Abstract
Novel COstatus system (Transonic Systems, Inc., NY), based on ultrasound dilution (UD), works off in situ arterial and central venous catheters in pediatric patients to measure cardiac output (CO). The purpose of the present study was to validate CO measurement by UD (COUD) with pulmonary artery (PA) thermodilution (COTD) in a prospective animal study. Ten anesthetized pigs (16–45 kg) were instrumented with pediatric PA, central venous, and peripheral artery catheters. For COUD measurements, normothermic saline (0.5–1.0 ml/kg body weight, up to a maximum of 30 ml) was injected into the venous limb of an arteriovenous loop that was connected between in situ catheters. For COTD measurements, 5–10 ml cold saline was injected into the PA catheter. Sixty-four averaged sets were obtained for comparison. COTD mean was 2.98 ± 1.21 l/min (range 1.33–6.29), and COUD mean was 2.68 ± 1.16 l/min (range 1.33–5.85). This study yielded a correlation r = 0.96, COUD = 0.91*(COTD) − 0.04 l/min; bias was 0.3 l/min with limits of agreement as −0.39 to 0.99 l/min; and the percentage error was 23.73% between the methods. CO measurements by UD agreed well with thermodilution measurements in the pediatric swine model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Accurate measurement of cardiac output (CO) is an important part of hemodynamic monitoring in critically ill patients because it helps guide titration of therapies and is becoming increasingly important in pediatric intensive care and pediatric anesthesia [17, 19–21, 26]. Although CO is routinely measured in adult critically ill patients using the current clinical standard pulmonary artery (PA) thermodilution technique, it is rarely used with pediatric patients due to inherent risks of invasive catheterization, such as carotid artery puncture, cardiac arrhythmia, bleeding, embolism, etc. [17]. Other less invasive methods require placement of dedicated catheters in the femoral artery [19, 26]; involve blood loss and are not approved for patients <40 kg [19]; are technically demanding and time consuming (e.g., magnetic resonance imaging [MRI]) [26]; or are known to be less accurate (e.g., bioimpedance techniques, Doppler methods, CO2 re-breathing) [5, 11, 12, 17], etc. Thus, pediatric hemodynamic monitoring in some units is performed by clinical assessment using markers such as central venous pressure, heart rate, urine output, etc. However, studies [8, 22] have shown that such assessment could be inaccurate in children. Thus, there is a clear need for a bedside device that can be routinely used for precise and safe measurement of CO in critically ill children [4, 16, 18, 19, 22].
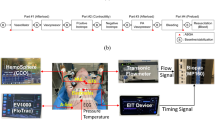
Recently, COstatus (Transonic Systems, Inc., Ithaca, NY), a minimally invasive system to routinely measure CO and blood volumes in intensive care unit (ICU) patients, was introduced. This system is based on ultrasound dilution (UD) technology [15] and uses isotonic saline as the indicator. COstatus requires connecting a primed extracorporeal arteriovenous (AV) tube set (AV loop; priming volume approximately 5 ml) between the in situ standard arterial and central venous catheters in the patient using three-way stopcocks (Fig. 1). These catheters will continue to be used for their routine purposes of patient treatment while they are in the ICU. Two UD flow-dilution sensors are placed on the arterial and venous ends of the AV loop. The sensors are clamped on the tubing and do not come in contact with the blood. These reusable sensors detect both transit-time blood flow and ultrasound velocity. For the duration of CO measurements, which last approximately 5–8 min, a small roller pump is used to circulate blood through the AV loop from the artery to vein at a slow rate of 8–12 ml/min. This pump allows for a stable flow through the loop. Isotonic saline, heated to body temperature using a bag warmer (HFW1000; Transonic Systems), in the order of 0.5–1 ml/kg (up to a maximum of 30 ml), is injected quickly and smoothly into the venous side of the AV loop to obtain COstatus measurements. The venous sensor measures the exact amount of injected indicator, monitors the quality of injection, and performs automatic calibration of the patient’s blood properties. The arterial sensor measures the change in the concentration of the saline after passing through the cardiopulmonary system, and a UD curve is obtained [4, 14, 15]. Cardiac output is then calculated using the following equation (Eq. 1) based on the Stewart-Hamilton principle
where CO is the cardiac output (ml/min), V inj is volume of injected isotonic saline as measured by the venous sensor, and ∫ Ca(t) dt is the area under the dilution curve of the saline concentration in arterial blood (ml [saline]/ml [blood] × min) as measured by the arterial sensor [15]. At the end of each measurement session, the loop is flushed with heparinized saline, and the blood is returned from the loop into the venous side of the patient.
Schematic of the COstatus AV loop connected to the standard arterial and central venous catheters and the location of UD sensors. Using the roller pump, blood is circulated through the sterile AV loop from the artery to the vein without any blood loss for the duration (5–8 min) of measurements. During this time isotonic saline is injected into the venous end of the AV loop using the injection syringe. The venous sensor measures the injection volume, and the arterial sensor measures the dilution curve, from which the CO is calculated by Stewart-Hamilton equation. At the end of the session, the AV loop is flushed with heparinized saline from the flush syringe, and the blood from the AV loop is returned back to the venous side of the patient
COstatus was previously validated against the “gold-standard” perivascular probe measurements in different animal models [4, 9]. It was also validated in adult humans against the PA thermodilution [7, 24] and transpulmonary thermodilution methods [9]. However, to our knowledge, COstatus was not validated in children or in young animals against the PA thermodilution method. Thus, the purpose of this study was to assess the feasibility and accuracy of CO measurement by the UD method (COUD) compared with the “clinical- standard” PA thermodilution method (COTD) in a pediatric animal model.
Materials and Methods
The protocol was approved by the Committee for the Humane Use of Animals at SUNY-Upstate Medical University. All animals received care in compliance with the Guide for the Care and Use of Laboratory Animals (NIH Publications 85-23, revised 1985). Ten healthy Yorkshire pigs were anesthetized with intravenous sodium pentobarbital (50 ml/kg), and intubation was performed. Animals were ventilated using a Galileo ventilator (Hamilton Medical, Reno, NV). Continuous anaesthesia with sodium pentobarbital was maintained at 6 mg/kg/min using a pump (model 907; Harvard Apparatus, Mills, MA), and intermittent boluses of pancuronium bromide were given to maintain paralysis when indicated. A pediatric model Swan–Ganz catheter (132F5; Edwards Lifesciences, Irvine, CA) was introduced through the jugular vein into the PA for thermodilution measurements. A dual-lumen central venous catheter (4F or 7F) and arterial (20G) catheters were inserted into the animal. These catheters were used for UD measurements, and between measurements they were used for routine monitoring of pressure and for infusing medications.
For COUD measurements, 0.5–1 ml/kg (maximum of 30 ml) body-temperature isotonic saline was manually injected into the venous side of the AV loop. Two to three measurements were performed randomly throughout the breathing cycle. One extra measurement was performed if a “repeat” message was displayed on the monitor. For COTD measurements, 5–10 ml cold isotonic saline was injected into the PA catheter. Three to four measurements were obtained per session, and the odd one was left out. Measurements from each method were averaged for comparison. COUD and COTD measurements were performed within 3–5 min.
Standard descriptive statistics, including CO means and SDs, were calculated, and the results are expressed as means ± SDs. Correlation between averaged measurements of COUD and COTD was determined by linear regression analysis using the least-squares method. Bias was defined as the mean difference between the two methods. Limits of agreement were calculated as bias ± 1.96 SD between the methods defining 95% confidence limits (CIs; precision). Comparison of the bias and limits of agreement between the two methods was assessed by Bland–Altman [1] analysis. This statistical method is used for comparing two measurement techniques when neither can be considered a true gold standard [1]. The percentage error was calculated as two SDs of the bias divided by the mean of COTD (as recommended by Critchley and Critchley [3]). A percentage error <±30% is recommended to be clinically acceptable when comparing a new technique with the current reference method [3].
Results
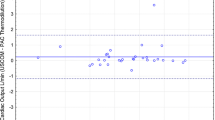
A total of 64 averaged measurement sets were compared between COUD and COTD. These measurements were obtained from 10 pigs weighing between 16 to 45 kg. (mean ± SD 32.8 ± 10.6). The mean of COTD measurements was 2.98 ± 1.21 l/min (range 1.33–6.29; median = 2.65), whereas the mean of COUD measurements was 2.68 ± 1.16 l/min (range 1.33–5.85; median = 2.36). The correlation between the methods was r = 0.96, COUD = 0.91*(COTD) − 0.04 l/min (Fig. 2). The bias between COTD and COUD was 0.3 l/min, and the limits of agreement were found to be −0.39 to 0.99 l/min. Bland–Altman analysis showed that COUD measurements matched with reference COTD measurements equally well over the entire range of CO measured (Fig. 3). Percentage error was calculated per Critchley and Critchley’s methodology [3] for all of the data and was found to be 23.7%, which is less than the recommended clinically acceptable limit of ±30%.
Discussion
Measurement of CO is of considerable interest for cardiovascular assessment and hemodynamic management of critically ill pediatric patients [4, 16, 18, 19, 22]. Researchers have shown several indications [13, 23] for which CO monitoring in pediatrics should be considered: (1) hemodynamically unstable patients who need invasive pressure monitoring; (2) patients with acute lung injury and acute respiratory distress syndrome; (3) patients with severe trauma, polytrauma, and burn injury; (4) patients with heart insufficiency; (5) patients in shock or with multiple-organ dysfunction syndrome; (6) patients undergoing surgeries with suspected high blood loss, such as liver and other organ transplantation surgeries; (7) patients with congenital and acquired heart diseases; and (8) patients with cardiopulmonary interactions and on mechanical ventilator, etc. Despite its clinical importance, CO is infrequently measured in children due to various risks and other complications involved with the existing methods [8, 19, 22].
The recently introduced COstatus system offers an opportunity to routinely measure CO in ICU patients at the bedside [7, 9, 24]. To our knowledge, the current study is the first animal validation of the COstatus system in a pediatric model with the standard thermodilution method. Animals with structurally normal hearts with no known cardiac defects were studied to ensure stable hemodynamic conditions were presented for measurement of CO by both methods. We chose to compare COstatus with PA thermodilution in a pediatric animal model because PA thermodilution is the current clinical standard for CO measurement in adults [11, 16, 20], and because it is rarely used in young children, validation of a new technique with thermodilution is less feasible in pediatric patients [18, 19, 22]. The study results demonstrate that COstatus measured CO equally well as PA thermodilution. When comparing measurements obtained by two different techniques, a correlation coefficient ≥0.8 indicates a strong positive relationship [17, 27]. In this study, comparing COUD and COTD yielded r = 0.96, which represents a strong positive correlation. Previous studies in adult humans [7, 9, 24] and in animals [4, 10] also yielded a high positive correlation. Bias and limits of agreement observed in this study were close to the results observed by Ruperez et al. [20], who compared COTD with CO measured by transpulmonary thermodilution. Percentage error as determined by Critchley and Critchley’s criteria [3] in this study was 23.7%, which is lower than the clinically acceptable limit of 30% and also similar to that observed in adult studies [7, 9, 24]. No complications or adverse events were noticed during the study.
With every injection performed, COstatus system also measures and automatically displays blood volumes: total end diastolic, central blood, and active circulation [15]. These volumes could be used as indicators of cardiac preload and fluid responsiveness, which would be crucial in the management of the critically ill [6, 9]. Additionally, it also alerts the clinician about the presence of left-to right or right-to-left shunts [15]. Boehne et al. [2] concluded that COstatus identified left-to-right shunts in children with high sensitivity. In an experimental lamb study, Vranken et al. [25] found that COstatus-measured CO was reliable even in the presence of a significant left-to-right shunt.
Major advantages of COstatus system compared with other techniques include (1) works off in situ standard catheters in ICU patients, which allows it to be used with pediatric ICU patients with both lines; (2) involves no blood loss; (3) quick to set up; and (4) uses normothermic isotonic saline as indicator. Limitations of this system include (1) the arterial pressure display from the arterial line is not available for the 5–8 min when the pump is running. Many PICUs have cuff pressure available, and hence that could be used to monitor pressure. The system does not provide continuous CO measurements. Also, the need to inject 0.5–1 ml/kg (maximum of 30 ml) saline could be less preferable in patients who are hypervolemic. de Boode et al. [4] showed that there is no significant difference in measurement accuracy between 0.5 ml/kg versus 1 ml/kg; therefore, in hypervolemic patients it might be better to inject less volume. Other limitations of this technique have been discussed in the literature [4, 15].
In conclusion, COstatus-measured CO agreed well with PA thermodilution CO measurements in this pig study. Although more studies are necessary in children and newborn animals to confirm the accuracy of this method under various conditions, such as shock, heart defects, etc., we believe that the COstatus system, based on UD technology, could potentially be used to measure CO in critically ill pediatric patients at the bedside.
References
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Boehne M, Baustert M, Happel C et al (2010) Novel ultrasound dilution technique detects left-to-right shunts with high accuracy in children [abstract]. Pediatr Res 1:117
Critchley LA, Critchley JA (1999) A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput 15:85–91
de Boode WP, van Heijst AF, Hopman JC et al (2010) Cardiac output measurement using an ultrasound dilution method: a validation study in ventilated piglets. Pediatr Crit Care Med 11:103–108
de Wall EE, Wappler F, Buhre WF (2009) Cardiac output monitoring. Curr Opin Anaesthesiol 22:71–77
Eremenko AA, Safarov PN (2007) Central blood volume index and total end-diastolic volume index as indicators of cardiac preload [abstract]. Intensive Care Med 35:A83
Eremenko AA, Safarov PN (2010) Flow-regulated extracorporeal arteriovenous tubing loop for cardiac output measurements by ultrasound velocity dilution: validation in post-cardiac surgery intensive care unit patients. ASAIO J 56:616–623
Fanconi S, Burger R (1992) Measurement of cardiac output in children. Intensive Care World 9:8–12
Galstyan G, Bychynin M, Alexanyan M et al (2010) Comparison of cardiac output and blood volumes in intrathoracic compartments measured by ultrasound dilution and transpulmonary thermodilution methods. Intensive Care Med 36:2140–2144
Gleed RD, Smith T, Callahan M et al (2006) Validation of novel ultrasound dilution cardiac output method for pediatric and neonatal patients [abstract]. Intensive Care Med 32:0659
Hirschl MM, Kittler H, Woisetchlager C et al (2000) Simultaneous comparison of thoracic bioimpedance and arterial pulse waveform-derived cardiac output with thermodilution measurement. Crit Care Med 28:1798–1802
Jakovljevic DG, Nunan D, Donovan G et al (2008) Comparison of cardiac output determined by different rebreathing methods at rest and at peak exercise. Eur J Appl Physiol 102:593–599
Karovic KJ, Podhoransky B, Csomor D et al (2007) Hemodynamic monitoring using PiCCO system in a 10 months old infant suffering from serious burn injury. Bratisl Lek Listy 108:359–363
Krivitski NM (1995) Novel method to measure access flow during hemodialysis by ultrasound dilution technique. ASAIO J 41:M741–M745
Krivitksi NM, Kislukhin VV, Thuramalla NV (2008) Theory and in vitro validation of a new extracorporeal arteriovenous loop approach for hemodynamic assessment in pediatric and neonatal intensive care unit patients. Pediatr Crit Care Med 9:423–428
Lemson J, de Boode WP, Hopman JCW et al (2008) Validation of transpulmonary thermodilution cardiac output measurement in a pediatric animal model. Pediatr Crit Care Med 9:313–319
Levy RJ, Chiavacci RM, Nicolson SC et al (2004) An evaluation of a noninvasive cardiac output measurement using partial carbon dioxide rebreathing in children. Anesth Analg 99:1642–1647
Mohan UR, Britto J, Habibi P et al (2002) Noninvasive measurement of cardiac output in critically ill children. Pediatr Cardiol 23:58–61
Nusmeier A, Hoeven JG, Lemson J (2010) Cardiac output monitoring in pediatric patients. Expert Rev Med Devices 7:503–510
Ruperez M, Lopez-Herce J, Garcia C et al (2004) Comparison between cardiac output measured by the pulmonary arterial thermodilution technique and that measured by the femoral arterial thermodilution technique in a pediatric animal model. Pediatr Cardiol 25:119–123
Skowno JJ, Broadhead M (2008) Cardiac output measurement in pediatric anesthesia. Pediatr Anaesth 18:1019–1028
Tibby SM, Hatherill M, Marsh MJ et al (1997) Clinician’s abilities to estimate cardiac index in ventilated children and infants. Arch Dis Child 77:516–518
Tibby SM, Murdoch IA (2003) Monitoring cardiac function in intensive care. Arch Dis Child 88:46–52
Tsutsui M, Matsuoka N, Ikeda T et al (2009) Comparison of a new cardiac output ultrasound dilution method with thermodilution technique in adult patients under general anesthesia. J Cardiothorac Vasc Anesth 23:835–840
Vranken S, de Boode W, Hopman J et al (2010) Cardiac output measurement is feasible in the presence of left-to-right shunt with ultrasound dilution method: a validation study in lambs [abstract]. Crit Care 14:97
Wiegand G, Kerst G, Baden W et al (2010) Noninvasive cardiac output determination for children by the inert-gas rebreathing method. Pediatr Cardiol 31:1214–1218
Zou KH, Tuncali K, Silverman SG (2003) Correlation and simple linear regression. Radiology 2003:2338–2345
Acknowledgments
This work was supported by National Institutes of Health Small Business Innovation Research Grant No. R44 HL061994.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darling, E., Thuramalla, N. & Searles, B. Validation of Cardiac Output Measurement by Ultrasound Dilution Technique With Pulmonary Artery Thermodilution in a Pediatric Animal Model. Pediatr Cardiol 32, 585–589 (2011). https://doi.org/10.1007/s00246-011-9915-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-011-9915-x