance. These findings suggest the potential for use of TDI to help identify IVIG resistant patients earlier in order to provide optimal treatment to turn off inflammation to prevent CAL.
Study Limitations
Our study had several limitations. First, because it is a retrospective study, it may have been subject to selection bias; however, patients were selected based on their diagnosis and responsiveness to the IVIG. Second, TDI acquisition in children with KD may be difficult during the acute phase when patients were fussy, inconsolable, and tachycardic; however, the echocardiograms were obtained when patients were asleep, distracted, or intranasal versed was used for anxiolytics. Third, fusion of E′ and A′ velocities was excluded from analysis, but this accounted for only 1 % of our patient population, and acquisition of TDI velocities was obtained as parallel as possible to limit the inaccuracies of the velocities obtained.
Conclusions
In summary, this study found that IVIG resistant patients had more diastolic dysfunction than IVIG responder patients and suggests that the diastolic dysfunction may be a consequence of myocarditis rather than coronary artery involvement. TDI may be a useful diagnostic tool in the early identification of IVIG resistant patients, and a prospective study should be done to validate the usefulness of TDI in predicting IVIG resistance. Early identification of patients at high risk of IVIG resistance may lead to development of adjunctive therapeutic approaches to decrease the risk of CALs and deleterious effect of coronary artery dilation in the long-term follow-up of these patients.
References
Amoozgar H, Mehdizadeh S, Ajami G, Alyasin S, Borzoee M, Abtahi S, Cheriki S (2009) Evaluation of myocardial function by pulsed tissue Doppler in Kawasaki disease. Pediatr Cardiol 30(7):936–940
Anderson TM, Meyer RA, Kaplan S (1985) Long-term echocardiographic evaluation of cardiac size and function in patients with Kawasaki disease. Am Heart J 110(1 Pt 1):107–115
Ashouri N, Takahashi M, Dorey F, Mason W (2008) Risk factors for nonresponse to therapy in Kawasaki disease. J Pediatr 153(3):365–368
Daniels LB, Gordon JB, Burns JC (2012) Kawasaki disease: late cardiovascular sequelae. Curr Opin Cardiol 27(6):572–577
Daniels LB, Tjajadi MS, Walford HH, Jimenez-Fernandez S, Trofimenko V, Fick DB Jr, Phan HA, Linz PE, Nayak K, Kahn AM, Burns JC, Gordon JB (2012) Prevalence of Kawasaki disease in young adults with suspected myocardial ischemia. Circulation 125(20):2447–2453
Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, Matsuishi T (2006) Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr 149(2):237–240
Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, Morikawa A (2006) Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 113(22):2606–2612
Kobayashi T, Saji T, Otani T, Takeuchi K, Nakamura T, Arakawa H, Kato T, Hara T, Hamaoka K, Ogawa S, Miura M, Nomura Y, Fuse S, Ichida F, Seki M, Fukazawa R, Ogawa C, Furuno K, Tokunaga H, Takatsuki S, Hara S, Morikawa A (2012) Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet 379(9826):1613–1620
Koteda Y, Suda K, Kishimoto S, Ito S, Kudo Y, Nishino H, Ishii H, Iemura M, Matuishi T (2009) Impact of intravenous immunoglobulin infusion on longitudinal left ventricular performance in patients with acute Kawasaki disease of usual course. J Cardiol 54(1):45–51
Kurotobi S, Kawakami N, Shimizu K, Aoki H, Nasuno S, Takahashi K, Kogaki S, Ozono K (2005) Brain natriuretic peptide as a hormonal marker of ventricular diastolic dysfunction in children with Kawasaki disease. Pediatr Cardiol 26(4):425–430
Marcella JJ, Ursell PC, Goldberger M, Lovejoy W, Fenoglio JJ Jr, Weiss MB (1983) Kawasaki syndrome in an adult: endomyocardial histology and ventricular function during acute and recovery phases of illness. J Am Coll Cardiol 2(2):374–378
Matsuura H, Ishikita T, Yamamoto S, Umezawa T, Ito R, Hashiguchi R, Saji T, Matsuo N, Takano M (1987) Gallium-67 myocardial imaging for the detection of myocarditis in the acute phase of Kawasaki disease (mucocutaneous lymph node syndrome): the usefulness of single photon emission computed tomography. Br Heart J 58(4):385–392
Moffett BS, Syblik D, Denfield S, Altman C, Tejtel-Sexson K (2015) Epidemiology of immunoglobulin resistant Kawasaki disease: results from a large, national database. Pediatr Cardiol 36(2):374–378
Moran AM, Newburger JW, Sanders SP, Parness IA, Spevak PJ, Burns JC, Colan SD (2000) Abnormal myocardial mechanics in Kawasaki disease: rapid response to gamma-globulin. Am Heart J 139(2 Pt 1):217–223
Muta H, Ishii M, Furui J, Nakamura Y, Matsuishi T (2006) Risk factors associated with the need for additional intravenous gamma-globulin therapy for Kawasaki disease. Acta Paediatr 95(2):189–193
Nakano H, Ueda K, Saito A, Nojima K (1985) Left ventricular systolic function in children with coronary arterial lesion following Kawasaki disease. Heart Vessels 1(2):89–93
Newburger JW, Fulton DR (2004) Kawasaki disease. Curr Opin Pediatr 16(5):508–514
Newburger JW, Sanders SP, Burns JC, Parness IA, Beiser AS, Colan SD (1989) Left ventricular contractility and function in Kawasaki syndrome. Effect of intravenous gamma-globulin. Circulation 79(6):1237–1246
Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP et al (1991) A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med 324(23):1633–1639
Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 110(17):2747–2771
Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics 114(6):1708–1733
Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, Kogaki S, Hara J (2007) Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr 166(2):131–137
Selamet Tierney ES, Newburger JW, Graham D, Baker A, Fulton DR, Colan SD (2011) Diastolic function in children with Kawasaki disease. Int J Cardiol 148(3):309–312
Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, Atz AM, Printz BF, Baker A, Vetter VL, Newburger JW (2011) Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr 158(5):831–835 e833
Takeuchi D, Saji T, Takatsuki S, Fujiwara M (2007) Abnormal tissue doppler images are associated with elevated plasma brain natriuretic peptide and increased oxidative stress in acute Kawasaki disease. Circ J Off J Jpn Circ Soc 71(3):357–362
Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, Martin DD, Newburger JW, Burns JC (2008) Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr 153(1):117–121
Vogel M, Smallhorn JF, Freedom RM (1992) Serial analysis of regional left ventricular wall motion by two-dimensional echocardiography in patients with coronary artery enlargement after Kawasaki disease. J Am Coll Cardiol 20(4):915–919
Yanagawa H, Nakamura Y, Yashiro M, Uehara R, Oki I, Kayaba K (2006) Incidence of Kawasaki disease in Japan: the nationwide surveys of 1999–2002. Pediatr Int Off J Jpn Pediatr Soc 48(4):356–361
Yutani C, Go S, Kamiya T, Hirose O, Misawa H, Maeda H, Kozuka T, Onishi S (1981) Cardiac biopsy of Kawasaki disease. Arch Pathol Lab Med 105(9):470–473
Conflict of interest
None.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phadke, D., Patel, S.S., Dominguez, S.R. et al. Tissue Doppler Imaging as a Predictor of Immunoglobulin Resistance in Kawasaki Disease. Pediatr Cardiol 36, 1618–1623 (2015). https://doi.org/10.1007/s00246-015-1206-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-015-1206-5