Abstract
This study aims to estimate plasma levels of acylated ghrelin in children with pulmonary hypertension (PH) associated with congenital heart disease (CHD) and to correlate the levels of acylated ghrelin with endothelin-1 (ET-1), nitric oxide (NO), and clinical hemodynamic parameters. We investigated the plasma concentration of acylated ghrelin, ET-1, NO, and the hemodynamic parameters in 20 children with CHD, 20 children with PH-CHD, and 20 normal children. Plasma-acylated ghrelin and NO levels were significantly higher in CHD group than in control subjects (P < 0.001). Moreover, plasma-acylated ghrelin, ET-1, and NO levels were significantly elevated in PH-CHD group compared with the CHD group (P < 0.05). In PH-CHD children, plasma-acylated ghrelin levels correlated positively with pulmonary artery systolic pressure (PASP; r = 0.740, P < 0.001), pulmonary artery diastolic pressure (PADP; r = 0.613, P = 0.004), right ventricular systolic pressure (RVSP; r = 0.642, P = 0.002), mean pulmonary arterial hypertension (mPAP; r = 0.685, P = 0.001), right ventricle diameter (RVD; r = 0.473, P = 0.035), pulmonary artery trunk diameter (PAD; r = 0.613, P = 0.004), NO (r = 0.463, P = 0.04), and ET-1 (r = 0.524, P = 0.018). Plasma-acylated ghrelin levels were elevated both in CHD and in PH-CHD. Increased acylated ghrelin levels correlated positively with ET-1, NO, PASP, PADP, RVSP, mPAP, RVD, and PAD. Acylated ghrelin may be a new biomarker of PH-CHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary hypertension (PH) is a fatal disease characterized by a progressive increase in pulmonary vascular resistance with remodeling of the pulmonary vasculature, which previously carried a poor prognosis in children with congenital heart disease (CHD) [6, 12, 25]. Endothelial dysfunction contributes to sustained pulmonary vasoconstriction and endothelial and vascular smooth muscle cell proliferation, playing a very important role in the complex pathology of PH [4, 7]. Ghrelin, a 28-amino acid peptide released from the stomach, has recently been identified as a natural ligand of the growth hormone secretagogue receptor (GHSR); it increases food intake and effects physical development and growth [8, 36]. Ghrelin is broadly expressed in many tissues and organs [16], including cardiovascular tissues [11]. Ghrelin has two subtypes: an acylated form and a deacylated form [2, 3]. Recently, it was found that ghrelin could exert a protective effect on the cardiovascular [35, 38] and digestive systems [17], including decreasing mean arterial pressure, raising cardiac output in patients with congestive heart failure (CHF), improving left ventricle function in rats with CHF [28], and reducing hepatic injury [17]. Most importantly, exogenous ghrelin attenuated pulmonary hypertension, pulmonary vascular remodeling [31], and right ventricular hypertrophy and decreased both right ventricular systolic pressure (RVSP) and right ventricular diastolic pressure (RVDP) in experimental model of PH [13]. Recently, researchers found that total plasma ghrelin levels were elevated in adult patients with idiopathic pulmonary arterial hypertension, and increased plasma ghrelin levels correlated positively with pulmonary artery systolic pressure (PASP) and nitric oxide (NO) [40]. Although total plasma ghrelin levels were higher in children with CHD [1, 18, 42], none of the study subjects had PH. In addition, the acylated ghrelin which possesses biological activity has not been investigated in children with PH-CHD.
Based on these understandings, the objective of this study was to estimate plasma levels of acylated ghrelin, NO, and ET-1 in children with PH-CHD and to correlate the levels of acylated ghrelin with pulmonary artery pressure and right ventricular pressure, which have not been confirmed in the literature to date.
Materials and Methods
Study Population
This study was conducted on 20 children with CHD, 20 children with PH-CHD, and 20 healthy children. All patients’ cardiac diagnoses were made on the basis of clinical and laboratory examinations (including right heart catheterization). The diagnosis of PH-CHD was established according to Stephen et al. [32] and Currie et al. [9]. This study was conducted in accordance with the Declaration of Helsinki. The local ethics committee approved the study protocol. Informed consents were obtained from the parents of the subjects.
Anthropometry
The following clinical characteristics were obtained from the patients’ medical chart: age, sex, body weight (in kilograms), height (in meters), and medical history. Body mass index (BMI) was calculated as the ratio of body weight (kg) and squared height (m2).
Echocardiography
All patients underwent a complete M-mode and 2D echocardiography examination according to the recommendations for quantification methods during the performance of a pediatric echocardiogram [24]. The right ventricle diameter (RVD), pulmonary artery trunk diameter (PAD), and ejection fraction (EF) were measured.
Cardiac Catheterization
All patients underwent right heart catheterization under general anesthesia and systemic heparinization. Details of the procedure were described previously [10]. PASP, PADP, RVSP, RVDP, and mPAP were assessed during the process of catheterization, and the above data were retrievable from operation reports.
Blood Sampling and Hormone Assay
After overnight fasting, specimens were collected at 08–09 a.m. Blood samples for the measurement of acylated ghrelin, ET-1, and NO were immediately transferred to chilled polypropylene tubes containing EDTA-2Na (1 mg/ml) and aprotinin (500 U/ml), centrifuged at 4 °C and 1600×g for 15 min, and then plasma samples were stored at −80 °C until assayed. The commercially available human acylated ghrelin enzyme immunoassay kit (Cayman Chemical Co., Michigan, USA) was used to assay the acylated ghrelin levels. Plasma ET-1 levels were analyzed with ELISA kit (ET-1 kit was purchased from Shang Hai Westang Bio-Tech. CO. LTD, China). Plasma NO levels were assessed with a nitric acid reduction method according to the instructions of the NO detection kit (Jian Cheng, China).
Statistical Analyses
Statistical Package for Social Sciences (SPSS) program version 13.0 was used in the analysis of data. Quantitative data were used to describe mean ± standard error. Statistical differences between the two groups were analyzed using an independent Student’s t test or Chi-square test. Correlations between variables were explored using Pearson’s coefficient. To assess the relative strength of the association, a multiple linear regression analysis was performed to examine the possible factors that impact acylated ghrelin. P < 0.05 was considered statistically significant.
Results
Subjects
Table 1 depicts the baseline characteristics of the study population. There were no significant differences in age and sex for these three groups. Height, weight, and BMI were significantly lower in both the CHD group and PH-CHD group compared with the control group, while the difference between CHD group and PH-CHD group was not significant. Table 2 shows that compared with the CHD group, the PH-CHD group had higher PASP, PADP, RVSP, mPAP, RVD, and PAD.
Differences in Acylated Ghrelin, ET-1, and NO Levels
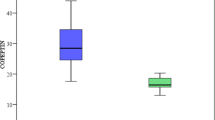
As shown in Fig. 1, plasma-acylated ghrelin and NO levels were significantly higher both in CHD group and in PH-CHD group than in control subjects. Moreover, plasma-acylated ghrelin, ET-1, and NO levels were significantly elevated in PH-CHD group compared with the CHD group (86.8 ± 21.7 ng/ml vs. 27.4 ± 9.5, P < 0.001; 185.5 ± 50 pg/ml vs. 73.2 ± 18.5 pg/ml, P < 0.001; 67.1 ± 17.7 μmol/l vs. 53.5 ± 20 μmol/l, P < 0.001 respectively).
Correlations of Acylated Ghrelin, ET-1, and NO with Various Parameters
Table 3 indicates the results of Pearson’s correlation of the variables in the PH-CHD patients. PASP, PADP, RVSP, mPAP, RVD, and PAD were positively correlated with plasma-acylated ghrelin levels. Plasma ET-1 levels also correlated positively with PASP and RVDP. Furthermore, PASP and RVD were found to be positively correlated with plasma NO levels. However, there were no significant correlations between plasma-acylated ghrelin levels and age, height, weight, RVDP, or EF. Additionally, both plasma ET-1 and NO levels were also positively correlated with acylated ghrelin levels, while there were no significant correlations between ET-1 and NO plasma levels.
Based on the sample correlation coefficients, the relationship between acylated ghrelin, ET-1, NO, and variables were further assessed in a multiple regression analysis. In a multiple regression model including age, weight, height, BMI, PASP, PADP, RVSP, RVDP, mPAP, RVD, PAD, EF, ET-1, and NO, none of the variables had significant correlations with acylated ghre lin.
Discussion
Although the available information confirms that the two ghrelin isoforms: acylated ghrelin and deacylated ghrelin have different metabolic effects [14], only acylated ghrelin owns physiologic activity and can promote growth hormone release and food intake [19]. So, we focused our investigation on acylated ghrelin. In this study, we demonstrated for the first time that plasma-acylated ghrelin levels were elevated in all the children with CHD. PASP, PADP, RVSP, mPAP, RVD, and PAD were positively correlated with plasma-acylated ghrelin levels in the children with PH-CHD. Additionally, both ET-1 and NO plasma levels were also positively correlated with acylated ghrelin levels.
It has been recently demonstrated that plasma ghrelin concentration was inversely correlated with BMI. Consistent with this conclusion, acylated ghrelin levels were increased and were negatively associated with BMI in all the children with CHD. Several studies agreed that increased plasma ghrelin levels were a compensatory mechanism in response to anabolic–catabolic imbalance. However, our results reveal that since there was no significant difference in BMI between CHD group and PH-CHD group, acylated ghrelin levels were significantly elevated in PH-CHD group. Lund et al. [26] showed that total plasma ghrelin levels were elevated both in cachectic [5] and in non-cachectic heart failure and did not correlate with BMI. Researchers also found that increased total plasma ghrelin in adult patients with idiopathic pulmonary arterial hypertension had no correlation with BMI [40]. Obviously, malnutrition and growth retardation cannot explain this phenomenon. The mechanism for elevated acylated ghrelin levels is not well understood [3], but total plasma ghrelin levels were decreased in patients with advance cancer and weight loss compared with the health controls, and the reduction in ghrelin levels might be attribute to the severity and progression of the disease [21].
Ghrelin is implicated as a cardiovascular hormone that can directly regulate cardiopulmonary function, including regulation of blood pressure [20, 27]. Yang et al. [40] reported that elevated total plasma ghrelin levels were positively associated with PASP and RVD in adult patients with idiopathic pulmonary arterial hypertension. Interestingly, a study by Rodriguez et al. [30] showed that increased acylated ghrelin was positively correlated with blood pressure in adult patients with metabolic syndrome. We also found a similar phenomenon that children with PH-CHD had higher plasma-acylated ghrelin levels compared with the CHD, and PASP, PADP, RVSP, mPAP, RVD, and PAD were positively correlated with acylated ghrelin. It is known that patients with CHD have left-to-right shunting which causes the increased pulmonary blood flow and right ventricular dilation. PAD and RVD are important indicators for PH. Echocardiography shows that PH-CHD group had higher PAD and RVD. It is absolutely possible that there is no difference in EF between CHD group and PH-CHD group because of the left-to-right shunting. So, acylated ghrelin may predict the severity of PH-CHD in children. However, the available data demonstrate completely opposite results in PH with atrial septal defect in adult patients [23]. The differences were completely reasonable due to the different cohorts, different assays, and different ages.
It is not completely clear, however, how ghrelin regulates PH-CHD. ET-1 contributes to endothelial dysfunction both directly, through its vasoconstrictor effects, and indirectly, through inhibition of NO production [34]. Interestingly, ghrelin, a potent physiological antagonist of ET-1 [37], was demonstrated to reverse endothelial dysfunction in adult patients with metabolic syndrome by increasing NO bioactivity [33]. In our study, both plasma ET-1 and NO levels were elevated in PH-CHD. Our study also showed that plasma ET-1 and NO levels were in accordance with increased plasma-acylated ghrelin levels. From previous findings, it is known that ghrelin participates in ET-1-mediated biological processes [4, 7]. We suppose that, in children with PH-CHD, acylated ghrelin improved endothelial dysfunction and regulated PH via decreasing the vasoconstrictor effect of ET-1 [31] and increasing NO bioactivity [22]. This conclusion is supported by recent evidence that ghrelin has the ability to activate endothelial eNOS in endothelial cells dependent on the phosphatidylinositol 3-kinase (PI3 K) pathway [39, 41]. Ghrelin also has been demonstrated to reduce chronic liver injury and fibrogenesis by increased eNOS expression [17]. Rocha-Sousa et al. speculated that ghrelin’s hypotensive effect in ocular hypertension animal models may be associated with NO [29]. Additionally, ghrelin stimulates the production of NO by PI3 K, Akt, and eNOS pathways [15]. Collectively, these findings imply that elevated plasma-acylated ghrelin levels had some effect on the pulmonary vasculature. Our findings, along with those of previous studies, indicate that ghrelin may be a new therapeutic approach for PH-CHD.
Of course, more research is needed to further verify this assumption. Firstly, we need to assay plasma-deacylated ghrelin levels and total plasma ghrelin levels. Secondly, it is necessary to obtain data using increased/varied numbers, races, regions, and ages of patients. Thirdly, a study with a longer follow-up period should be executed to evaluate the relationship between acylated ghrelin and clinical hemodynamic parameters. Fourthly, more work is required to validate the supposition that acylated ghrelin is a novel prognostic biomarker, and further understanding of the pathways controlling ghrelin secretion will be useful to develop new therapeutic approaches for PH-CHD.
In conclusion, plasma-acylated ghrelin, ET-1, and NO levels are elevated in children with PH-CHD. PASP, PADP, RVSP, MPAP, RVD, PAD, plasma NO, and ET-1 levels were positively correlated with acylated ghrelin. Acylated ghrelin may predict the severity of PH-CHD and be a new therapeutic approach for PH-CHD.
References
Afify MF, Mohamed GB, El-Maboud MA, Abdel-Latif EA (2009) Serum levels of ghrelin, tumor necrosis factor-alpha and interleukin-6 in infants and children with congenital heart disease. J Trop Pediatr 55:388–392
Akamizu T, Shinomiya T, Irako T, Fukunaga M, Nakai Y, Nakai Y, Kangawa K (2005) Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J Clin Endocrinol Metab 90:6–9
Al Massadi O, Lear PV, Müller TD, Lopez M, Dieguez C, Tschop MH, Nogueiras R (2014) Review of novel aspects of the regulation of ghrelin secretion. Curr Drug Metab 15:398–413
Amabile N, Guignabert C, Montani D, Yeghiazarians Y, Boulanger CM, Humbert M (2013) Cellular microparticles in the pathogenesis of pulmonary hypertension. Eur Respir J 42:272–279
Attanasio P, Anker SD, Doehner W, von Haehling S (2011) Hormonal consequences and prognosis of chronic heart failure. Curr Opin Endocrinol Diabetes Obes 18:224–230
Barst RJ, Ertel SI, Beghetti M, Ivy DD (2011) Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J 37:665–677
Coggins MP, Bloch KD (2007) Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol 27:1877–1885
Cummings DE, Shannon MH (2003) Roles for ghrelin in the regulation of appetite and body weight. Arch Surg 138:389–3961
Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ (1985) Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol 6:750–756
Feltes TF, Bacha E, Beekman RH 3rd et al (2011) Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation 123:2607–2652
Granata R, Isgaard J, Alloatti G, Ghigo E (2011) Cardiovascular actions of the ghrelin gene-derived peptides and growth hormone-releasing hormone. Exp Biol Med (Maywood) 236:505–514
Gupta V, Tonelli AR, Krasuski RA (2012) Congenital heart disease and pulmonary hypertension. Heart Fail Clin 8:427–445
Henriques-Coelho T, Correia-Pinto J, Roncon-Albuquerque R Jr, Baptista MJ, Lourenço AP, Oliveira SM, Brandão-Nogueira A, Teles A, Fortunato JM, Leite-Moreira AF (2004) Endogenous production of ghrelin and beneficial effects of its exogenous administration in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 287:H2885–H2890
Heppner KM, Tong J, Kirchner H, Nass R, Tschöp MH (2011) The ghrelin O-acyltransferase–ghrelin system: a novel regulator of glucose metabolism. Curr Opin Endocrinol Diabetes Obes 18:50–55
Iantorno M, Chen H, Kim JA, Tesauro M, Lauro D, Cardillo C, Quon MJ (2007) Ghrelin has novel vascular actions that mimic PI 3-kinase-dependent actions of insulin to stimulate production of NO from endothelial cells. Am J Physiol Endocrinol Metab 292:E756–E764
Jarkovska Z, Rosicka M, Krsek M, Sulkova S, Haluzik M, Justova V, Lacinova Z, Marek J (2005) Plasma ghrelin levels in patients with end-stage renal disease. Physiol Res 54:403–408
Kabil NN, Seddiek HA, Yassin NA, Gamal-Eldin MM (2014) Effect of ghrelin on chronic liver injury and fibrogenesis in male rats: possible role of nitric oxide. Peptides 52:90–97
Kandil ME, Elwan A, Hussein Y, Kandeel W, Rasheed M (2009) Ghrelin levels in children with congenital heart disease. J Trop Pediatr 55:307–312
Kim YS, Lee JS, Lee TH, Cho JY, Kim JO, Kim WJ, Kim HG, Jeon SR, Jeong HS (2012) Plasma levels of acylated ghrelin in patients with functional dyspepsia. World J Gastroenterol 18:2231–2237
Kishimoto I, Tokudome T, Hosoda H, Miyazato M, Kangawa K (2012) Ghrelin and cardiovascular diseases. J Cardiol 59:8–13
Legakis I, Stathopoulos J, Matzouridis T, Stathopoulos GP (2009) Decreased plasma ghrelin levels in patients with advanced cancer and weight loss in comparison to healthy individuals. Anticancer Res 29:3949–3952
Leite-Moreira AF, Soares JB (2007) Physiological, pathological and potential therapeutic roles of ghrelin. Drug Discov Today 12:276–288
Li ZF, Zhou DX, Pan WZ, Zhang L, Ge JB (2013) Circulating ghrelin was negatively correlated with pulmonary arterial pressure in atrial septal defect patients. Chin Med J 126:3936–3939
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 23:465–495 quiz 576–577
Lourenço AP, Fontoura D, Henriques-Coelho T, Leite-Moreira AF (2012) Current pathophysiological concepts and management of pulmonary hypertension. Int J Cardiol 155:350–361
Lund LH, Williams JJ, Freda P, LaManca JJ, LeJemtel TH, Mancini DM (2009) Ghrelin resistance occurs in severe heart failure and resolves after heart transplantation. Eur J Heart Fail 11:789–794
Mao Y, Tokudome T, Kishimoto I (2014) Ghrelin as a treatment for cardiovascular diseases. Hypertension 64:450–454
Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K (2004) Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 110:3674–3679
Rocha-Sousa A, Pereira-Silva P, Tavares-Silva M, Azevedo-Pinto S, Rodrigues-Araújo J, Pinho S, Avelino A, Falcão-Reis F, Leite-Moreira A (2014) Identification of the ghrelin-GHSR 1 system and its influence in the modulation of induced ocular hypertension in rabbit and rat eyes. Peptides 57:59–66
Rodriguez A, Gomez-Ambrosi J, Catalan V, Becerril S, Sáinz N, Gil MJ, Silva C, Salvador J, Barba J, Colina I, Frühbeck G (2010) Association of plasma acylated ghrelin with blood pressure and left ventricular mass in patients with metabolic syndrome. J Hypertens 28:560–567
Schwenke DO, Gray EA, Pearson JT, Sonobe T, Ishibashi-Ueda H, Campillo I, Kangawa K, Umetani K, Shirai M (2011) Exogenous ghrelin improves blood flow distribution in pulmonary hypertension-assessed using synchrotron radiation microangiography. Pflugers Arch 462:397–406
Stephen B, Dalal P, Berger M, Schweitzer P, Hecht S (1999) Noninvasive estimation of pulmonary artery diastolic pressure in patients with tricuspid regurgitation by Doppler echocardiography. Chest 116:73–77
Tesauro M, Schinzari F, Iantorno M, Rizza S, Melina D, Lauro D, Cardillo C (2005) Ghrelin improves endothelial function in patients with metabolic syndrome. Circulation 112:2986–2992
Tesauro M, Schinzari F, Rovella V, Di Daniele N, Lauro D, Mores N, Veneziani A, Cardillo C (2009) Ghrelin restores the endothelin 1/nitric oxide balance in patients with obesity-related metabolic syndrome. Hypertension 54:995–1000
Tokudome T, Kishimoto I, Miyazato M, Kangawa K (2014) Ghrelin and the cardiovascular system. Front Horm Res 43:125–133
Ueno H, Yamaguchi H, Kangawa K, Nakazato M (2005) Ghrelin: a gastric peptide that regulates food intake and energy homeostasis. Regul Pept 126:11–19
Wiley KE, Davenport AP (2002) Comparison of vasodilators in human internal mammary artery: ghrelin is a potent physiological antagonist of endothelin-1. Br J Pharmacol 136:1146–1152
Wu R, Chaung WW, Dong W, Ji Y, Barrera R, Nicastro J, Molmenti EP, Coppa GF, Wang P (2012) Ghrelin maintains the cardiovascular stability in severe sepsis. J Surg Res 178:370–377
Xu X, Jhun BS, Ha CH, Jin ZG (2008) Molecular mechanisms of ghrelin-mediated endothelial nitric oxide synthase activation. Endocrinology 149:4183–4192
Yang D, Liu Z, Yang Z (2013) Ghrelin and its relation with N-terminal brain natriuretic peptide, endothelin-1 and nitric oxide in patients with idiopathic pulmonary hypertension. Cardiology 124:241–245
Yang D, Liu Z, Zhang H, Luo Q (2013) Ghrelin protects human pulmonary artery endothelial cells against hypoxia-induced injury via PI3-kinase/Akt. Peptides 42:112–117
Yilmaz E, Ustundag B, Sen Y, Akarsu S, Kurt AN, Dogan Y (2007) The levels of Ghrelin, TNF-alpha, and IL-6 in children with cyanotic and acyanotic congenital heart disease. Mediators Inflamm 2007:32403
Acknowledgments
We thank Ying Fan (Department of Ultrasound, the Affiliated Hospital of Luzhou Medical College, Luzhou, China). This study was supported by the Talent Foundation of the affiliated Hospital of Luzhou Medical College (2012).
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gang Li and Jiyi Xia have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, G., Xia, J., Jia, P. et al. Plasma Levels of Acylated Ghrelin in Children with Pulmonary Hypertension Associated with Congenital Heart Disease. Pediatr Cardiol 36, 1423–1428 (2015). https://doi.org/10.1007/s00246-015-1178-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-015-1178-5