Abstract
We report our experience with the use of covered stents for the management of coarctation of the aorta. From December 2001 to March 2004, nine patients (seven males; median age, 31 years; mean weight, 65 ± 15 kg) underwent implantation. Indications included critical or atretic native coarctation (n = 4), patients >50 years of age (n = 2), associated patent ductus arteriosus (n = 1) or adjacent aneurysm (n = 1), and the presence of a circumferential fracture within a previously implanted stent (n = 1). The covered balloon-expandable Cheatham-Platinum stent and the self-expandable stent graft Braile were employed. Adequate implantation was observed in all patients. Gradients were reduced from 54 ± 14 to 3 ± 8 mmHg and the coarctation site increased from 2.4 ± 2.9 to 15.9 ± 4.3 mm. The patent ductus arteriosus was immediately closed and the aneurysm excluded. Two patients >35 years with aneurysmal ascending aorta and metallic aortic prosthesis had aneurysm formation at follow-up, with one undergoing aneurysm exclusion using a Braile stent. Although covered stents are useful in the management of selected patients with coarctation, aneurysm formation may still occur in patients with markers of aortic wall weakness. Refinements in the deployment technique and/or the stent design are needed to eliminate this risk.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Although coarctation of the aorta (CoA) is usually diagnosed and managed in infancy and childhood, patients may occasionally remain asymptomatic for a long time and the diagnosis is only made in adolescence and adulthood in the context of investigation for hypertension [15]. Balloon angioplasty of a discrete lesion has become an established alternative treatment in patients older than 1 year of age in most centers [9, 10, 17, 22]. However, it has been suggested that due to the presence of intrinsic aortic wall abnormalities secondary to aging, persistent residual gradients or aneurysm formation may follow balloon dilatation in adolescents and adults, leading to suboptimal outcomes [21]. During the past 10 years, stent implantation has been performed safely in these older age groups with excellent medium-term outcomes [1, 13, 14, 20, 30]. Although this technique is probably more effective for abolishing the aortic gradient than balloon dilatation alone, the risks of aneurysm formation, aortic dissection, and even with death have not been completely eliminated [18, 31]. In addition to the increasing use of stent grafts to treat atherosclerotic dissections and aneurysms in the aorta of adults [6], a covered balloon-expandable stent from NuMED has become available for clinical purposes [4, 25]. The use of these novel intravascular implants in patients with CoA is appealing since it may help to minimize the previously mentioned complications [5, 11, 12, 16]. Therefore, this study was conducted to determine the feasibility, safety, and efficacy of this management strategy in a small series of patients with CoA.
Materials and Methods
Patient Selection
Since September 1997, patients older than 10 years of age with a clinical diagnosis of CoA have been referred to our institution for possible stent implantation. Covered stent technology became available in mid-2001 in our pediatric catheterization laboratory. Thereafter, candidates for covered stent implantation as a primary procedure included older children, adolescents, and adults weighing at least 40 kg with a discrete or a diffuse form of native, recurrent, or persistent CoA and with one or more of the following underlying morphological characteristics:
-
“Critical” obstruction, defined by a minimum diameter at the CoA site ≤3 mm at catheterization
-
CoA associated with atresia of the aortic lumen detected at catheterization (“blind” CoA)
-
CoA associated with a patent ductus arteriosus (PDA), as previously described [28]
-
CoA associated with degenerative changes of the aortic wall, suggested by the presence of an aneurysmal ascending aorta and/or transverse arch
-
CoA in patients older than 30 years of age
Covered stent implantation was also indicated as a secondary procedure in two additional situations:
-
Aneurysm formation following standard stent implantation, detected acutely (as a “bailout” procedure) or at follow-up
-
The presence of circumferential fractures within a previously implanted stent in the aorta with malalignment between the proximal and distal ends and/or protrusion of the stent struts into the aortic wall at a repeat catheterization during follow-up
Covered Stents
Two types of covered stents were used in this series. The covered Cheatham-Platinum (CP) stent (NuMED, Nicholville, NY, USA) is a handmade stent composed of heat-tempered 90% platinum and 10% iridium metal alloy, with the 0.013-in. metal wire arranged in a “zig” pattern. Additional characteristics and potential advantages of this implant have been discussed in previous reports [3, 4, 25]. This stent is covered with an ultrathin stretchable ePTFE membrane applied to the stent using biodegradable adhesives. Stents with eight zigs were used to enable expansion up to 25 mm if needed [4]. Lengths from 39 to 60 mm were used to allow reliable stent placement, adequate coverage of coarctation or diseased segment, and incorporation of its proximal end into the isthmus.
The Braile stent is composed of a self-expandable stainless-steel cylindrical framework, made in Z segments, covered externally with polyester (Braile Biomedica, SJ Rio Preto, São Paulo, Brazil). Such structure is compressed in a delivery catheter made of polytetrafluoroethylene, ranging in size from 17 to 24 Fr. Length and diameter of the stent varied according to the extension and size of the diseased segment to be treated. These stents are available in lengths of 5–9 cm and, when fully expanded, their diameter ranges from 18 to 34 mm. Also, they can be custom made. In general, the selected diameter of the Braile stent is 2–4 mm or 10–20% larger than the diameter of the isthmus at the level of the take-off of the left subclavian artery. The last row of the Z segments is not covered, allowing free flow through the stent framework. Previous reports have shown the feasibility, safety, and efficacy of this stent in the treatment of atherosclerotic aortic disease in the adult [23, 27].
Procedure Protocol and Technique of Stent Implantation
Informed consent was obtained from parents or patients following the guidelines of the Human Subject Protection Committee at our institution. The procedure was performed under general anesthesia. Right femoral accesses were obtained and a 5-Fr and a 7-Fr sheath were inserted in the artery and vein, respectively. Heparin sulfate (100–150 IU/kg; maximum, 10,000 IU) was given. Standard right and left heart catheterization was carried out and angiograms in the aortic arch were taken in left lateral, shallow left anterior oblique, and shallow right anterior oblique with mild degree of caudal angulation views to best profile the lesion. To help proper stent deployment and take simultaneous pressure measurements after implantation, an angiographic catheter was positioned in the aortic arch through a transeptal approach or through a short 5-Fr sheath inserted in the right brachial artery. A marked pigtail catheter (Royal-Flush, Cook Cardiology, Bloomington, IN, USA) or a 10-mm metallic sphere was left on the chest for calibration purposes. The lesion was crossed in a retrograde manner using a 5-Fr right coronary Judkins catheter (Cordis Corporation, Miami, FL, USA) with the aid of a hydrophilic guidewire (Road-Runner, Cook Cardiology). The hydrophilic wire was exchanged for an Amplatz superstiff 0.035-in. 260-cm wire (Cook Cardiology), which was left either in the ascending aorta or in the right or left subclavian artery, enabling the straightest wire course possible for subsequent stent deployment. Critical lesions were predilated using 6- to 8-mm-diameter balloons.
The covered CP stent (NuMED) was delivered in a similar manner to the standard stents using the conventional backload technique, as described in previously published protocols [3, 14]. However, manual crimping of the covered CP stent onto the catheter balloon and loading the system stent balloon into the long sheath required extra care. The superstiff guidewire was left in the catheter balloon during the crimping maneuver in order to straighten the system and avoid damage to the balloon caused by the stent struts. Wetting the ePTFE layer was avoided at all times to keep its original shape around the stent, preventing unfolding and damaging. A cut-off large profile sheath (1Fr less than the long sheath placed in the artery) was used to protect the stent while advancing the stent–catheter balloon unit into the long sheath through the hemostatic valve. The ultimate profile of the long sheath required for implantation depended on the profile of the catheter balloon but was generally 3Fr larger than the balloon. We used 10- to 14-Fr blue long sheaths (Cook Cardiology) for CP stent delivery, which were left in the artery for the least amount of time as possible.
The balloon diameter was selected to equal that of the proximal isthmus at the level of the take-off of the left subclavian artery, not exceeding the diameter of the aorta at the level of the diaphragm. If the CoA site was located immediately distal to the subclavian artery, the stent was deployed across the artery ostium with the least degree of straddling as possible. The BIB balloon (NuMED) (outer balloon, 12–20 mm in diameter; 45–55 mm long) was used in all but one patient, who had balloon-expandable covered stent implantation. In a single patient, a Fox balloon (8 × 80 mm; JOMED, Beringen, Switzerland) was used to expand the covered CP stent in a segmentar lesion with critical obstruction. The balloons were slowly inflated by hand. After stent deployment, repeat angiograms and hemodynamic measurements were obtained to assess the immediate dilatation results.
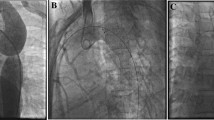
In patients with atresia of the aortic lumen (blind CoA), the distance between the proximal and distal ends of the aorta was assessed by simultaneous contrast injection in the aortic arch and the descending aorta just below the CoA site (Fig. 1). A right or left therapeutic Judkins coronary catheter (Medtronic, Minneapolis, MN, USA), was positioned in the proximal “beak” at the superior aortic end through either the right or the left subclavian artery. The atretic segment was then crossed or perforated using the soft (for “functional” atresia) or the stiff end (for “anatomical” atresia) of a 0.014-in. coronary wire. To monitor proper perforation and/or wire advancement, a Touhey-Borst was used to allow for repeat manual contrast injections through the therapeutic catheter. After perforation, the stiff end of the coronary wire was replaced by the soft end, which was then snared in the descending aorta to optimize support. A coronary angioplasty balloon (3 mm diameter, 2 cm long) was advanced through the therapeutic catheter over the wire to predilate the lesion. This enabled subsequent multiple catheter and wire exchange for further stent deployment from the femoral artery.
(Left) Simultaneous angiograms in the ascending and descending aorta in steep left anterior oblique view showing an atretic coarctation of the aorta with a short atretic segment between the proximal and distal ends, which was perforated using the stiff end of a coronary wire. (Middle) Shallow left anterior oblique view showing the immediate result after a covered CP stent implantation dilated to 18 mm. There is a slight line of dissection at the base of the origin of the left subclavian artery. (Right) Repeat angiogram after 4 months demonstrating distal progression of the dissection associated with a small aneurysm around the stent.
The Braile stent was advanced over the guidewire through a cut-down in the right or left femoral artery performed by the vascular surgeon. Once the system was adequately positioned, the delivery catheter was retracted, allowing stent expansion and apposition to the aortic wall.
Cephazolin (20 mg/kg) was given during the procedure and at 8-hour intervals (total of three doses). Heparin effect was partially neutralized (30–60%) with protamine. Hemostasis was achieved by manual compression in the balloon-expandable stent procedures. The femoral artery was repaired by the vascular surgeon in the self-expandable stent procedures. The patients were awakened in the catheterization laboratory and transferred to the recovery room for routine clinical observation and monitoring. Hospital discharge on the following day was planned for most patients. Upon discharge, patients were instructed to receive aspirin (100–200 mg/day) for 6 months, avoid contact sports for 3 months, and observe the recommendations for endocarditis prophylaxis for lifetime. A chest radiograph, an electrocardiogram, and a transthoracic echocardiogram were obtained before discharge.
Follow-Up
Follow-up visits were scheduled 1, 6, and 12 months after the procedure and yearly thereafter, with clinical, electrocardiographic, radiographic, and echocardiographic evaluation. A repeat catheterization under local anesthesia and/or a magnetic resonance imaging (MRI) study was scheduled after 6–18 months to assess stent position and the gradient across the stent, diameter at the dilated site, possible intimal hyperplasia, and the presence of an aneurysm (defined as a bulge ≥3 mm). Antihypertension medications were progressively discontinued, if possible, at the referring cardiologist's discretion.
Statistical Analysis
All values are described as frequencies, means (± standard deviations), or medians with range as applicable. A paired Student's t-test was used to compare changes in pressures and diameters before and after the procedure. An analysis of variance test was used to compare changes in pressures and diameters with time. The Sigmastat 2.0 (Jandel Corporation) statistical software, set to default settings, was used to perform the statistical analysis. The level of statistical significance was set at p < 0.05.
Results
Patient Population
From September 1997 to March 2003, 47 patients underwent stent implantation for the management of CoA at our institution. Of these patients, seven underwent primary and two secondary covered stent implantation from December 2001 to March 2004. Patient and procedure characteristics are shown in Tables 1 and 2. Seven patients were male. The median age at the time of the procedure was 31 years (range, 8–57). The mean weight was 65 ± 15 kg. All patients were hypertensive and taking medications, with the mean blood pressure taken in the right arm being 165 ± 15/105 ± 12 mmHg before implantation.
Morphological Characteristics and Associated Conditions
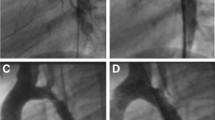
All patients except no. 9 had native CoA. Patient 1 had acute aneurysm formation at the CoA site after distal migration of a conventional stent (Palmaz 4014, Cordis) to the descending aorta (Fig. 2). No gradient was detected across the CoA site after stent migration. Patient 6 had a tubular form of CoA with critical obstruction associated with a hypoplastic aortic arch and isthmus (Fig. 3). Patient 7 had multiarterial coronary artery disease and was waiting surgical revascularization. Patient 9 had a circumferential fracture in a standard CP stent detected unexpectedly at a repeat catheterization performed 1 year after previous implantation. In this patient, a 5-mmHg gradient was recorded across the stent (Fig. 4).
(Left) Aortic angiogram in shallow left anterior oblique view demonstrating a small to moderate aneurysm at the left aspect of the aortic wall after distal migration of a standard stent (Palmaz 4014, Cordis), which was implanted just below the coarctation site. (Middle) Immediate exclusion of the aneurysm after implantation of a stent graft (Braile). (Right) Repeat angiogram after 1 year demonstrating no endovascular leak or significant neo-intimal proliferation.
(Left) Aortic angiogram in shallow left anterior oblique view demonstrating a tubular form of coarctation with critical obstruction associated with a hypoplastic aortic arch and isthmus. (Middle) Restoration of the aortic lumen after implantation of a long (6 cm) covered CP stent dilated to 8 mm. (Right) Follow-up (8 months) result after further stent dilatation to 10 mm. A 15-mmHg gradient persisted across the arch.
(Left) Circumferential fracture within a standard CP stent with malalignment of the two ends. (Right) Angiogram in shallow left anterior oblique view after deployment of a stent graft (Braile) within the fractured stent and a regular CP stent (with gold weldings) in the distal arch. Adequate coverage of the fractured area was achieved despite diminished flow to the left subclavian artery.
Immediate Results
The covered CP stent was used in seven patients and the Braile stent in the other two (Table 2). Adequate implantation was observed in all patients with no episodes of malposition or migration (Fig. 5). The CoA diameter increased from 2.4 ± 2.9 to 15.9 ± 4.3 mm (p < 0.001) and the pressure gradient across the CoA site decreased from 54.6 ± 14.5 to 2.9 ± 7.6 mmHg (p < 0.001) (excluding patients 1 and 9). The aortic aneurysm (patient 1) and the PDA (patient 4) were immediately and completely closed after stent implantation. In patient 9, after the initial deployment of a Braile stent, a regular new CP stent (with gold weldings) was implanted in the distal arch to optimize stent apposition, which resulted in diminished flow to the left subclavian artery (Fig. 4).
(Left) Angiogram in the transverse arch in lateral view showing an atretic coarctation. (Middle) Immediate result after stent expansion to the isthmus diameter (16) with restoration of the aortic lumen and no development of aortic wall abnormalities. (Right) Follow-up (1 year) angiogram demonstrating maintenance of the initial results.
Procedure Complications
In patient 2, a line of dissection was observed at the base of the origin of the left subclavian artery following stent deployment, as shown in Fig. 1. This patient also had significant blood loss and high arterial pressures after the procedure, requiring blood transfusion and nitroprusside infusion. There was no mortality, pulse loss, or neurological abnormalities after the procedure. Patient 9 had slightly diminished blood pressure in the left arm. Eight of the nine patients were discharged home the following day.
Clinical Follow-Up
At a mean follow-up of 12 ± 4 months, all patients had normal femoral and distal pulses. Patient 6 had a 20-mmHg systolic pressure gradient between upper and lower limbs. No arm–leg gradient was detected in the remaining patients. Systemic blood pressure was a mean of 130 ± 10/90 ± 10 mmHg. It was possible to either discontinue (n = 4) or reduce the dosages (n = 5) of antihypertensives at the last clinical assessment.
Hemodynamic and Angiographic Reassessment
Eight patients underwent repeat catheterization (n = 7) or an MRI study (n = 1) at a mean follow-up of 12 ± 5 months. The mean CoA gradient and diameter were 3.3 ± 8.2 mmHg and 15.5 ± 4.0 mm, respectively, not statistically different from the values found immediately after the procedure (both p > 0.05). There was no evidence of significant late neointimal growth within the stent, strut fractures, or recanalization of the PDA. The angio MRI study performed in patient 8 failed to demonstrate the aortic lumen within the stent (Fig. 6). Late aneurysms were observed in two patients. In patient 2, a small aneurysm was seen all around the covered CP stent (Fig. 1). A decision was made not to perform any additional procedures and imaging follow-up was planned. In patient 3, a large aneurysm was seen in the left posterior aspect of a covered CP stent (Fig. 7), filled through the inferior aspect of the stent.
(Top left) Angiogram in the transverse arch in lateral view showing a critical coarctation. (Top right) Immediate result after stent expansion to the isthmus diameter (12 mm). (Bottom left) Magnetic resonance imaging (MRI) study after 8 months demonstrating good stent position with no signs of recoarctation or aneurysm formation. (Bottom right) Angio MRI study demonstrating a drop off in the signal within the covered stent.
(Top left) Angiogram in shallow right oblique view showing a coarctation of the aorta measuring 5 mm at the narrowest site. (Top right) Immediate result after implantation of a covered CP stent dilated up to the isthmus diameter (18 mm). (Bottom left) Late (1 year) angiogram in shallow left oblique view demonstrating a large aneurysm at the left posterior aspect of the aorta. (Bottom right) Exclusion of the aneurysm after deployment of a stent graft (Braile).
Further Procedures
Patient 2 underwent a successful and uneventful repair of the aneurysmal ascending aorta under total circulatory arrest 4 months after stent implantation. Patient 3 underwent successful aneurysm exclusion using a custom-made self-expandable Braile stent (8 cm long) at a repeat catheterization (Fig. 7). Patient 6 underwent further dilatation of the previously implanted stent to 10 mm at repeat catheterization and remained with a 15-mmHg gradient across the aortic arch (Fig. 3). Patient 7 underwent a coronary revascularization operation 6 months after stent implantation and had an uneventful recovery.
Discussion
Although bare stents have been used successfully to abolish the aortic gradient in the management of CoA, aneurysm formation remains a concern, with a reported incidence of less than 5% [1, 13, 14, 20, 30]. Theoretically, covered stents may avoid this complication; however, there is a paucity of information in this regard [5, 11, 12, 16, 25]. This study demonstrated that the use of covered stents for the management of selected patients with CoA is feasible, safe, and generally effective. In this small series, all procedures were completed successfully and major complications were not encountered. However, in order to achieve the best results in terms of preventing aneurysm formation, we think that the type of stent and the technique for deployment should be tailored depending on the basic scenario in which the covered stents are utilized. First, in the young patient (<30–35 years) with a critical obstruction (associated or not with a PDA), immediate full dilatation of a covered CP stent was safe and effective in this series. Gradient relief was achieved with no development of aortic wall abnormalities in such patients. Also, immediate PDA obliteration was observed in one patient, avoiding the use of two separate devices to address the two lesions [2], as previously reported [28]. Second, in patients with markers of aortic wall weakness, such as an advanced age [29], aneurysmal ascending aorta [19], and a dysfunctional bicuspid aortic valve [8, 19, 26] requiring replacement, we speculate whether the initial strategy regarding the technique for stent implantation should be modified. In two of three such patients in this series, the use of a covered CP stent was not sufficient to prevent the development of dissections/aneurysms. In patient 2, the line of dissection at the base of the left subclavian artery progressed and a small aneurysm around the stent was observed at follow-up. In patient 3, late aneurysm formation occurred silently and unexpectedly and might have been related to tears or shrinkage in the ePTFE membrane during covered CP stent deployment. Staged stent dilatation has been recommended for patients with critical lesions [7, 20], avoiding an abrupt increase at the CoA site, which may result in aortic wall dissection, rupture, or aneurysm formation [18, 31]. Generally, it has not been our policy to routinely employ this strategy. In our experience, immediate full dilatation of the stent has not resulted in the development of dissections, bulges, or aneurysms in patients without markers of aortic wall weakness (unpublished data). However, in these high-risk patients a staged approach may be a more effective means to prevent the development of such aortic wall abnormalities. Alternatively, although a self-expandable stent does not have sufficient radial force to open a tight CoA, we speculate whether this type of implant should be used initially, creating a favorable substrate for further stent dilatation in a repeat catheterization performed after 6 months. Finally, in patients with acute or late aneurysm formation after CoA stenting, although the use of a balloon-expandable covered stent is likely to be safer than the use of coils to thrombose the aneurysm across the struts of a bare stent [24], the use of a self-expandable covered stent for aneurysm exclusion is probably even safer since it avoids an additional local vascular trauma caused by the radial forces of the balloon, which may result in aneurysm progression and rupture. Because the use of a covered stent does not eliminate the risk of late aneurysm formation, close follow-up using imaging techniques is mandatory. Interestingly, angio MRI was not an adequate means to delineate the aortic lumen within the covered CP stent in a patient in this series. The drop-off in the signal within the endovascular implant was likely caused by the barrier effect of the ePTFE membrane. Whether this may hinder the recognition of an aneurysm adjacent to the stent is speculative.
From a safety standpoint, the main concern with the use of covered stents in the aorta is the possibility of occlusion of side branches, especially the spinal artery resulting in paraplegia or paraparesis [6]. This complication is uncommon when the covered segment is short and limited to the thoracic aorta [6]. Avoiding the aortic territory below the ninth thoracic vertebra may also minimize these catastrophic events since the spinal artery usually arises below this point. Also, covered stents are less forgiving than the bared ones when distal migration occurs. One should avoid implantation at sites that may obstruct the origin of vessels, such as the mesenteric and renal arteries in the abdominal aorta. Although this was not evaluated in this study, we believe that the use of the BIB balloon (NuMED) yields a more controlled stent deployment, minimizing the risk of inadvertent migration. In this series, most covered stents were short (39–45 mm long) and implanted to the proximal thoracic aorta. Although in three patients longer covered stents (60–80 mm long) were used, no neurological complications were encountered. We speculate that the lack of significant peripheral atherosclerosis in patients with CoA, who are usually younger than patients with atherosclerotic thoracic and abdominal aneurysms, may be an important protective factor against these complications.
This study also illustrated the use of a stent graft to manage a circumferential fracture with protrusion of the stent struts into the aortic wall. The occurrence of fractures within the CP stent has been acknowledged in clinical practice [3, 4] but has been infrequently reported. Although a localized fracture between two rows is probably benign with limited clinical implications, a circumferential fracture may result in distal embolization of the fragments and unpredictable consequences. Because there was no change in the stent position at late catheterization, we speculate that this type of fracture occurred after endothelialization of the intravascular implant. Refinements in the welding process using gold have been employed by the manufacturer to manage this problem (John Cheatham, personal communication). However, a decision was made to implant a self-expandable Braile stent graft to minimize the risk of subsequent wall erosion and perforation due to protrusion of the stent struts. Previous reports have documented the resistance of this endovascular stent graft to fatigue [23, 27].
In conclusion, although covered stents are useful in the management of selected patients with CoA, abnormalities of the aortic wall may occur after implantation of such endovascular implants, which may require further procedures. These abnormalities are probably more common in high-risk patients with markers of aortic wall weakness, such as an advanced age, aneurysmal ascending aorta, and a previous dysfunctional bicuspid aortic valve requiring replacement. Refinements in the deployment technique and/or in the stent design are needed to eliminate this risk. A larger number of patients and longer follow-up are required to draw stronger conclusions.
References
ZR Bulbul E Bruckheimer JC Love JT Fahey WE Hellenbrand (1996) ArticleTitleImplantation of balloon-expandable stents for coarctation of the aorta: implantation data and short-term results Cathet Cardiovasc Diagn 39 36–42 Occurrence Handle8874943 Occurrence Handle1:STN:280:DyaK2s%2FivV2gsw%3D%3D
JP Cheatham (1999) ArticleTitleStents and Amplatzers: what's an interventionalist to do? Cathet Cardiovasc Interv 47 39–40 Occurrence Handle1:STN:280:DyaK1MzhtFyrsA%3D%3D
JP Cheatham (2001) ArticleTitleStenting of coarctation of the aorta Cathet Cardiovasc Interv 54 112–125 Occurrence Handle10.1002/ccd.1249 Occurrence Handle1:STN:280:DC%2BD3MrgtlKitg%3D%3D
JP Cheatham (2003) NuMED Cheatham platinum stents: role in the management of congenital heart defects PS Rao MJ Kern (Eds) Catheter Based Devices Lippincott Williams & Wilkins Philadelphia 353–368
JV Giovanni Particlede (2001) ArticleTitleCovered stents in the treatment of aortic coarctation J Interv Cardiol 14 187–190 Occurrence Handle12053302
RS Dieter (2001) ArticleTitleTransluminal endovascular stent grafting of aortic dissections and aneurysms: a concise review of the major trials Clin Cardiol 24 358–363 Occurrence Handle11346242 Occurrence Handle1:STN:280:DC%2BD3M3ltVChsw%3D%3D Occurrence Handle10.1002/clc.4960240503
C Duke E Rosenthal SA Qureshi (2003) ArticleTitleThe efficacy and safety of stent redilatation in congenital heart disease Heart 89 905–912 Occurrence Handle12860870 Occurrence Handle1:STN:280:DC%2BD3szjtVyjsQ%3D%3D
WD Edwards DS Leaf JE Edwards (1978) ArticleTitleDissecting aortic aneurysm associated with congenital bicuspid aortic valve Circulation 57 1022–1025 Occurrence Handle639201 Occurrence Handle1:STN:280:DyaE1c7ks1ygsA%3D%3D
ME Fawzy M Awad H Hegazy et al. (2003) ArticleTitleLong-term follow-up (15-year) results of balloon coarctation angioplasty in the adolescent and adult patient J Am Coll Cardiol 41 488 Occurrence Handle10.1016/S0735-1097(03)82667-0
SE Fletcher MR Nihill RG Grifka MP O'Laughlin CE Mullins (1995) ArticleTitleBalloon angioplasty of native coarctation of the aorta: midterm follow-up and prognostic factors J Am Coll Cardiol 25 730–734 Occurrence Handle7860921 Occurrence Handle10.1016/0735-1097(94)00437-U Occurrence Handle1:STN:280:DyaK2M7msVykuw%3D%3D
T Forbes D Matisoff J Dysart S Aggarwal (2003) ArticleTitleTreatment of coexistent coarctation and aneurysm of the aorta with covered stent in a pediatric patient Pediatr Cardiol 24 289–291 Occurrence Handle12522654 Occurrence Handle10.1007/s00246-002-0262-9 Occurrence Handle1:STN:280:DC%2BD3s3mt12muw%3D%3D
J Gunn T Cleveland P Gaines (1999) ArticleTitleCovered stent to treat co-existent coarctation and aneurysm of the aorta in a young man Heart 82 351 Occurrence Handle10455088 Occurrence Handle1:STN:280:DyaK1Mzoslagtg%3D%3D
MA Hamdan S Maheshwari JT Fahey WE Hellenbrand (2001) ArticleTitleEndovascular stents for coarctation of the aorta: initial results and intermediate-term follow-up J Am Coll Cardiol 38 1518–1523 Occurrence Handle11691533 Occurrence Handle10.1016/S0735-1097(01)01572-8 Occurrence Handle1:STN:280:DC%2BD3MnktVOrsw%3D%3D
DA Harrison PR McLaughlin C Lazzam M Connelly LN Benson (2001) ArticleTitleEndovascular stents in the management of coarctation of the aorta in the adolescent and adult: one year follow up Heart 85 561–566 Occurrence Handle11303011 Occurrence Handle10.1136/heart.85.5.561 Occurrence Handle1:STN:280:DC%2BD3M3ms1Wjsg%3D%3D
H Kaemmerer (2003) Aortic coarctation and interrupted aortic arch MA Gatzoulis GD Webb PEF Daubeney (Eds) Diagnosis and Management of Adult Congenital Heart Disease Churchill Livingstone Edinburgh, UK 253–264
MS Khan JW Moore (2000) ArticleTitleTreatment of abdominal aortic pseudoaneurysm with covered stents in a pediatric patient Cathet Cardiovasc Interv 50 445–448 Occurrence Handle10.1002/1522-726X(200008)50:4<445::AID-CCD17>3.0.CO;2-1 Occurrence Handle1:STN:280:DC%2BD3cvjsFSnsQ%3D%3D
J Koerselnan H Vries Particlede W Jaarsma et al. (2000) ArticleTitleBalloon angioplasty of coarctation of the aorta: a safe alternative for surgery in adults: immediate and mid-term results Cathet Cardiovasc Interv 50 28–33
SJ Korkola CI Tchervenkov D Shum-Tim N Roy (2002) ArticleTitleAortic rupture after stenting of a native coarctation in an adult Ann Thorac Surg 74 936 Occurrence Handle12238877 Occurrence Handle10.1016/S0003-4975(01)03160-5
J Lindsay SuffixJr (1988) ArticleTitleCoarctation of the aorta, bicuspid aortic valve and abnormal ascending aortic wall Am J Cardiol 61 182–184 Occurrence Handle3276120 Occurrence Handle10.1016/0002-9149(88)91327-6
AG Magee G Brzezinska-Rajszys SA Qureshi et al. (1999) ArticleTitleStent implantation for aortic coarctation and recoarctation Heart 82 600–606 Occurrence Handle10525517 Occurrence Handle1:STN:280:DyaK1Mvlslaiug%3D%3D
BW McCrindle TK Jones WR Moaow et al. (1996) ArticleTitleAcute results of balloon angioplasty of native coarctation versus recurrent aortic obstruction are equivalent. Valvuloplasty and Angioplasty of Congenital Anomalies (VACA) Registry Investigators J Am Coll Cardiol 28 1810–1817 Occurrence Handle8962571 Occurrence Handle1:STN:280:DyaK2s7it1ahug%3D%3D
C Ovaert BW McCrindle D Nykanen et al. (2000) ArticleTitleBalloon angioplasty of native coarctation: clinical outcomes and predictors of success J Am Coll Cardiol 35 988–996 Occurrence Handle10732899 Occurrence Handle10.1016/S0735-1097(99)00646-4 Occurrence Handle1:STN:280:DC%2BD3c7psFSgtQ%3D%3D
JH Palma JA Souza Particlede CM Rodrigues Alves et al. (2002) ArticleTitleSelf-expandable aortic stent-grafts for treatment of descending aortic dissections Ann Thorac Surg 73 1138–1141 Occurrence Handle11996254 Occurrence Handle10.1016/S0003-4975(02)03397-0
CA Pedra CB Pilla SL Braga CA Esteves VF Fontes (2002) ArticleTitleManagement of a large pseudoaneurysm secondary to balloon dilatation for native coarctation of the aorta with coil occlusion after stent implantation in a child Cathet Cardiovasc Interv 56 262–266 Occurrence Handle10.1002/ccd.10185
SA Qureshi M Zubrzycka G Brzezinska-Rajszys A Kosciesza J Ksiazyk (2004) ArticleTitleThe use of covered Cheatham-Platinum stents in aortic coarctation and recoarctation Cardiol Young 14 50–54 Occurrence Handle15237671 Occurrence Handle10.1017/S104795110400109X
CS Roberts WC Roberts (1991) ArticleTitleDissection of the aorta associated with congenital malformation of the aortic valve J Am Coll Cardiol 17 712–716 Occurrence Handle1993792 Occurrence Handle1:STN:280:DyaK3M7jtF2juw%3D%3D Occurrence Handle10.1016/S0735-1097(10)80188-3
CM Rodrigues Alves JH da Fonseca JA Souza Particlede AC Camargo Carvalho E Buffolo (2002) ArticleTitleEndovascular treatment of thoracic disease: patient selection and a proposal of a risk score Ann Thorac Surg 73 1143–1148 Occurrence Handle11996255
M Sadiq NH Malick SA Qureshi (2003) ArticleTitleSimultaneous treatment of native coarctation of the aorta combined with patent ductus arteriosus using a covered stent Cathet Cardiovasc Interv 59 387–390 Occurrence Handle10.1002/ccd.10501
TJ Schlatmann AE Becker (1977) ArticleTitleHistologic changes in the normal aging aorta: implications for dissecting aortic aneurysm Am J Cardiol 39 13–20 Occurrence Handle831420 Occurrence Handle1:STN:280:DyaE2s%2FosV2hsQ%3D%3D
BD Thanopoulos L Hadjinikolaou GN Konstadopoulou et al. (2000) ArticleTitleStent treatment for coarctation of the aorta: intermediate term follow up and technical considerations Heart 84 65–70 Occurrence Handle10862593 Occurrence Handle10.1136/heart.84.1.65 Occurrence Handle1:STN:280:DC%2BD3czitFajtg%3D%3D
C Varma LN Benson J Butany PR McLaughlin (2003) ArticleTitleAortic dissection after stent dilatation for coarctation of the aorta: a case report and literature review Cathet Cardiovasc Interv 59 528–535 Occurrence Handle10.1002/ccd.10548 Occurrence Handle1:STN:280:DC%2BD3szlslyiuw%3D%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pedra, C., Fontes, V., Esteves, C. et al. Use of Covered Stents in the Management of Coarctation of the Aorta. Pediatr Cardiol 26, 431–439 (2005). https://doi.org/10.1007/s00246-004-0814-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-004-0814-2