Abstract
The purpose of this study was to assess how red blood cell (RBC) transfusions impact hemodynamic parameters in infants with single-ventricle lesions. This was a retrospective chart review. The setting was a pediatric cardiac intensive care unit at a tertiary care children’s hospital. Fifty-nine patients <1 year of age with single-ventricle physiology who received a blood transfusion between December 2007 and April 2009 were analyzed. They received a total of 183 transfusions. Exclusion criteria included transfusions given within 72 h of cardiac surgery or transfusions given to patients with active bleeding. There were no interventions. The study population was divided into terciles based on pretransfusion hemoglobin (Hgb) concentration. The pretransfusion Hgb concentration in group A was 7.8 to 12.3 gm/dl, in group B was 12.4 to 13.2 gm/dl, and in group C was 13.3 to 15.7 gm/dl. Heart rate, blood pressure, arterial saturation, and cerebral near-infrared spectroscopy (cNIRS) values before transfusion, as well as at 1, 2, 4, 8, and 12 h after transfusion, were collected. There was significant improvement in diastolic blood pressure, arterial saturation, and cNIRS in group A after 12 h. Transfusions given in group B also resulted in improvement in diastolic blood pressure and arterial saturation, with less robust response of cNIRS. In group C, only arterial saturation values increased significantly. RBC transfusions can improve hemodynamics and markers of oxygen delivery in infants with single-ventricle physiology, but further studies are needed to determine an optimal Hgb level in this population. Interventions to increase Hgb above this level may be of limited benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the setting of chronic hypoxemia, children with cyanotic congenital heart disease (CCHD) often develop an increased hematocrit. This process is thought to represent a compensatory mechanism to achieve adequate oxygen delivery to tissue beds. Based on this physiologic adaptation, most congenital heart centers have adopted a strategy of red blood cell (RBC) transfusion among children with cyanotic heart disease to achieve greater serum hemoglobin (Hgb) concentration among hospitalized patients. Although there are no published guidelines, RBC transfusion is typically undertaken to achieve serum Hgb levels >13 gm/dl.
However, studies have demonstrated increased risks associated with RBC transfusions in adults after cardiac surgery [8, 26]. Although a more liberal transfusion strategy has been shown to have greater rates of worsening organ failure and other complications in adults, a randomized study of conservative versus liberal RBC transfusion strategies in critically ill children without congenital heart disease has demonstrated no difference in adverse outcomes [15]. Recent prospective studies have reported that significant hemodilution during surgical repair of infants with congenital heart defects was associated with poorer neurodevelopmental outcome [13, 22, 37]. Another prospective trial suggested that low hematocrit during cardiopulmonary bypass may affect early postoperative lactate levels [24]. It is not known if there are potential benefits and risks of RBC transfusions outside of the immediate postoperative period in patients with CCHD. Analysis of the effect of RBC transfusion is complex because of the time-dependent nature of the decision to transfuse. The clinical decision to transfuse is largely based on bleeding complications and low Hgb concentrations occurring during the hospital course, which in turn are dependent on multiple clinical variables and therapies.
At our institution, we have generally pursued a strategy of “proactive transfusion” by maintaining serum Hgb levels >13 gm/dl in young children with CCHD. Although this strategy may improve overall oxygen delivery, the efficacy of this approach has not been evaluated. Our goal was to assess how transfusions impact regional oxygen saturation (rSO2), arterial saturation, and hemodynamic markers based on Hgb levels before blood is transfused.
Materials and Methods
This retrospective study was performed with the approval of the Institutional Review Board of Emory University School of Medicine and Children’s Healthcare of Atlanta. We reviewed the hospital records of patients <1 year of age with cyanotic single-ventricle lesions and arterial saturations <90% who were admitted to the cardiac intensive care unit at Children’s Healthcare of Atlanta at Egleston and who received a blood transfusion between December 2007 and April 2009. We included all patients who were admitted before and after surgery as well as and those who did not undergo cardiac surgery during the same hospitalization. At our institution, general criteria for blood transfusions include patients who are believed to have low Hgb concentration (usually we maintain Hgb concentration >13 gm/dl), low arterial saturations (saturation <70% in the setting of CCHD), low blood pressure (mean arterial pressure <40 mm Hg in the neonate or 50 mm Hg in children ≤1 year of age), active bleeding (sanguinous chest tube output >2 ml/kg/h), or by clinician preference. Exclusion criteria included blood transfusions given within 72 h of cardiac surgery or transfusions given to patients with sanguinous chest tube output >2 ml/kg/h. The primary outcome variable was change in cerebral rSO2 as measured by near-infrared spectroscopy (NIRS). This is routinely used at our institution on all infants with congenital heart disease in the cardiac intensive care unit, and trends are followed-up as a noninvasive proxy for mixed venous oxygen saturation (SVO2). Secondary outcome variables included blood pressure, arterial saturation, heart rate, and adverse events. Complete blood counts, creatinine, and lactic acid levels before and after transfusion were obtained. We divided the data points into terciles based on pretransfusion Hgb concentration. Heart rate, blood pressure, arterial saturation, and cerebral NIRS (cNIRS) values before transfusion, as well as at 1, 2, 4, 8, and 12 h after the start of the transfusion, were collected.
Data Analysis
Data are presented as means or medians and ranges where appropriate. Comparisons of different groups were made by χ2 or Fisher exact test. Changes in hemodynamic data were evaluated by analysis of variance with the values before transfusion and at 8 and 12 h after transfusion. Significance was determined at a p < 0.05.
Results
During the study period, 59 patients and 183 transfusions were identified (Tables 1, 2). Several patients had transfusions during different hospitalizations, and these were all included. Forty-five patients received blood transfusions after surgery during the same hospitalization as their cardiac surgery, which accounted for 133 transfusions. Eight patients received transfusions during readmissions after surgery. Two were readmitted for sternal wound infections, 2 for respiratory distress, 3 for decreased arterial saturations, and 1 for decreased oral intake. Four patients received a blood transfusion as an inpatient after catheterization.
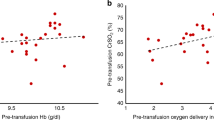
Pretransfusion Hgb concentration in group A was 7.8 to 12.3 gm/dl, in group B was 12.4 to 13.2 gm/dl, and in group C was 13.3 to 15.7 gm/dl. Further patient characteristics of the three groups are listed in Table 3. There was no statistical difference among the groups with regard to those receiving mechanical ventilation or vasoactive drips (Fig. 1). Sixty controls were obtained. These were periods when the patients had an Hgb value >13.3 gm/dl and did not receive a blood transfusion. They were matched with the study groups in terms of mechanical ventilation and inotropic support. There was no significant change of any parameter during 12 h. Mean heart rate decreased by 1 beat/minute; mean systolic blood pressure decreased by 2.5 mm Hg; mean diastolic pressure decreased by 1 mm Hg; mean arterial saturation was unchanged; and mean NIRS value decreased by 1.5.
After RBC transfusion, there was no statistically significant change in heart rate regardless of Hgb concentration (Figs. 2, 3, 4, 5, and 6). There was, however, significant improvement in diastolic blood pressure, arterial saturation, and cNIRS when an RBC transfusion was given at Hgb < 12.3 gm/dl (group A). There was an almost 8% increase in cNIRS. Transfusions given in group B also resulted in statistically significant improvement in diastolic blood pressure and arterial saturation. However, there was a less robust response in cNIRS. There were little to no statistically significant improvements when transfusions were given at Hgb > 13.2 gm/dl (Group C).
Because the decision to transfuse RBCs in some centers may relate more to clinical status than baseline Hgb, we also examined whether responses varied by whether subjects received ventilatory support or inotropic medications. The improvement in cNIRS was similar in both patients who were ventilated and those who were breathing spontaneously (5.9% vs. 6.2%, p = 0.55). Those subjects on inotropic support demonstrated an increase in cNIRS of 6.7% compared with 7.1% for those not on inotropic medications, p = 0.80.
Fourteen adverse events occurring within 48 h of a blood transfusion were identified: 5 from group A, 4 from group B, and 5 from group C. These events are listed in Table 4. They occurred in 11 patients (one patient had 3 events, and 1 patient had 2 events). There were no occurrences of acute renal failure or significant changes in lactic acid levels after transfusion. In group C, there was an increase in median lactic acid concentration after blood transfusions, but this did not approach statistical significance (p = 0.27), and the levels all remained clinically low. Three deaths occurred during the hospitalization in which a blood transfusion was received, but all occurred >48 h after transfusion. One patient who had undergone a Norwood procedure died after cardiopulmonary arrest 3 days after receiving a blood transfusion at postoperative day 56. The patient also had long-standing renal failure resulting in anuria. A second patient died after cardiopulmonary arrest at 7 days after the last blood transfusion, and a third patient died 1 month after the last blood transfusion because care was withdrawn.
Discussion
The rationale for RBC transfusion is to achieve improvement of oxygen transport and, ultimately, tissue oxygenation. In the setting of hypoxemia, increasing the Hgb concentration will increase arterial oxygen content and oxygen delivery (DO2) at a given cardiac output. Oxygen consumption (VO2) can remain constant despite decrease in oxygen delivery until a critical value is reached, at which point tissue hypoxia develops [19]. This critical level varies among individuals, but it is important to keep oxygen delivery above this level. In patients with single-ventricle physiology, their physiology limits their arterial saturation and oxygen content.
Our analysis demonstrated that RBC transfusions do appear to improve a number of important physiologic parameters. In those subjects with pretransfusion Hgb values <12.3 gm/dl, RBC transfusions favorably impacted blood pressure, arterial saturation, and, most importantly, rSO2. Not surprisingly, at greater Hgb levels, transfusion of RBCs appeared to have less of an impact. These findings should be of value in devising a uniform RBC transfusion strategy for this population.
A recent prospective study compared a restrictive versus liberal RBC-transfusion strategy in the immediate postoperative period in children undergoing either a Glenn anastomosis or Fontan completion [4]. The strategies were divided to maintain an Hgb concentration either >9 gm/dl or >13 gm/dl, respectively. Cholette et al. demonstrated no difference in arterial lactate levels or in arteriovenous and arteriocerebral oxygen content during the first 48 h after surgery. These data suggests that patients with palliated single-ventricle physiology may not require as high an Hgb concentration as previously believed.
In adult cardiac surgery patients, several parameters have been suggested to help determine the need for blood transfusion. These include relative tachycardia, relative hypotension, decrease in VO2 > 10% from baseline, oxygen extraction ratio (VO2/DO2) > 50%, SVO2 < 50%, and a mixed venous oxygen partial pressure (PvO2) < 32 mm Hg [30]. However, these suggested guidelines may not be applicable for clinicians caring for children with CCHD. SVO2 is thought to be a single statistically independent variable that accurately reflects the adequacy of systemic perfusion [1] and may improve survival when used as goal-directed therapy [36]. Studies have looked at the use of cNIRS to monitor continuous cerebral oxygen saturation as an estimation of SVO2 in patients with CCHD. Although a correlation is present, cNIRS is more useful to follow as a trend than taken as an absolute measurement [2, 12, 16, 21]. Furthermore, a positive correlation has also been shown between rSO2 and Hgb concentration in children with and without congenital heart disease [2, 17]. Low rSO2 and SVO2 in patients after a Norwood procedure has an association with worse outcomes in terms of organ failure [35], neurodevelopment [7, 11], and mortality [25].
However, there is no agreement as to the appropriate threshold to maintain Hgb concentration in patients with single-ventricle physiology. Polycythemia leads to an increase in O2 content, but this is offset by a decrease in cardiac output, leading to no change or even a decrease in O2 delivery. This has been previously attributed to hyperviscosity of blood and increased afterload [6, 9, 23], but newer evidence suggests that this may be due to regulation of oxygen delivery and not solely on blood viscosity [18].
There is growing literature that stored RBCs have a diminished ability for oxygen delivery compared with native RBCs. There is some debate as to the efficacy of newer versus older stored RBCs in terms of O2 transport [30, 33], and it has been suggested that older stored blood may increase the risk of postoperative complications [14]. An animal model has suggested that circulation of stored RBCs result in significantly malperfused and underoxygenated microvasculature not detected at the systemic level [34]. Storage of RBCs affects cell deformability and depletes nitric oxide, which impairs the vasodilatory activity of RBCs due to the decrease in S-nitroso-Hgb concentration [27].
Transfusions also carry risk of adverse effects. Blood product exposure has been demonstrated as an independent risk factor for the development of central line-associated bloodstream infection [5]. Sepsis due to contaminated blood has been documented at 1/250,000 transfusions, but this is probably underreported as a complication [10]. Immune modulation may be a more important mechanism for sepsis than contaminated blood. Exposure to blood products is thought to decrease natural killer cell function, affect antigen presentation, decrease helper-to-suppressor T-lymphocyte ratio, and decrease cell-mediated immunity [3, 29]. Studies have demonstrated increased morbidity, mortality, and cost associated with RBC transfusions due to infections, acute respiratory distress syndrome, and organ failure after cardiac surgery [20, 32]. We identified 14 adverse events in 11 patients. These patients received a mean of 5.7 transfusions each within the study period. Due to their high number of transfusions, they likely were more critically ill and more prone to the effects of immune modulation. We had 4 incidents of culture-positive sepsis (2.5%) but no acute increases in serum creatinine or lactic acid levels. There is also some evidence that greater levels of Hgb is associated with increased incidence of systemic–to–pulmonary shunt thrombosis [28].
There are alternatives to the use of RBC transfusion in patients with evidence of hypovolemia. They can include crystalloid fluid, albumin, and hydroxyethyl starch. A prospective, randomized trial using hydroxyethyl starch or albumin in the setting of noncardiac surgery demonstrated effective hemodynamic stabilization without adverse impact on coagulation or other safety parameters in both groups [31]. Using these fluids may improve cardiac output without the detriment of increasing blood viscosity or decreasing DO2 to a level at which tissue hypoxia develops. Patients may have sufficient oxygen-carrying capacity at lower Hgb concentrations than initially expected. Children with normal two-ventricle circulation do not demonstrate an increase in adverse events with maintaining Hgb as low as 7 gm/dl [15]. This threshold is likely greater in patients with single-ventricle physiology.
Limitations
Limitations of this study include its retrospective design and lack of randomization. There may be selection bias in terms of which patients received blood transfusions because our institution does not have a set protocol for the administration of blood products. The decision for blood transfusion is strictly clinician derived. More critically ill patients, especially those with a more tenuous postoperative course, are more likely to receive blood. Patients who clinically demonstrate improvement with previous RBC transfusions are also more likely to receive further transfusions. In addition, adverse events are listed but may not be solely linked to transfusions.
Although liberal versus restrictive transfusion strategies have been examined in relatively stable patients in a pediatric intensive care unit [15] and after cavopulmonary connections [4], we believe there is a role for larger prospective studies looking at these strategies to optimize oxygen delivery and demonstrate survival benefit and improved clinical outcome measures in patients with CCHD.
Conclusion
In summary, blood transfusions improve markers of oxygen delivery, such as arterial saturation and cNIRS, when given to patients with Hgb concentration <13.2 gm/dl. Further studies are needed to determine optimal Hgb levels in hospitalized infants with single-ventricle physiology. Interventions to augment Hgb above this level may be of limited benefit.
References
Alston RP, Anderson A, Sanger K (2006) Is body surface area still the best way to determine pump flow rate during cardiopulmonary bypass? Perfusion 21:139–147
Bhutta AT, Ford JW, Parker JG et al (2007) Noninvasive cerebral oximeter as a surrogate for mixed venous saturation in children. Pediatr Cardiol 28:34–41
Blajchman MA (2002) Immunomodulation and blood transfusion. Am J Ther 9:389–935
Cholette JM, Rubenstein JS, Alfieris GM et al (2011) Children with single-ventricle physiology do not benefit from greater hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red-cell transfusion strategy. Pediatr Crit Care Med 12(1):39–45
Costello JM, Graham DA, Morrow DF et al (2009) Risk factors for central line-associated bloodstream infection in a pediatric cardiac intensive care unit. Pediatr Crit Care Med 10(4):453–459
DeFilippis AP, Law K, Curtin S, Eckman JR (2007) Blood is thicker than water: the management of hyperviscosity in adults with cyanotic heart disease. Cardiol Rev 15:31–34
Dent CL, Spaeth JP, Jones BV et al (2005) Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg 130:1523–1530
Despotis G, Eby C, Lublin DM (2008) A review of transfusion risks and optimal management of perioperative bleeding with cardiac surgery. Transfusion 48(Suppl):2S–30S
Erslev AJ, Caro J (1984) Secondary polycythemia: a boon or a burden? Blood Cells 10:177–191
Hendrickson JE, Hillyer CD (2009) Noninfectious serious hazards of transfusion. Anesth Analg 108:759–769
Hoffman GM, Mussatto KA, Brosig CL et al (2005) Systemic venous oxygen saturation after the Norwood procedure and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg 130:1094–1100
Johnson BA, Hoffman GM, Tweddell JS et al (2009) Near-infrared spectroscopy in neonates before palliation of hypoplastic left heart syndrome. Ann Thorac Surg 87:571–577
Jonas RA, Wypij D, Roth SJ et al (2003) The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg 126:1765–1774
Koch CG, Li L, Sessler DI et al (2008) Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 358:1229–1239
Lacroix J, Hebert PC, Hutchison JS et al (2007) Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 356:1609–1619
Li J, Van Arsdell GS, Zhang G et al (2006) Assessment of the relationship between cerebral and splanchnic oxygen saturations measured by near-infrared spectroscopy and direct measurements of systemic haemodynamic variables and oxygen transport after the Norwood procedure. Heart 92:1678–1685
Li J, Zhang G, Holtby H et al (2008) The influence of systemic hemodynamics and oxygen transport on cerebral oxygen saturation in neonates after the Norwood procedure. J Thorac Cardiovascular Surg 136(1):83–90
Lindenfeld J, Weil JV, Travis VL et al (2005) Regulation of oxygen delivery during induced polycythemia in exercising dogs. Am J Physiol Heart Circ Physiol 289:H1821–H1825
Madjdpour C, Spahn DR (2007) Allogeneic red blood cell transfusion: physiology of oxygen transport. Best Pract Res Clin Anaesthesiol 21:163–171
Marik PE, Corwin HL (2008) Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med 36:2667–2674
McQuillen PS, Nishimoto MS, Bottrell CL et al (2007) Regional and central venous oxygen saturation monitoring following pediatric cardiac surgery: concordance and association with clinical variables. Pediatr Crit Care Med 8:154–160
Newburger JW, Jonas RA, Soul J et al (2008) Randomized trial of hematocrit 25% versus 35% during hypothermic cardiopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg 135:347–354
Nihill MR, McNamara DG, Vick RL (1976) The effects of increased blood viscosity on pulmonary vascular resistance. Am Heart J 92:65–72
Ootaki Y, Yamaguchi M, Yoshimura N et al (2004) Efficacy of a criterion-driven transfusion protocol in patients having pediatric cardiac surgery. J Thorac Cardiovasc Surg 127:953–958
Phelps HM, Mahle WT, Kim DW et al (2009) Postoperative cerebral oxygenation in hypoplastic left heart syndrome following the Norwood procedure. Ann Thorac Surg 87(5):1490–1494
Reeves BC, Murphy GJ (2008) Increased mortality, morbidity, and cost associated with red blood cell transfusion after cardiac surgery. Curr Opin Cardiol 23:607–612
Reynolds JD, Aheam GS, Angelo M et al (2007) S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci USA 104:17058–17062
Sahoo TK, Chauhan S, Sahu M et al (2007) Effects of hemodilution on outcome after modified Blalock-Taussig shunt operation in children with cyanotic congenital heart disease. J Cardiothorac Vasc Anesth 21:179–183
Shorr AF, Jackson WL (2005) Transfusion practice and nosocomial infection: assessing the evidence. Curr Opin Crit Care 11:468–472
Slight RD, Alston P, McClelland DBL, Mankad PS (2009) What factors should we consider in deciding when to transfuse patients undergoing elective cardiac surgery? Transfus Med Rev 23:42–54
Standl T, Lochbuehler H, Galli C et al (2008) HES 130/0/4 (Voluven) or human albumin in children younger than 2 yr undergoing noncardiac surgery. A prospective randomized, open-label, multicentre trial. Euro J Anaesthesth 25:437–445
Szekely A, Cserep Z, Sapi E et al (2009) Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg 87:187–197
Tinmouth A, Fergusson D, Yee IC et al (2006) Clinical consequences of red cell storage in the critically ill. Transfusion 46:2014–2027
Tsai AG, Cabrales P, Intaglietta M (2004) Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion 44:1626–1634
Tweddell JS, Ghanayem NS, Mussatto KA et al (2007) Mixed venous saturation monitoring after stage 1 palliation for hypoplastic left heart syndrome. Ann Thorac Surg 84:1301–1310
Tweddell JS, Ghanayem NS, Hoffman GM (2010) Pro: NIRS is “standard of care” for postoperative management. Semin Thorac Cardiovasc Surg Pediatr Cardiol Surg Ann 13:44–50
Wypij D, Jonas RA, Bellinger DC (2008) The effect of hematocrit during hypothermic cardiopulmonary bypass in infant heart surgery: results from the combined Boston hematocrit trials. J Thorac Cardiovasc Surg 135:355–360
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuo, J.A., Maher, K.O., Kirshbom, P.M. et al. Red Blood Cell Transfusion for Infants With Single-Ventricle Physiology. Pediatr Cardiol 32, 461–468 (2011). https://doi.org/10.1007/s00246-011-9901-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-011-9901-3