Abstract
Left ventricular noncompaction (LVNC) is a form of cardiomyopathy resulting from a disorder of endomyocardial morphogenesis. It has been associated with significant morbidity and mortality. The aim of this study was to characterize associated cardiac findings in children with LVNC and to identify risk factors associated with increased mortality. From our echocardiography database, we identified 46 patients diagnosed with LVNC between December 1999 and February 2005. The mean age at presentation was 3.6 ± 5.6 years, and the mean duration of follow-up was 1.9 ± 2.1 years. Left ventricular ejection fraction was decreased in 24 patients (52%; mean 39.5% ± 13.1%). Thirty-six patients (78%) had associated cardiac lesions, including atrial septal defect (n = 16 [35%]), ventricular septal defect (n = 17 [37%]), patent ductus arteriosus (n = 14 [30%]), and Ebstein’s anomaly (n = 5 [11%]). Electrocardiogram abnormalities were found in 80% of patients; most commonly they included left (n = 15 [43%]) and right ventricular hypertrophy (n = 19 [54%]). Documented arrhythmias included ectopic atrial rhythm (n = 2), junctional rhythm (n = 2), supraventricular tachycardia (n = 2), and ventricular tachycardia (n = 1). Overall mortality was 20%, and there was no association with ejection fraction, morphologic defect, or arrhythmia. Mean age at diagnosis in survivors (4.5 ± 6.1 years) was higher than nonsurvivors (0.4 ± 0.7 years) (p < 0.0001). LVNC is a rarely isolated form of cardiomyopathy, and it is associated with significant additional cardiac abnormalities. Although it does not have an invariably fatal course, early presentation in infancy does carry an increased risk of mortality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Noncompaction of the ventricular myocardium, previously referred to as “spongy myocardium,” is now better known as left ventricular noncompaction (LVNC). It is believed to result from the arrest of normal endomyocardial morphogenesis. The disorder is characterized by the gross anatomic appearance of numerous prominent trabeculations and deep intertrabecular recesses within the left ventricle [3, 7]. Isolated noncompaction of the myocardium is defined as LVNC with no associated congenital cardiac malformations. This is a rare form of cardiomyopathy, with few reports in the literature [3, 10], and it is currently classified as a genetic cardiomyopathy by the American Heart Association [9]. Diagnostic echocardiographic criteria have recently been proposed [8].
Clinical complications of ventricular dysfunction, systemic embolism, and ventricular tachyarrhythmias have been described in both adults and children [3, 6, 12]. Fewer than 200 cases of LVNC have been reported in case studies or series of children. This study represents one of the largest reported series of pediatric patients to date. We describe characteristics of LVNC in children and seek to identify risk factors associated with morbidity and mortality.
Methods
The echocardiographic database of Riley Children’s Hospital was searched for all patients diagnosed with LVNC between December 1999 and February 2005 who were followed-up through May 2007. Medical records were reviewed to document clinical presentations, including heart failure symptoms, treatment with cardiac medications, and interventional procedures. Comorbidities, such as facial dysmorphisms, familial inheritance patterns, developmental delay, failure to thrive, and associated medical illnesses, were also evaluated. Twelve-lead electrocardiograms (ECGs) and Holter monitoring were analyzed for conduction abnormalities and arrhythmias. Two-dimensional ECGs were reviewed for severity of noncompaction, left ventricular systolic function, and other features of congenital heart disease. This study was approved by the Institutional Review Board at the Indiana University School of Medicine.
Diagnostic Criteria
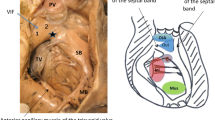
LVNC was diagnosed by the characteristic presence of multiple, excessively prominent trabeculations associated with deep intertrabecular recesses on echocardiography as previously described [2]. An X-to-Y ratio was measured using the previously reported method, where X represents depth of the trabecular recess, and Y represents total free-wall thickness to the peak of the trabeculation (Fig. 1). This method has been shown to correspond with necropsy specimens of patients with LVNC [13]. Two independent reviewers calculated X-to-Y ratios and characterized the echocardiographic studies as representing mild, moderate, or severe LVNC.
Statistical Analysis
Descriptive data for continuous variables are presented as mean ± SD. Log rank tests compared survival between categorical groups. Cox regression analysis was performed to identify associations between continuous variable measurements and survival; p < 0.05 was considered significant. Cohen’s kappa was calculated to test for interobserver accuracy in noncompaction severity scores.
Results
Study Population
Forty-six cases of LVNC were identified from approximately 40,000 echocardiograms and 3,500 cases of cardiomyopathy during the study period (Table 1). Of the 46 children with LVNC, 23 were boys, and 23 were girls. Median age at presentation was 0.44 years (range birth to 18.5 years). The mean duration of follow-up was 1.9 ± 2.1 years (range 0 to 6.9 years).
Clinical Data
Twenty-five patients (54%) presented with symptoms of heart failure. Treatment with cardiac medications-including furosemide (n = 30 [65%]), digoxin (n = 22 [48%)], angiotensin-converting enzyme (ACE) inhibitors (n = 21 [46%]), aldactone (n = 13 [28%]), and beta-blockers (n = 10 [22%])-was common. Six patients (13%) were on aspirin therapy, and seven patients (15%) required inotropic agents to augment cardiac output at some point in their life.
Thirty-six patients (78%) had associated congenital cardiac abnormalities. Cardiac surgeries or interventions were performed on 16 patients (35%). These included atrial septal defect (ASD) repair (n = 5 [11%]), ventricular septal defect (VSD) repair (n = 4 [9%]), patent ductus arteriosus (PDA) ligation (n = 5 [11%]), pulmonary artery banding (n = 5 [11%]), modified Blalock-Taussig shunt placement (n = 4 [9%]), hemi-Fontan palliation (n = 1 [2%]), and Fontan palliation (n = 1 [2%]).
Clinical comorbidities included failure to thrive (n = 7 [15%]), developmental delay (n = 6 [13%]), facial dysmorphisms (n = 7 [15%]), and renal/genitourinary abnormalities (n = 6 [13%]). Neurologic abnormalities, including seizure disorder, encephalopathy, ventricular hemorrhage, and/or hearing loss, occurred in 15 (33%) patients. Two cases of cardiomyopathy in first-degree relatives were identified.
Electrocardiographic Findings
ECG and Holter data were available in 35 of 46 patients. Of those, 28 (80%) were noted to have ECG and/or rhythm abnormalities. The most frequent findings were right (n = 19 [54%]) and left ventricular hypertrophy (n = 15 [43%]). Conduction abnormalities included first-degree atrioventricular block (n = 4 [11%]), prolonged QTc (n = 4 [11%]), interventricular conduction delay (n = 3 [9%]), right bundle branch block (n = 2 [6%]), and Wolff-Parkinson-White syndrome (n = 3 [9%]). All 3 patients with Wolff-Parkinson-White syndrome had Ebstein’s anomaly and decreased ventricular function. Arrhythmias included ectopic atrial rhythm (n = 2 [6%]), junctional rhythm (n = 3 [9%]), supraventricular tachycardia (n = 3 [9%]), and ventricular tachycardia (n = 2 [6%]).
Two-Dimensional Echocardiography
Isolated left ventricular noncompaction was found in 10 patients (22%). Thirty-six patients (78%) had additional cardiac defects: VSD (n = 17 [37%]), ASD (n = 16 [35%]), PDA (n = 14 [30%]), and Ebstein’s anomaly of the tricuspid valve (n = 5 [11%]). Three patients had “Swiss cheese” VSD morphology with multiple muscular defects. Other complex abnormalities identified included anomalous left coronary artery from the pulmonary artery (n = 1), dextrocardia (n = 1), d-transposition of the great vessels (n = 1), and double-inlet left ventricle (n = 1). Patients also had left-sided (cor triatriatum, subaortic stenosis, coarctation of the aorta; n = 2), and right-sided obstruction abnormalities (tricuspid atresia, pulmonary atresia; n = 2). Mean presenting left ventricular ejection fraction was 50% ± 18% (range 15 to 76). Left ventricular systolic function was decreased in 24 patients (52%).
The mean calculated X-to-Y ratio was 0.50 ± 0.09 for observer 1 and 0.49 ± 0.09 for observer 2, with significant agreement in severity scores (p < 0.001). However, there was no statistically significant relation between severity score and mortality.
Outcomes
Survival at follow-up was 80% (mean 1.9 ± 2.1 years). No patients underwent cardiac transplantation. Thrombus was identified in only one patient. The need for inotropic support during hospitalization was associated with an increased incidence of mortality (p = 0.0002). Mean age of diagnosis in survivors (4.5 ± 6.1 years) was higher that of than nonsurvivors (0.4 ± 0.7 years) and was the only significant predictor of mortality (p < 0.001).
Discussion
LVNC is a congenital cardiomyopathy that has been recognized with increasing frequency because of advances in awareness and diagnostic imaging. Newer noninvasive imaging modalities, including computed axial tomography and magnetic resonance imaging (Fig. 2), have been shown to correlate well with images seen on ECG [4, 5, 11]. Isolated left ventricular noncompaction is even more rare, and its natural history and clinical manifestations have not been well defined. Several recent reports have bee published since the initial descriptions [6, 10]. Our series represents one of the largest reported in the pediatric population.
The use of echocardiography, and the X-to-Y ratio, in diagnosing LVNC has been duplicated successfully in multiple studies and found to correlate with necropsy specimens [13]. Interobserver variation must be considered, however, in assessing the severity of noncompaction. Our study demonstrated a significant interobserver agreement in both quantitative and qualitative assessment.
Compared with the children initially reported by Chin et al. [3], patients in this and other reported series were diagnosed at an earlier median age (Table 2). This may be attributed to a previously unrecognized correlation between LVNC and additional congenital heart defects that present at an earlier age. More than half of patients have decreased systolic function at the time of diagnosis, but the presence of heart failure symptoms vary by study. Three major cardiac risks associated with ventricular noncompaction were initially reported: decreased systolic function of the noncompacted left ventricle, endocardial clot with systemic embolization, and ventricular arrhythmias. Our study confirms previous findings [6, 12] that the incidence of these events, especially systemic thromboembolism and ventricular arrhythmia, is not as significant as originally reported by Chin et al. [3].
Clinical Characteristics and Outcomes
A large proportion of patients do appear to have associated structural cardiac abnormalities. We also found several ECG abnormalities, as has been previously described [1]. Overall mortality rates appear to have improved. This may be explained by earlier recognition of LVNC and improved therapeutic intervention. However, the presence of associated abnormalities does not appear to significantly correlate with increased risk of mortality. Rates of heart transplantation vary and may be dependent on institutional practice.
Much work has gone into understanding the genetic cause of LVNC. In the original report, Chin et al. [3] described familial recurrence in half of their patients. Mutations in the gene G4.5, which maps to chromosome Xq28, was initially identified as the gene associated with Barth syndrome and encodes a novel protein family called “tafazzins.” Many familial and sporadic cases have now been reported, and the highly heterogeneous genetic nature has been well described [15]. Autosomal dominant inheritance appears to be the prominent mode of transmission, but X-linked and mitochondrial inheritance has also been described [2, 8, 14]. Xing et al. [15] demonstrated familial disease in approximately 25% of the LVNC patients enrolled in their study. Our group showed only 2 cases of first-degree relatives with LVNC; however, a detailed genetic analysis was not performed in every patient.
Conclusion
LVNC is a slightly rare cardiomyopathy that is commonly associated with additional cardiac abnormalities. Systemic embolic events and ventricular tachycardia are less common than previously reported. Although LVNC does not have an invariably fatal course, earlier presentation in infancy does predict an increased risk of mortality. Further study, with longer follow-up, is needed to clarify long-term prognosis.
References
Celiker A, Ozkutlu S, Dilber E et al (2005) Rhythm abnormalities in children with isolated ventricular noncompaction. Pacing Clin Electrophysiol 28:1198–1202
Chen R, Tsuji T, Ichida F et al (2002) Mutation analysis of the G4.5 gene in patients with isolated left ventricular noncompaction. Mol Genet Metab 77:319–325
Chin TK, Perloff JK, Williams RG et al (1990) Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 82:507–513
Daimon Y, Watanabe S, Takeda S et al (2002) Two-layered appearance of noncompaction of the ventricular myocardium on magnetic resonance imaging. Circ J 66:619–621
Hany TF, Jenni R, Debatin JF (1997) MR appearance of isolated noncompaction of the left ventricle. J Magn Reson Imaging 7:437–438
Ichida F, Hamamichi Y, Miyawaki T et al (1999) Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 34:233–240
Jenni R, Goebel N, Tartini R et al (1986) Persisting myocardial sinusoids of both ventricles as an isolated anomaly: echocardiographic, angiographic, and pathologic anatomical findings. Cardiovasc Intervent Radiol 9:127–131
Jenni R, Oechslin E, Schneider J et al (2001) Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 86:666–671
Marron BJ, Towbin JA, Thiene G et al (2006) Contemporary definitions and classification of the cardiomyopathies. An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113:1807–1816
Oechslin EN, Attenhofer Jost CH, Rojas JR et al (2000) Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 36:493–500
Petersen SE, Selvanayagam JB, Wiesmann F et al (2005) Left ventricular noncompaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 46:101–105
Pignatelli RH, McMahon CJ, Dreyer WJ et al (2003) Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation 108:2672–2678
Ritter M, Oechslin E, Sutsch G et al (1997) Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 72:26–31
Sasse-Klaassen S, Gerull B, Oechslin E et al (2003) Isolated noncompaction of the left ventricular myocardium in the adult is an autosomal dominant disorder in the majority of patients. Am J Med Genet A 119:162–167
Xing Y, Ichida F, Matsuoka T et al (2006) Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol Genet Metab 88:71–77
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsai, S.F., Ebenroth, E.S., Hurwitz, R.A. et al. Is Left Ventricular Noncompaction in Children Truly an Isolated Lesion?. Pediatr Cardiol 30, 597–602 (2009). https://doi.org/10.1007/s00246-008-9382-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-008-9382-1