Abstract
With longer duration of follow-up, as many as 50% of Fontan patients will develop atrial tachycardia, usually in association with significant hemodynamic abnormalities. Arrhythmia management in the Fontan patient is reviewed. The incidence and type of arrhythmia occurrence are examined, including macro-reentrant rhythm which involves the right atrium, reentrant rhythm localized to the pulmonary venous atrium (seen in patients with lateral tunnel procedures), and atrial fibrillation. Risk factors for development of these arrhythmias are considered, and short- and long-term therapeutic options for medical and surgical treatment are discussed. Surgical results are presented for 117 patients undergoing Fontan conversion and arrhythmia surgery (isthmus ablation (9), modified right atrial maze (38) or Cox-maze III (70)). Operative mortality is low (1/117, 0.8%). Seven late deaths occurred, and include two patients who died shortly following cardiac transplantation (2/6, 33%) after Fontan conversion and arrhythmia surgery. Overall arrhythmia recurrence is 12.8% during a mean follow-up of 56 months. Fontan conversion with arrhythmia surgery can be performed with low operative mortality, low risk of recurrent tachycardia, and marked improvement in functional status in most patients. Because the development of tachycardia is usually an electromechanical problem, attention to only the arrhythmia with medications or ablation may allow progression of hemodynamic abnormalities to either a life-threatening outcome or a point at which transplantation is the only potential option. Because cardiac transplantation in Fontan patients is associated with high early mortality, earlier consideration for surgical intervention is warranted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The incidence of atrial arrhythmias following Fontan-type surgery increases steadily with the postoperative interval, with at least 50% of patients experiencing problematic atrial tachycardia by 20 years of follow-up [22, 47, 62]. Although the arrhythmias are generally believed to be a consequence of surgical interventions, electron microscopy has demonstrated that the atria of patients with tricuspid atresia show a distinctly abnormal atrial fiber array compared with normal hearts [50]; this unusual fiber orientation may predispose the atria to the slowing of conduction necessary for reentrant rhythms. In addition, in natural history studies of unoperated adults with tricuspid atresia, at least 40% of patients experienced tachycardia by the fourth decade [58, 59]. In postoperative Fontan patients, supraventricular tachycardia is usually a macro-reentrant rhythm involving the right atrium, although in lateral tunnel-type repairs the reentrant rhythm may be localized to the pulmonary venous atrium [16]. Slowed conduction with reentry is facilitated by anatomic barriers, such as the orifices of the inferior and superior vena cavae, the os of the coronary sinus, and the atrial septal defect, further compounded by extensive atrial suture lines of either the atriopulmonary anastomosis or the lateral tunnel repair. Residual hemodynamic abnormalities (or simply the dissipation of energy forces within the atria) result in marked distention with fibrosis, which is complicated by sinus node dysfunction; an irregular atrial rhythm serves as a trigger for the onset of tachycardia.
Risk Factors
Surgical variables identified as risk factors for the development of tachycardia include an older age at the time of initial repair, early postoperative arrhythmias, sinus node dysfunction, and double-inlet left ventricle [10, 20, 22, 60]. Modifications of the atriopulmonary anastomosis to the lateral tunnel repair were developed in an effort to improve hemodynamics and limit late arrhythmias. Although initial reports suggested a decreased incidence of arrhythmias following the lateral tunnel technique [6, 10, 22, 46], this difference has become less significant with longer follow-up [19]. Limitation of atrial suture lines by performance of the extracardiac total cavopulmonary anastomosis has a theoretical advantage in limiting the development of tachycardia, which is supported by the early follow-up report of Amodeo et al. [3] showing a 5-year arrhythmia-free rate of 92%.
Significant hemodynamic abnormalities are present in many Fontan patients with recurrent atrial tachycardia. Marked atrial dilatation with stasis of atrial flow and limited forward output predisposes patients to thrombus development and the risk of stroke or pulmonary embolus. The dilated right atrium may impinge on pulmonary venous return and distort ventricular anatomy sufficiently to depress systolic function. Anti-arrhythmic medications frequently exacerbate bradycardia and further depress ventricular function. Thus, it is essential to view the presence of atrial tachycardia in most cases as an electromechanical problem, which is not adequately addressed by either arrhythmia therapy or Fontan revision in isolation. Additionally, ventricular function may be further depressed by the development of chronic atrial tachycardia or atrial fibrillation, and repeated attempts at catheter ablation may allow a window of opportunity to pass for surgical intervention with low risk.
Medical Treatment of Atrial Tachycardia
Early Postoperative Tachycardia
A small subset of patients develops atrial tachycardia during the acute postoperative period following Fontan completion. At that time, it is important to determine whether tachycardia is a consequence of inotropic medications used to maintain cardiac output or related to either significant residual anatomic problems or marked sinus node dysfunction. Persistent atrial arrhythmias in the absence of inotropic medications are usually related to structural hemodynamic sequelae; attention to these abnormalities by early diagnosis and possible emergent reoperation is of critical importance and should not be delayed. Acute management after structural hemodynamic residua have been excluded includes removal of inotropic agents and providing a regular atrial rhythm with pacing, as necessary. Intravenous amiodarone is highly effective for rhythm control in this setting; diltiazem may be used for acute rate control as needed.
Late Postoperative Tachycardia
For patients developing late postoperative arrhythmias, the mean time to presentation is usually 6–11 years post-repair [16, 52]. Following the initial presentation with tachycardia, many patients will experience isolated episodes of tachycardia during the next few years [16, 36]. However, as time progresses, tachycardia recurrences become more frequent and prolonged, with the potential development of atrial fibrillation after several years [32].
Recognition of the acute onset of tachycardia is confounded by the usual presence of 2:1 atrioventricular conduction resulting in ventricular rates of 110–130 beats per minute (bpm); such patients may present with vague symptoms of unexplained fatigue or respiratory symptoms. In contrast, patients with rapid 1:1 atrioventricular conduction of tachycardia frequently experience syncope or rarely experience cardiac arrest. The vagal response during syncope may terminate tachycardia, creating uncertainty as to the cause of symptoms. In either setting, patients with single ventricle physiology do not tolerate persistent tachycardia and may develop profound congestive failure within 12–36 hours of the onset of persistent heart rates greater than 100 bpm. In addition to depressed ventricular systolic function and atrioventricular valve regurgitation, tachycardia increases the risk of developing atrial thrombi resulting in pulmonary or cerebral emboli. Therefore, the acute termination of tachycardia within 24 hours of presentation is of high importance; delaying termination of tachycardia for 4–6 weeks of anticoagulation, as is recommended for tachycardia of uncertain but prolonged duration in adult patients with two ventricles, may have life-threatening consequences. In the setting of prolonged tachycardia, echocardiograms (usually transesophageal) are performed to determine the presence of atrial thrombi and establish the risk of acute emboli with cardioversion. Although this is the most sensitive technique for detecting atrial thrombi, our experience with transesophageal echocardiograms immediately preceding Fontan conversion suggests that this technique is neither sensitive nor specific for the detection of thrombi in the dilated right atria [31]. The risk of cardioversion with embolic phenomena must be weighed against the risk of delaying cardioversion and developing profound ventricular dysfunction or sudden cardiac arrest.
Acute Therapy
Therapeutic choices for acute cardioversion include pharmacologic agents, transesophageal or intra-atrial pacing, and synchronized direct-current cardioversion. Data regarding the efficacy of acute pharmacologic cardioversion are not available, and there are only anecdotal reports of efficacy [22]. In the hemodynamically stable patient, a trial of small doses of intravenous calcium channel blocking medication, such as diltiazem, may be effective in terminating tachycardia or at least slowing the ventricular response. Ibutilide, a class III anti-arrhythmic medication, may be acutely successful but generally is avoided in patients with electrolyte abnormalities or those receiving other medications such as sotalol or amiodarone, which may prolong the QT interval. Transesophageal pacing conversion is frequently effective, particularly in younger patients or patients with lateral tunnel-type repairs [8]. Placement of cardioversion pads in the anterior–posterior configuration is useful to facilitate cardioversion of the massively dilated atria, and energy doses of 1 or 2 J/kg, or 200 J in adult patients, are used.
Chronic Therapy
The choice of anti-arrhythmic therapy is determined by the severity of symptoms associated with tachycardia and also the reliability of detection of tachycardia and delivery of prompt medical care. Patients presenting with symptoms of palpitations and seeking care at the earliest onset of infrequent episodes of hemodynamically stable tachycardia may be treated with periodic cardioversion, without chronic anti-arrhythmic drugs beyond beta-blockers or digoxin. Once tachycardia becomes recurrent, medications such as procainamide, sotalol, and propafenone are initially effective, but with time tachycardia usually becomes refractory to medications. In our experience, a combination of sotalol with low-dose beta blockade has been the most effective chronic pharmacologic treatment. Due to the high incidence of pulmonary and thyroid side effects with chronic use, amiodarone is not recommended for Fontan patients other than as a bridge to more definitive therapy. Based on the risk of thrombosis, patients with atrial tachycardia should receive chronic anticoagulation therapy with warfarin.
Hemodynamic Assessment
Following the initial presentation with tachycardia, allowing for a period of recovery of ventricular function, a careful assessment is performed to detect hemodynamic abnormalities. Patients presenting with severe symptoms, such as congestive failure, syncope, or cardiac arrest, undergo invasive cardiac catheterization with electrophysiology testing and progress to more definitive therapy. Noninvasive evaluation including chest radiograph, echocardiogram, and exercise testing is performed for patients with milder symptoms and physical examinations consistent with acceptable hemodynamics. The presence of cardiomegaly, decreasing exercise tolerance, peripheral edema or lower leg discoloration, ascites, protein-losing enteropathy, or progressive cyanosis with resting saturations less than 92% should prompt aggressive evaluation. Patients with Fontan physiology typically do not complain of exercise intolerance until advanced stages of failure; therefore, exercise testing provides objective documentation of declining maximal oxygen consumption, desaturation, or arrhythmia occurrence with exertion [49].
The transthoracic echocardiogram frequently underestimates the degree of atrial dilatation or areas of obstruction to atrial flow, which are more accurately assessed by cardiac catheterization with angiography. Cardiologists may be misled by assessing hemodynamic status on the basis of right atrial pressure measurement alone; an otherwise acceptable right atrial pressure of 15 or 16 mmHg may be associated with angiographic evidence of marked atrial dilatation with poor forward flow from the atria to the pulmonary arteries. In this setting of passive filling of the pulmonary circulation, a right atrial-to-pulmonary artery gradient as low as 2 mmHg may represent significant obstruction. Full hemodynamic assessment in addition to pressure measurements includes angiographic estimation of right atrial emptying; right-to-left atrial shunting; kinking and distortion of the pulmonary arteries, particularly of the right pulmonary artery; and the degree of pulmonary arteriovenous malformations. Obstruction of pulmonary venous return is assessed by both angiography and measurement of the pressure difference between bilateral wedge pressures and ventricular end diastolic pressure. Ventricular angiograms should assess atrioventricular valve regurgitation and provide a qualitative assessment of contractility, in addition to catheterization for measurement of end diastolic pressure and cardiac index.
Catheter Ablation
Catheter ablation is recommended in patients without significant hemodynamic abnormalities or in patients considered very high-risk surgical candidates due to ventricular dysfunction or multiorgan disease. In our series of Fontan patients undergoing electrophysiologic mapping of right atrial macro-reentry, three primary exit sites of atrial tachycardia circuits identified are the low lateral right atrial wall, the perimeter of the atrial septal defect patch, and the inferomedial right atrial isthmus [16]; others have reported similar findings [4, 18, 63]. The atriopulmonary anastomosis is a less common site of exit. In patients with a lateral tunnel-type Fontan repair, the tachycardia circuit may be partitioned to the pulmonary venous atrium; these patients often have negative P waves in lead I [16]. Infrequently, focal atrial tachycardia may be present.
Acute success rates for ablation of atrial tachycardia in Fontan patients range from 33% to 100% [9, 11–13, 25, 27, 35, 44, 53, 63]; however, recurrence of tachycardia during short-term follow-up is reported as 33%–100% (Table 1). Acute and long-term results of catheter ablation in Fontan patients are significantly worse than results achieved in most other forms of congenital heart disease. Improvement in acute success rates is expected with the use of three-dimensional mapping and larger tipped, irrigated catheters with more powerful energy delivery. The technique of scar mapping of the right atrium with ablation lines delivered to eliminate channels between scars has provided promising results [44]. Limitations to the catheter technique specific to Fontan patients include the multiplicity of tachycardia circuits, restricted catheter access, distorted anatomy, hemodynamic instability, and the inability to deliver lesions of sufficient depth to achieve transmural penetrance. In our experience with more than 100 patients undergoing repeat surgery, right atrial wall thicknesses as measured in the operating room average 12 mm, extending to 20 mm, indicating the difficulty of completion of transmural lines of block with lesion depths of 3–8 mm. Thrombogenicity of extensive ablation lesions in the stagnant right atrium is of concern, as evidenced by the image of thrombus obtained in an adolescent months after a partially effective ablation procedure (Fig. 1). In addition, focal wall thinning due to extensive ablation may produce an additional area of slowed conduction, promoting tachycardia. The most significant concern regarding ablation in Fontan patients is the possibility of overlooking significant hemodynamic problems and allowing ventricular dysfunction, atrial thrombosis, or protein-losing enteropathy to develop and/or progress to either a life-threatening condition or beyond the possibility of surgical intervention.
Surgical Treatment of Arrhythmias
Fontan Revision
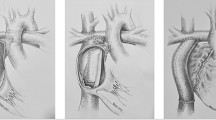
Because the majority of Fontan patients with arrhythmias have significant associated structural/hemodynamic problems, a number of centers have performed Fontan revisions without arrhythmia surgery, resulting in improved hemodynamic outcomes [7, 29, 34, 41, 48, 51, 55, 57]. In a meta-analysis of 77 reported cases of Fontan revisions (Table 2), tachycardia recurred in 76% of patients during short-term follow-up. The inability to eliminate tachycardia by only improving hemodynamics led our group to attempt to incorporate ablation techniques into the operative revision of the Fontan circulation. Initially, we performed limited ablation of the inferomedial right atrial isthmus, with implantation of an atrial anti-tachycardia pacemaker [17]. Due to recurrent tachycardia in several of these patients, a modified right atrial maze procedure was developed to eliminate all right atrial macro-reentrant circuits, essentially eliminating tachycardia recurrence during medium-term follow-up [16]. The surgical technique of the Fontan conversion to a total cavopulmonary extracardiac connection has been extensively reported by our group [15–17, 37–40].
Patients considered for arrhythmia surgery undergo preoperative electrophysiology studies with mapping of atrial macro-reentrant circuits; epicardial mapping of the right atrium is then repeated in the operating room in an attempt to correlate the findings of the endocardial studies with observed anatomic findings. Careful review of the tachycardia history and tracings is performed because the presence of clinical atrial fibrillation indicates that more extensive arrhythmia surgery in the left atrium will be necessary. Details of the original surgical Fontan procedure are reviewed because lateral tunnel-type repairs, incisions in the roof of the left atrium, or deviation of the atrial septal patch to the left may partition the anatomic right atrium, and the reentrant circuit, to the pulmonary venous chamber. In addition, focal atrial tachycardia is not addressed by the right atrial maze and requires direct elimination of the tachycardia focus.
Right Atrial Reentry Tachycardia
The modified right atrial maze surgery involves an incision from the superior vena cava to the inferior vena cava, with resection of a large portion of the anterior right atrial wall and the right atrial appendage. Linear cryoablation lesions are delivered as follows: (1) from the os of the coronary sinus to the transected inferior vena cava; (2) from a right-sided atrioventricular valve orifice, when present, to the transected inferior vena cava; (3) from the posterior rim of the atrial defect to the lateral rim of the resected atrial wall; (4) from the base of the resected right atrial appendage to the superior rim of the atrial defect; and, more recently, (5) an additional lesion delivered from the inferior rim of the atrial defect to the posterior border of the coronary sinus. Cryoablation lesions were originally delivered using a circular probe (Frigitronics) at −60°C for 90 seconds each; currently, a malleable 12-Fr linear probe is used to deliver temperatures of −155°C for 60 seconds (CryoCath Technologies, Montreal, Quebec). The total time necessary to complete the ablation lesions is 5 or 6 minutes. Care is taken to avoid the conducting system near the atrioventricular node. Based on the findings of the electrophysiology testing and the operative history, additional lesions may be necessary in the partitioned left atrium. Patients with atrial reentry tachycardia undergo postoperative atrial pacing studies after cessation of inotropic support to assess the efficacy of surgery and to program atrial anti-tachycardia pacemakers, if necessary. Patients subsequently receive beta-blocker medications for a period of 3 months postoperatively.
Atrial Fibrillation
The presence of atrial fibrillation mandates performance of the Cox maze III procedure in the left atrium; right atrial surgery decreases but does not eliminate atrial fibrillation [5, 23, 24]. In addition to the modified right atrial maze procedure reported previously, a left atrial maze procedure is performed as described by Cox et al. [14]. Lesions were initially delivered by incision, but the use of linear cryoablation lesions with a malleable probe has significantly decreased the operative cross-clamp time.
The pulmonary veins are isolated with an encircling lesion. The left atrial appendage is excised, with a cryoablation lesion connecting the base of the resected appendage to the encircling pulmonary vein lesion. A cryoablation lesion is delivered from the inferior pulmonary veins to the mitral valve annulus; a corresponding lesion is placed epicardially on the coronary sinus. Performance of the left atrial maze requires an additional 30–45 minutes using the cryoablation technique and more than 60 minutes using incisions, with attendant increased risk of renal failure and myocardial dysfunction. In patients with multiple prior surgeries and markedly distorted anatomy, adequate exposure of the left atrial appendage is difficult and may not be possible. In these circumstances, a circular cryoablation lesion can be placed at the base of the left atrial appendage within the atrium to isolate the left atrial appendage without resection. Patients with atrial fibrillation receive intravenous amiodarone postoperatively, followed by oral amiodarone for a period of 3 months postoperatively; atrial pacing studies are not performed prior to hospital discharge.
Pacemaker Implantation
Of our initial 13 patients, atrial anti-tachycardia pacemakers were implanted in 12 (Intertach II, Intermedics). Because this pacemaker became unavailable, and due to the decreased incidence of tachycardia with the more extensive right atrial maze and the need for rate responsiveness, atrial rate-responsive pacemakers (Medtronic Kappa SR, Medtronic, Inc., Minneapolis, MN) were implanted in the subsequent 31 patients. More recently, dual-chamber anti-tachycardia pacemakers (Medtronic Gem III AT) have been implanted due to the potential need for ventricular pacing during late follow-up and to avoid the risk of reoperation; most of these pacemakers are programmed for atrial pacing only. Atrial pacing rates are programmed to consistently maintain a regular atrial rhythm faster than the intrinsic rhythm, usually 70–80 beats per minute during late follow-up. One patient received an epicardial defibrillator in addition to multisite ventricular pacing; this patient had a prior cardiac arrest secondary to ventricular tachycardia, and ventricular dysfunction associated with left bundle branch block.
Surgical Results
Since 1994, 117 patients have undergone arrhythmia surgery with Fontan conversion at Children’s Memorial Hospital; the surgical outcome is summarized in Table 3. There was one early death (0.8%), occurring in an adolescent male with heterotaxy syndrome and marked ventricular diastolic dysfunction. Late mortality has been 5.9% due to intractable heart failure (1), coronary artery disease (1), discontinuation of renal dialysis (1), injuries from a motor vehicle collision (1), and suddenly after sedation administration (1). Two additional patients died following cardiac transplantation 7 and 10 months after Fontan conversion.
Overall arrhythmia recurrence is 12.8% during a mean follow-up of 56 months. In patients with atrial reentry tachycardia, we initially performed isthmus ablation on 9 patients. Since initiation of the modified right atrial maze procedure, 3/34 patients (8.8%) have had atrial tachycardia recurrence. These surgical arrhythmia results have been duplicated and reported by other centers with similar outcomes; variations in arrhythmia recurrence may be due to the absence of preoperative characterization of the arrhythmia circuits, variable lesions, and the use of radiofrequency versus cryoablation [1, 33, 52, 56, 61]. In the group of patients with atrial fibrillation who underwent the Cox maze III procedure, no patient has had late recurrence of atrial fibrillation. However, the late development of isolated episodes of atrial reentry tachycardia has occurred in 9/70 patients (12.8%) at a mean postoperative period of 36 months; recurrences are usually controlled with beta-blocking medications alone. The occurrence of late atrial reentry tachycardia following catheter ablation procedures for atrial fibrillation is reported to be 11%–43% [2, 25, 28]. The possibility of atrial conduction superiorly near Bachmann’s bundle or via atrial fibers in the coronary sinus is speculated.
Functional classification improved dramatically in most patients, as demonstrated by exercise testing [30]. However, a small number of patients experienced progressive decline in ventricular dysfunction, usually associated with significant atrioventricular valve regurgitation. Six patients have undergone cardiac transplantation at a mean postoperative interval of 12 months; one patient died awaiting transplantation, as noted previously. Overall, the incidence of late transplantation (5.1%) is similar to that reported in other series for Fontan patients [55]; in certain patients, Fontan conversion may substantially delay the timing of transplantation.
Cardiac Transplantation
Of note, early mortality following transplantation for Fontan patients is higher than for other forms of congenital heart disease and is reported to be 33%–48% [21, 26, 42, 43]. In our center, 12 Fontan patients were referred initially for consideration for cardiac transplantation: 7 underwent Fontan conversion for potentially correctable hemodynamic abnormalities, and 5 were listed for transplantation. Of the 7 undergoing surgery, all survived, and 5 are alive and considered to be in New York Heart Association class I or II; 2 patients went on to cardiac transplantation 23 months postoperatively. Patients with systemic right ventricles, heterotaxy syndrome, severe atrioventricular valve regurgitation, and protein-losing enteropathy appear to be at the highest risk for poor outcome. Of the 5 patients listed for transplantation, 2 patients died waiting, and 3 patients underwent transplantation, 2 of whom died of rejection and 1 is alive and well. These results indicate that earlier intervention to correct residual hemodynamic and arrhythmia problems is an important adjunct to improve overall survival.
Prophylactic Surgical Techniques
Prior to the performance of the initial Fontan repair, atrial tachycardia may be induced in as many as 20% of patients [45]. As described previously, avoidance of intra-atrial suture lines by performance of the extracardiac total cavopulmonary connection may decrease the incidence of arrhythmias during medium-term follow-up. Based on studies in animal models showing the importance of atrial reentry between the inferior atriotomy and the tricuspid valve annulus, centers performing the lateral tunnel-type repair have delivered prophylactic incisions from the atriotomy across the anterior atrial wall to the right-sided valve annulus to decrease arrhythmia development [45]. However, in postoperative patients, multiple centers have documented similar areas of tachycardia reentry: the isthmus between the inferior vena cava and the coronary sinus, the low lateral right atrial wall, and the superior rim of the atrial defect, sometimes extending to the superior vena cava [44]. Lesions in the inferior isthmus may be effective for at least one of these circuits. The loss of a regular sinus rhythm was shown by Fishberger et al. [20] to be the most important predictor of the subsequent development of atrial tachycardia; placement of an atrial pacemaker at the initial Fontan repair may thus delay the development of tachycardia.
Conclusions
With longer durations of follow-up, as many as 50% of Fontan patients will develop atrial tachycardia, usually in association with significant hemodynamic abnormalities. Catheter ablation in Fontan patients has a high risk of recurrent tachycardia during short-term follow-up and allows underlying hemodynamic problems to progress. Fontan revision without arrhythmia surgery results in improved hemodynamic status but recurrent tachycardia. When tachycardia recurrence becomes frequent or is associated with significant symptoms and/or when significant hemodynamic problems exist, Fontan conversion with arrhythmia surgery can be performed with low operative mortality, low risk of recurrent tachycardia, and marked improvement in functional status in most patients. Because the development of tachycardia is usually an electromechanical problem, attention to only the arrhythmia with medications or ablation may allow progression of hemodynamic abnormalities to either a life-threatening outcome or a point at which transplantation is the only potential option. Because cardiac transplantation in Fontan patients is associated with high early mortality, earlier consideration for surgical intervention is warranted.
By limiting atrial incisions and suture burden, the extracardiac total cavopulmonary connection may decrease the incidence of late atrial arrhythmias. In addition, “prophylactic arrhythmia surgery” should be considered in patients undergoing initial lateral tunnel-type Fontan repairs or Fontan conversion for structural/hemodynamic problems without problematic arrhythmias, based on the likelihood of developing tachycardia over time and the lack of future transvenous access to the right atrium. Performance of the modified right atrial maze lesions requires less than 6 minutes and does not increase the morbidity of the surgical procedure. Finally, we speculate that earlier atrial pacing with lead implantation performed at the time of the initial Fontan procedure may delay the appearance of atrial tachycardia by avoiding irregular and slow atrial rhythms.
References
Agnoletti G, Borghi A, Vignati G, Crupi GC (2003) Fontan conversion to total cavopulmonary connection and arrhythmia ablation: clinical and functional results. Heart 89:193–198
Aman C, Oral H, Hall B, et al. (2004) Prevalence and clinical significance of left atrial flutter after left atrial ablation for atrial fibrillation. Heart Rhythm 1:S238 [abstract]
Amodeo A, Galletti L, Marianeschi S, et al. (1997) Extracardiac Fontan operation for complex cardiac anomalies: seven years’ experience. J Thorac Cardiovasc Surg 114:1020–1030
Anné W, van Rensburg H, Adams J, et al. (2002) Ablation of post-surgical intra-atrial reentrant tachycardia. Predilection target sites and mapping approach. Eur Heart J 23:1609–1616
Ashburn DA, Harris L, Downar EH, et al. (2003) Electrophysiologic surgery in patients with congenital heart disease. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 6:51–58
Balaji S, Gewillig M, Bull C, de Leval MR, Deanfield JE (1991) Arrhythmias after the Fontan procedure. Comparison of total cavopulmonary connection and atriopulmonary connection. Circulation 84:III-162–III-167
Balaji S, Johnson TB, Sade RM, Case CL, Gillette PC (1994) Management of atrial flutter after the Fontan procedure. J Am Coll Cardiol 23:1209–1215
Benson DW Jr (1987) Transesophageal electrocardiography and cardiac pacing: state of the art. Circulation 75:III-86–III-92
Betts TR, Roberts PR, Allen SA, et al. (2000) Electrophysiological mapping and ablation of intra-atrial reentry tachycardia after Fontan surgery with the use of a noncontact mapping system. Circulation 102:419–425
Cecchin F, Johnsrude CL, Perry JC, Friedman RA (1995) Effect of age and surgical technique on symptomatic arrhythmias after the Fontan procedure. Am J Cardiol 76:386–391
Chan DP, Van Hare GF, Mackall JA, Carlson MD, Waldo AL (2000) Importance of atrial flutter isthmus in postoperative intra-atrial reentrant tachycardia. Circulation 102:1283–1289
Chinitz LA, Bernstein NE, O’Connor B, Glotzer TV, Skipitaris NT (1996) Mapping reentry around atriotomy scars using double potentials. Pacing Clin Electrophysiol 19:1978–1983
Collins KK, Love BA, Walsh EP, et al. (2000) Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol 86:969–974
Cox JL, Jaquiss RD, Schuessler RB, Boineau JP (1995) Modification of the maze procedure for atrial flutter and atrial fibrillation: II. Surgical technique of the maze III procedure. J Thorac Cardiovasc Surg 110:485–495
Deal BJ, Mavroudis C, Backer CL (2002) Surgical treatment of postoperative atrial reentry tachycardia. Prog Pediatr Cardiol 14:229–235
Deal BJ, Mavroudis C, Backer CL, Buck SH, Johnsrude C (2002) Comparison of anatomic isthmus block with the modified right atrial maze procedure for late atrial tachycardia in Fontan patients. Circulation 106:575–579
Deal BJ, Mavroudis C, Backer CL, Johnsrude CL, Rocchini AP (1999) Impact of arrhythmia circuit cryoablation during Fontan conversion for refractory atrial tachycardia. Am J Cardiol 83:563–568
Delacretaz E, Ganz LI, Soejima K, et al. (2001) Multiple atrial macro-re-entry circuits in adults with repaired congenital heart disease: entrainment mapping combined with three-dimensional electroanatomic mapping. J Am Coll Cardiol 37:1665–1676
Durongpisitkul K, Porter CJ, Cetta F, et al. (1998) Predictors of early- and late-onset supraventricular tachyarrhythmias after Fontan operation. Circulation 98:1099–1107
Fishberger SB, Wernovsky G, Gentles TL, et al. (1997) Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg 113:80–86
Gamba A, Merlo M, Fiocchi R, et al. (2004) Heart transplantation in patients with previous Fontan operations. J Thorac Cardiovasc Surg 127:555–562
Gelatt M, Hamilton RM, McCrindle BW, et al. (1994) Risk factors for atrial tachyarrhythmias after the Fontan operation. J Am Coll Cardiol 24:1735–1741
Ghai A, Harris L, Harrison DA, Webb GD, Siu SC (2001) Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol 37:585–592
Greason KL, Dearani JA, Theodoro DA, et al. (2003) Surgical management of atrial tachyarrhythmias associated with congenital cardiac anomalies: Mayo Clinic experience. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 6:59–71
Jais P, Hse L, Sanders P, et al. (2004) Determinants of the type of recurrent arrhythmia after ablation of chronic atrial fibrillation. Heart Rhythm Suppl 1:S56–S57 [abstract]
Jayakumar KA, Addonizio LJ, et al. (2004) Cardiac transplantation after the Fontan or Glenn procedure. J Am Coll Cardiol 44:2065–2072
Kalman JM, Van Hare GF, Olgin JE, et al. (1996) Ablation of “incisional” reentrant atrial tachycardia complicating surgery for congenital heart disease. Use of entrainment to define a critical isthmus of conduction. Circulation 93:502–512
Kannankeril PJ, Anderson ME, Rottman JN, Wathen MS, Fish FA (2003) Frequency of late recurrence of intra-atrial reentry tachycardia after radiofrequency catheter ablation in patients with congenital heart disease. Am J Cardiol 92:879–881
Kao JM, Alejos JC, Grant PW, et al. (1994) Conversion of atriopulmonary to cavopulmonary anastomosis in management of late arrhythmias and atrial thrombosis. Ann Thorac Surg 58:1510–1514
Kavey RE, Franklin WH, Deal BJ, et al. (2003) Exercise performance after Fontan conversion to total cavopulmonary connection. J Am Coll Cardiol 41:476A [abstract]
Kim PJ, Franklin WH, Duffy E, et al. (2003) Accuracy of transesophageal echocardiography in detecting right atrial thrombus in patients after the Fontan operation. Circulation 108:IV-677 [abstract]
Kirsh JA, Walsh EP, Triedman JK (2002) Prevalence of and risk factors for atrial fibrillation and intra-atrial reentrant tachycardia among patients with congenital heart disease. Am J Cardiol 90:338–340
Kopf GS, Mello DM, Kenney KM, et al. (2002) Intraoperative radiofrequency ablation of the atrium: effectiveness for treatment of supraventricular tachycardia in congenital heart surgery. Ann Thorac Surg 74:797–804
Kreutzer J, Keane JF, Lock JE, et al. (1996) Conversion of modified Fontan procedure to lateral atrial tunnel cavopulmonary anastomosis. J Thorac Cardiovasc Surg 111:1169–1176
Lesh MD, Kalman JM, Saxon LA, Dorostkar PC (1997) Electrophysiology of “incisional” reentrant atrial tachycardia complicating surgery for congenital heart disease. Pacing Clin Electrophysiol 20:2107–2111
Li W, Somerville J (2000) Atrial flutter in grown-up congenital heart (GUCH) patients. Clinical characteristics of affected population. Int J Cardiol 75:129–137
Mavroudis C, Backer CL, Deal BJ, Johnsrude CL (1998) Fontan conversion to cavopulmonary connection and arrhythmia circuit cryoablation. J Thorac Cardiovasc Surg 115:547–556
Mavroudis C, Backer CL, Deal BJ, Johnsrude C, Strasburger J (2001) Total cavopulmonary conversion and maze procedure for patients with failure of the Fontan operation. J Thorac Cardiovasc Surg 122:863–871
Mavroudis C, Deal BJ, Backer CL (2002) The beneficial effects of total cavopulmonary conversion and arrhythmia surgery for the failed Fontan. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 5:12–24
Mavroudis C, Deal BJ, Backer CL, Johnsrude CL (1999) The favorable impact of arrhythmia surgery on total cavopulmonary artery Fontan conversion. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2:143–156
McElhinney DB, Reddy VM, Moore P, Hanley FL (1996) Revision of previous Fontan connections to extracardiac or intraatrial conduit cavopulmonary anastomosis. Ann Thorac Surg 62:1276–1282
Michielon G, Parisi F, Di Carlo D, et al. (2003) Orthotopic heart transplantation for failing single ventricle physiology. Eur J Cardiothorac Surg 24:502–510
Michielon G, Parisi F, Squitieri C, et al. (2002) Orthotopic heart transplantation for congenital heart disease: an alternative for high-risk Fontan candidates? Circulation 107:II-411 [abstract]
Nakagawa H, Shah N, Matsudaira K, et al. (2001) Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: isolated channels between scars allow “focal” ablation. Circulation 103:699–709
Ohye RG, Bradley D, Law I, Bove EL (2002) Primary surgical prevention of post-operative atrial re-entry tachycardia. Prog Pediatr Cardiol 14:223–228
Pearl JM, Laks H, Stein DG, et al. (1991) Total cavopulmonary anastomosis versus conventional modified Fontan procedure. Ann Thorac Surg 52:189–196
Peters NS, Somerville J (1992) Arrhythmias after the Fontan procedure. Br Heart J 68:199–204
Petko M, Myung RJ, Wernovsky G, et al. (2003) Surgical reinterventions following the Fontan procedure. Eur J Cardiothorac Surg 24:255–259
Piran S, Veldtman G, Siu S, Webb GD, Liu PP (2002) Heart failure and ventricular dysfunction in patients with single or systemic right ventricle. Circulation 105:1189–1194
Sanchez-Quintana D, Climent V, Ho SY, Anderson RH (1999) Myoarchitecture and connective tissue in hearts with tricuspid atresia. Heart 81:182–191
Scholl FG, Alejos JC, Laks H (1997) Revision of the traditional atriopulmonary Fontan connection. Adv Card Surg 9:217–227
Sheikh AM, Tang ATM, Roman K, et al. (2004) The failing Fontan circulation: successful conversion of atriopulmonary connections. J Thorac Cardiovasc Surg 128:60–66
Triedman JK, Alexander ME, Love BA, et al. (2002) Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol 39:1827–1835
Triedman JK, Saul JP, Weindling SN, Walsh EP (1995) Radiofrequency ablation of intra-atrial reentrant tachycardia after surgical palliation of congenital heart disease. Circulation 91:707–714
van Son JAM, Mohr FW, Hambsch J, et al. (1999) Conversion of atriopulmonary or lateral atrial tunnel cavopulmonary anastomosis to extracardiac conduit Fontan modification. Eur J Cardiothorac Surg 15:150–157
Vignati G, Crupi G, Vanini V, et al. (2003) Surgical treatment of arrhythmias related to congenital heart disease. Ann Thorac Surg 75:1194–1199
Vitullo DA, DeLeon SY, Berry TE, et al. (1996) Clinical improvement after revision in Fontan patients. Ann Thorac Surg 61:1797–1804
Warnes CA, Somerville J (1986) Tricuspid atresia in adolescents and adults: current state and late complications. Br Heart J 56:535–543
Warnes CA, Somerville J (1987) Tricuspid atresia with transposition of the great arteries in adolescents and adults: current state and late complications. Br Heart J 57:543–547
Weber HS, Hellenbrand WE, Kleinman CS, Perlmutter RA, Rosenfeld LE (1989) Predictors of rhythm disturbances and subsequent morbidity after the Fontan operation. Am J Cardiol 64:762–767
Weinstein S, Cua C, Chan D, Davis JT (2003) Outcome of symptomatic patients undergoing extracardiac Fontan conversion and cryoablation. J Thorac Cardiovasc Surg 126:529–536
Weipert J, Noebauer C, Schreiber C, et al. (2004) Occurrence and management of atrial arrhythmia after long-term Fontan circulation. J Thorac Cardiovasc Surg 127:457–464
Zrenner B, Ndrepepa G, Schneider MAE, et al. (2001) Mapping and ablation of atrial arrhythmias after surgical correction of congenital heart disease guided by a 64-electrode basket catheter. Am J Cardiol 88:573–578
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deal, B.J., Mavroudis, C. & Backer, C.L. Arrhythmia Management in the Fontan Patient. Pediatr Cardiol 28, 448–456 (2007). https://doi.org/10.1007/s00246-007-9005-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-007-9005-2