Abstract
Left ventricular noncompaction is a rare form of cardiomyopathy, which results from multiple trabeculations in the left ventricular myocardium. The clinical presentation is highly variable, and spectrum includes asymptomatic patients diagnosed during family screening on one end to patients with depressed systolic function, heart failure, thromboembolic complications, and cardiac arrhythmias on the other (Kim et al in J Am Coll Cardiol 53: 2009, 2009). Further, the progression of the condition is highly variable. Hence, these patients require close follow-up, and management for each patient needs to be individualized and periodically reevaluated. Here, we present a series of five cases that have been followed in our practice and present our experience. A literature review of this rare form of congenital cardiomyopathy is also presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
LV noncompaction is a rare form of congenital cardiomyopathy. It was first described in 1932 in a newborn with aortic atresia, and the first case report of LVNC as an isolated finding was published in 1984 [1].
Since then, there have been several case reports and small studies mostly in adults, aimed at identifying etiology including genetic pattern, clinical course, diagnosis, management, and prognosis [1–16].
Despite all the effort, a clear understanding of this cardiomyopathy is lacking, frustrating the efforts to diagnose and prognosticate the condition with certainty, especially in young children who are mostly healthy and asymptomatic.
Here, we describe a series of five cases that we are following in our clinics, emphasizing the variations in clinical presentation and how this affects the management decisions. A literature review of this rare form of congenital cardiomyopathy is also presented.
Case 1
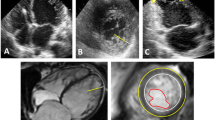
Two-month-old, ex 25-week premature baby was seen in cardiology clinic for an incidental finding of a small coronary cameral fistula from the left coronary artery (LCA) to the right ventricle (RV) noted on echocardiography performed because of clinical suspicion of significant patent ductus arteriosus (PDA) (Fig. 1). At the initial evaluation, the fistula was thought to be small and hemodynamically insignificant (normal left-sided chamber dimensions, normal left ventricular (LV) function, no murmurs or signs of Congestive heart failure on examination, and normal Electrocardiogram) and a decision was made to follow the patient clinically.
Patient was also noted to have other complications related to prematurity including retinopathy of prematurity and bronchopulmonary dysplasia. A concern was also raised for a Progressive Familial Intrahepatic Cholestasis (PFIC) deficiency, which was ruled out.
This patient was followed by cardiology and at around 4 months of age was identified to have LV noncompaction by echocardiogram using the Jenni criteria [2] (Figs. 2, 3). Subsequently, she was noted to have a decrease in her LV systolic function from normal (Ejection Fraction of 64%) to borderline low (Ejection Fraction of about 52%) with a dilatation of the left-sided chambers. She was referred for a cardiac MRI that confirmed the diagnosis of the LV noncompaction (Figs. 4, 5) as well as the hemodynamic nonsignificance of the coronary artery fistula.
The patient was started on Enalapril with stabilization of her LV function. She had a subsequent 24-h holter monitoring which was normal.
She is closely followed in the cardiology clinic and is 2 years old now. She continues to remain asymptomatic with a stable, low normal LV systolic function (Ejection Fraction of 54%).
Case 2
Eighteen-year-old young man presented for cardiology evaluation due to symptoms of chest pain, dizziness, and palpitations. He had an unremarkable physical exam. An electrocardiogram (ECG) and echocardiogram were performed as a part of his work up. ECG showed borderline short PR intervals (Fig. 6). The echocardiogram was significant for increased LV trabeculations and met the criteria for LVNC (Figs. 7, 8). LV function and volume were noted to be normal, and no other structural defects were seen. He subsequently had a 30-day event monitoring performed that showed few premature ventricular contractions (PVCs) and aberrantly conducted premature atrial contractions (PACs). A follow-up MRI was performed confirming the diagnosis of LV noncompaction (Fig. 9) with normal function and no regional wall motion abnormality. Nonspecific diffuse areas of delayed enhancement were noted. Due to normal function, no medications were started and he was restricted from sports. A follow-up was scheduled with an electrophysiologist, and an exercise stress test was recommended. However, he was subsequently lost to follow-up.
Case 3 and Case 4
These two patients were siblings of the patient described in case 2. The mother had requested an evaluation on the younger siblings due to the diagnosis of LVNC in their older brother. Both siblings were noted to meet the echocardiographic criteria for LV noncompaction. However, in the absence of symptoms with a normal physical exam, normal ECG, and normal ventricular systolic function, a decision was made to follow them clinically and no further testing was performed and no exercise restrictions were prescribed.
Case 5
This is a 2-year-old female who was referred to cardiology for an episode of “turning blue.” No other symptoms were reported, and she had otherwise been healthy. Her physical exam revealed a functional (Still’s) murmur but was otherwise unremarkable. Her ECG was normal, and the echo met the criteria for the diagnosis of LVNC (Figs. 10, 11) with normal systolic function and no regional wall motion abnormalities or other structural cardiac defects. She was prescribed a 24-h holter that was normal. Hence, we decided to follow her clinically without any further testing or intervention.
Discussion
Left ventricular noncompaction is a rare form of cardiomyopathy, which results from multiple trabeculations in the left ventricular myocardium. It is characterized by prominent trabeculae, deep intertrabecular recesses, and thickening of the myocardium in 2 distinct layers (compacted and noncompacted) [17]. Due to this, the left ventricle may demonstrate impaired systolic function, with or without dilatation [5]. It can also present with cardiac arrhythmias and conduction defects such as atrial fibrillation, ventricular arrhythmias, atrio-ventricular or bundle branch blocks, and Wolff-Parkinson-White syndrome [15]. In some cases, it has also been known to present with cardioembolic complications, resulting from atrial fibrillation or clot formation within the myopathic left ventricle [18].
This cardiomyopathy has been seen both in an isolated form [10, 17] as well as in association with other congenital heart defects such as ventricular septal defects, double-orifice mitral valve, Ebstein’s anomaly of the tricuspid valve, and bicuspid aortic valves [18].
In recent decades, the knowledge regarding this important cardiomyopathy has been increasing though the heterogeneity of presentation has frustrated efforts to form unifying guidelines to diagnose, manage, and prognosticate the condition.
Epidemiology
The true prevalence of LVNC is unclear, with reported prevalence ranging between 0.05 and 0.24% [19]. The age at presentation is also extremely variable with cases reported in utero to as old as 94 years of age [20–22]. Overall, a male preponderance is seen [19]. Differences in prevalence amongst different ethnicities are also suggested, but the data on this are unclear.
Embryology and Genetics
LVNC is thought to result from an arrest in the normal myocardial development. The formation of the four-chambered heart begins with the formation of a linear myocardial tube lined by endocardium. The heart tube initially consists of two layers: an outer myoepicardial mantle and an inner endocardial cell layer separated by an intervening matrix of proteoglycans and glycosaminoglycans referred to as the acellular cardiac jelly. Once looping of the inner heart tube occurs, trabeculation commences, which is first evident at the end of fourth gestational week [18]. Between weeks 5 and 8, the myocardium is compacted and epicardial coronary arteries are formed. It is believed, a perturbation leading to arrest in normal myocardial development during this phase results in this form of cardiomyopathy [23, 24].
There is also a complex, heterogeneous genetic basis for LVNC, including both familial and sporadic forms. Several different genes have been linked to this condition including (1) taffazin, (2) Beta-Dystrobrevin (DTNA), (3) Cypher/ZASP (LDB3), (4) lamin A/C (LMNA), (5) SCN5A, (6) MYH7, and (7) MYBPC3 [10].
However, there is a significant overlap in the phenotypes and LVNC is also found to occur with dilated or hypertrophic cardiomyopathy [25]. Two hypotheses have been proposed to explain the development of noncompaction in this setting that proposes microcirculatory dysfunction and metabolic disorders or a response to a previous history of myocarditis as the responsible mechanism [26, 27].
Clinical Presentation
Even though LVNC is considered to be a congenital cardiomyopathy, clinical presentation is highly variable. Some individuals remain asymptomatic and are diagnosed during family screening or after an incidental diagnostic test [28, 29]. Other patients can present with depressed systolic function causing heart failure, thromboembolic complications, cardiac arrhythmias, and conduction defects including atrial fibrillation and ventricular arrhythmias, which can sometimes be fatal [15]. In patients with left ventricular systolic dysfunction, dyspnea is the most common presenting symptom. Other presenting symptoms can include chest pain, palpitations, syncope, cerebrovascular accidents, and other systemic or pulmonary embolic complications [10, 15].
Diagnosis
Transthoracic echocardiography remains the imaging modality of choice. Alternative imaging modalities include contrast-enhanced two-dimensional echocardiography, ventriculography [30], ultrafast computed tomography, and cardiac magnetic resonance imaging. These alternative modalities are very helpful particularly if the transthoracic echocardiographic windows are inadequate or the echocardiogram is suggestive of the diagnosis but does not fully meet the criteria. In addition, cardiac MRI provides information useful in prognostication by providing an accurate estimate of ventricular mass, function, regional wall motion, myocardial perfusion, and fibrosis.
Based on the findings of the imaging, several different criteria have been suggested for making the diagnosis of which the prominent four are as follows
-
1.
Chin et al. criteria [5] that focus on trabeculae at the left ventricular apex on the parasternal long-axis, subcostal, and apical views, and on left ventricular free-wall thickness measured at end-diastole. LVNC is defined by a ratio of X/Y ≤ 0.5, where X represents the distance from the epicardial surface to the trough of the trabecular recess, and Y is the distance from the epicardial surface to peak of trabeculation.
-
2.
Jenni et al. criteria [2] are based on the presence of a two-layer structure, with a thin compacted epicardial layer (C) and a thicker noncompacted endocardial layer (NC) measured at end-systole from the parasternal short-axis and apical approaches. LVNC conforms to a ratio of NC/C of 2 in adults or more than 1.4 in children as suggested by Pignatelli et al. [7]. It is also based on the finding of noncompaction, which is predominantly in mid-lateral/apical/mid-inferior areas; the evidence of deep perfused intertrabecular recesses on color Doppler and decreased thickening and hypokinesia present within, but not limited to, the noncompacted segments.
-
3.
Stollberger et al. criteria [19] are based on the echocardiographic presence of more than three trabeculations protruding from the left ventricular wall, apical to the papillary muscles, and visible in a single image plane. These trabeculations must move synchronously with the myocardium and must have same echogenicity. The intertrabcular spaces must be perfused from the ventricular cavity and be visualized on color Doppler.
-
4.
Petersen et al. criteria [21] are based on the NC:C ratio of >2.3 at end-diastole frames on CMRI. The segment with the most pronounced trabeculations is chosen for measurement of the thickness of the noncompacted and the compacted myocardium under these criteria.
Besides the imaging that is needed for diagnosis, ECG is another essential test for patients with a diagnosis of LVNC. While there are no ECG- based diagnostic criteria for LVNC, however these patients are at a higher risk for cardiac arrhythmias.
Management
Management of patients with LVNC is based on clinical presentation and associated findings. Patients who are asymptomatic and who have normal left ventricular systolic function do not need treatment but should be closely followed.
Participation in competitive sports may be considered for asymptomatic patients with a diagnosis of LVNC and normal systolic function and without important ventricular tachyarrhythmias [16].
For those patients who have a reduced ejection fraction and heart failure, the ACC/AHA guideline for treatment of heart failure [31] is used. This includes judicious use of diuretics, ACE inhibitors, and beta-blockers. Patients who fail medical therapy can also benefit from cardiac resynchronization therapy (CRT) with or without an implantable cardioverter defibrillator (ICD) [32]. Arrhythmias are treated with beta-blockers, calcium channel blockers, Amiodarone, and other agents as appropriate [18]. Patients with sustained ventricular tachycardia or ventricular fibrillation should be considered for ICD implantation.
For patients with a high susceptibility or history of previous thromboembolic events, coexistent atrial fibrillation, confirmed left ventricular thrombi, or severely impaired left ventricular systolic function, oral anticoagulation drugs should be considered [33, 34].
Echocardiographic evaluation of other family members should also be discussed and offered because of the high incidence of familial recurrence in LVNC [35]. Also due to the possible association of LVNC with neuromuscular disorders and mitochondrial myopathies, patients should be referred for a comprehensive genetic assessment [18].
Prognosis
The prognosis for LVNC is highly variable and is mainly dependent on the age of onset of symptoms and the severity of the clinical manifestations. In both pediatric and adult populations, asymptomatic individuals have a better prognosis compared to those with symptoms. The variables that in particular associated with worse prognosis are NYHA class III–IV heart failure, left ventricular end-diastolic diameter exceeding 60 mm, left bundle branch block, and persistent atrial fibrillation [18].
References
Engberding R, Bender F (1984) Identification of a rare congenital anomaly of the myocardium by two-dimensional echocardiography: persistence of isolated myocardial sinusoids. Am J Cardiol 53(11):1733–1734
Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA (2001) Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 86(6):666–671
Branton H, Warren AE, Penney LS (2011) Left ventricular noncompaction and coronary artery fistula in an infant with deletion 22q11.2. Pediatr Cardiol 32(2):208–210
Lofiego C, Biagini E, Pasquale F, Ferlito M, Rocchi G, Perugini E et al (2007) Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart 93(1):65–71
Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R (1990) Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 82(2):507–513
Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R (2000) Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 36(2):493–500
Pignatelli RH, McMahon CJ, Dreyer WJ, Denfield SW, Price J, Belmont JW et al (2003) Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation 108(21):2672–2678
Uribe S, Cadavid L, Hussain T, Parra R, Urcelay G, Heusser F et al (2012) Cardiovascular magnetic resonance findings in a pediatric population with isolated left ventricular non-compaction. J Cardiovasc Magn Reson 14:9
Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH et al (2005) Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 46(1):101–105
Sarma RJ, Chana A, Elkayam U (2010) Left ventricular noncompaction. Prog Cardiovasc Dis 52(4):264–273
Stollberger C, Gerecke B, Finsterer J, Engberding R (2013) Refinement of echocardiographic criteria for left ventricular noncompaction. Int J Cardiol 165(3):463–467
Captur G, Muthurangu V, Cook C, Flett AS, Wilson R, Barison A et al (2013) Quantification of left ventricular trabeculae using fractal analysis. J Cardiovasc Magn Reson 15:36
Jacquier A, Thuny F, Jop B, Giorgi R, Cohen F, Gaubert JY et al (2010) Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur Heart J 31(9):1098–1104
Ichida F, Hamamichi Y, Miyawaki T, Ono Y, Kamiya T, Akagi T et al (1999) Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 34(1):233–240
El-Menyar AA, Gendi SM, Numan MT (2007) Noncompaction cardiomyopathy in the State of Qatar. Saudi Med J 28(3):429–434
Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NA 3rd et al (2015) Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the american heart association and american college of cardiology. J Am Coll Cardiol 66(21):2362–2371
Stanton C, Bruce C, Connolly H, Brady P, Syed I, Hodge D, Asirvatham S, Friedman P (2009) Isolated left ventricular noncompaction syndrome. J Am Cardiol 104:1135–1138
Captur G, Nihoyannopoulos P (2009) Left ventricular non-compaction: genetic heterogeneity, diagnosis and clinical course. Int J Cardiol 140:145–153
Stollberger C, Finsterer J (2004) Left ventricular hypertrabeculation/noncompaction. J Am Soc Echocardiogr 17:91–100
McCaffrey F (2001) Around pediheart: noncompaction of the left ventricle. Pediatr Cardiol 22:399
Winer N, Lefevre M, Nomballais MF et al (1998) Persisting spongy myocardium: a case indicating the difficulty of antenatal diagnosis. Fetal Diagn Ther 13:227–232
Sato Y, Matsumoto N, Matsuo S et al (2007) Isolated noncompaction of the ventricular myocardium in a 94 year-old-patient: depiction of echocardiography and magnetic resonance imaging. Int J Cardiol 119:e32–e34
Bleyl SB, Mumford BR, Brown-Harrison MC et al (1997) Xq28-linked noncompaction of the ventricular myocardium: prenatal diagnosis and pathologic analysis of affected individuals. Am J Med Genet 72:257–265
Weiford B, Subbarao V, Mulhrn K (2004) Noncompaction of the ventricular myocardium. Circulation 109:2965–2971
Biagini E, Ragni L, Ferlito M et al (2006) Different types of cardiomyopathy associated with isolated ventricular non-compaction. Am J Cardiol 98:619–627
Finsterer J (2009) Cardiogenetics, neurogenetics and pathogenetics of left ventricular hypertrabeculation/noncompaction. Pediatr Cardiol 30:e659–e681
Toyono M, Kondo C, Nakajima, Nakazawa M, Momma K, Kuskabe K (2001) Effects of carvedilol on left ventricular function, mass and scintigraphic findings in isolated left ventricular non-compaction. Heart 86:e4
Kim H, Chang S, Kim Y et al (2009) Fireworks in the left ventricle: doppler manifestation of left ventricular noncompaction. J Am Coll Cardiol 53:2009
Koh YY, Seo YU, Woo JJ et al (2004) Familial isolated noncompaction of the ventricular myocardium in asymptomatic phase. Yonsei Med J 45:931–935
Jenni R, Goebel N, Tartini R, Schneider J, Arbenz U, Oelz O (1986) Persisting myocardial sinusoids of both ventricles as an isolated anomaly: echocardiographic, angiographic, and pathological anatomical findings. Cardiovasc Intervent Radiol 9:127–131
Jessup M, Abraham WT, Casey DE, et al. 2009 Focused update ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. J Am Coll Cardiol 2009; 54: 1343: 1382
Epstein A, DiMarco J, Ellenbogen K et al (2008) ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol 51:1–62
Stollberger C, Finsterer J (2004) Thrombi in left ventricular hypertrabeculation/non-compaction: review of the literature. Acta Cardiol 59:341–344
Pitta S, Thatai D, Afonzo L (2007) Thromboembolic complications of left ventricular noncompaction: case report and brief review of the literature. J Clin Ultrasound 25:465–468
Lorsheyd A, Cramer MJ, Velthuis BK et al (2006) Familial occurrence of isolated non-compaction cardiomyopathy. Eur J Heart Fail 8:826–831
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any potential conflict of interest to declare.
Human and Animal Rights
Since this is a retrospective case review, consent was not obtained and no research performed on human or animal subjects. Also, no data that would help identify the patients have been used in the case series and the data related to patient identity have been stored on a separate unlinked file on a password protected computer kept securely by the investigator.
Rights and permissions
About this article
Cite this article
Gupta, U., Makhija, P. Left Ventricular Noncompaction Cardiomyopathy in Pediatric Patients: A Case Series of a Clinically Heterogeneous Disease. Pediatr Cardiol 38, 681–690 (2017). https://doi.org/10.1007/s00246-016-1566-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-016-1566-5